Abstract
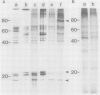
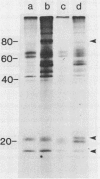
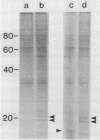
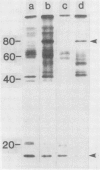
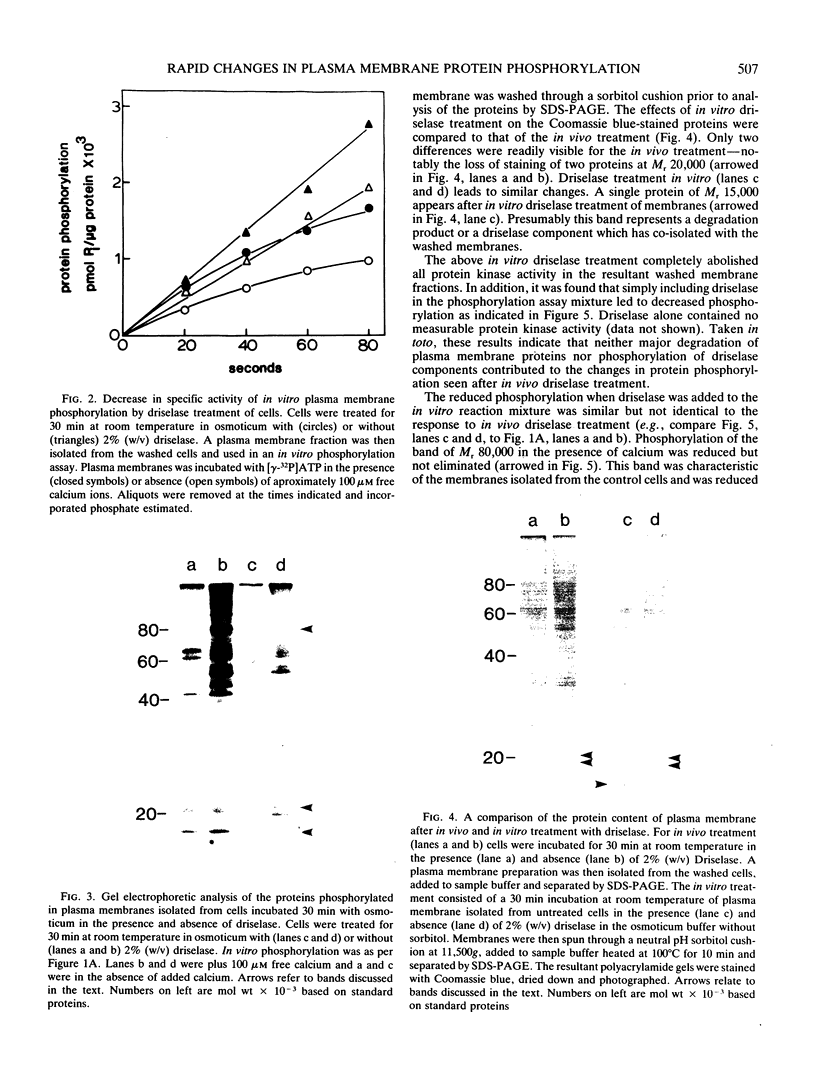
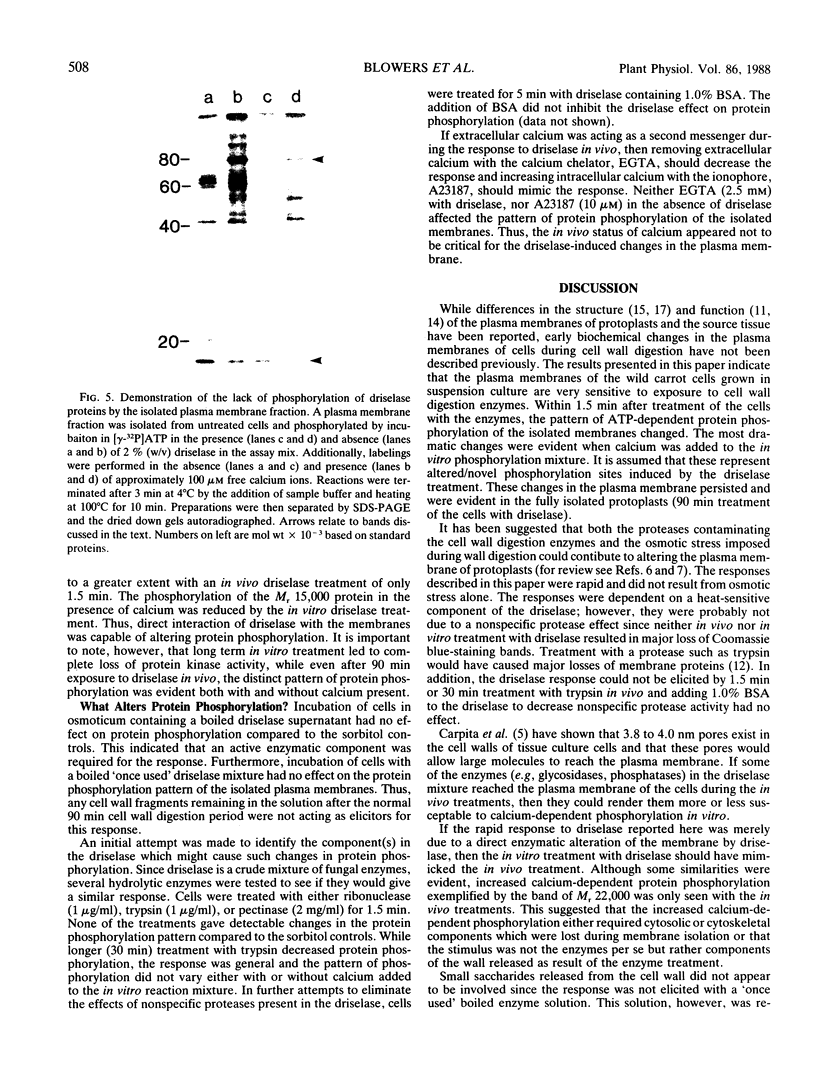
Plasma membrane vesicles from wild carrot cells grown in suspension culture were isolated by aqueous two-phase partitioning, and ATP-dependent phosphorylation was measured with [γ-32P]ATP in the presence and absence of calcium. Treatment of the carrot cells with the cell wall digestion enzymes, driselase, in a sorbitol osmoticum for 1.5 min altered the protein phosphorylation pattern compared to that of cells treated with sorbitol alone. Driselase treatment resulted in decreased phosphorylation of a band of Mr 80,000 which showed almost complete calcium dependence in the osmoticum treated cells; decreased phosphorylation of a band of Mr 15,000 which showed little calcium activation, and appearance of a new band of calcium-dependent phosphorylation at Mr 22,000. These effects appeared not to be due to nonspecific protease activity and neither in vivo nor in vitro exposure to driselase caused a significant loss of Coomassie blue-staining bands on the gels of the isolated plasma membranes. However, protein phosphorylation was decreased. Adding driselase to the in vitro reaction mixture caused a general decrease in the membrane protein phosphorylation either in the presence or absence of calcium which did not mimic the in vivo response. Cells labeled in vivo with inorganic 32P also showed a response to the Driselase treatment. An enzymically active driselase preparation was required for the observed responses.
Full text
PDF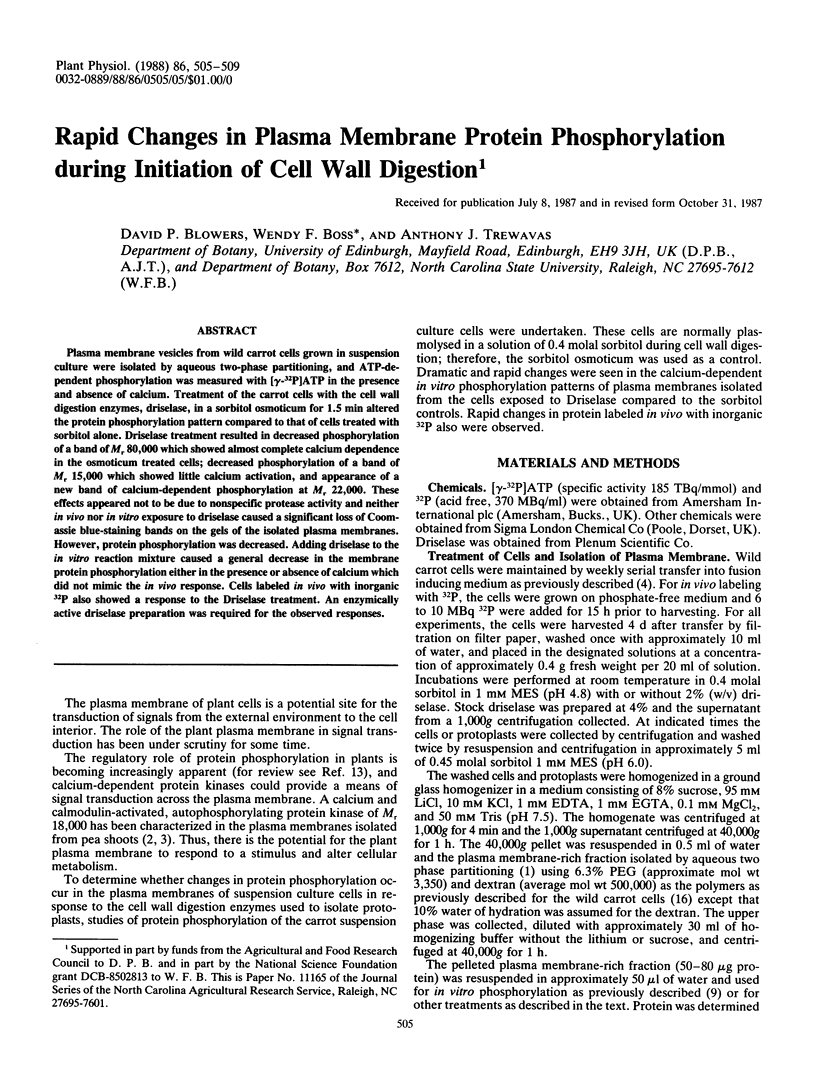


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTSSON P. A. Particle fractionation in liquid two-phase systems; the composition of some phase systems and the behaviour of some model particles in them; application to the isolation of cell walls from microorganisms. Biochim Biophys Acta. 1958 Feb;27(2):378–395. doi: 10.1016/0006-3002(58)90345-7. [DOI] [PubMed] [Google Scholar]
- Blowers D. P., Trewavas A. J. Autophosphorylation of plasma membrane bound calcium calmodulin dependent protein kinase from pea seedlings and modification of catalytic activity by autophosphorylation. Biochem Biophys Res Commun. 1987 Mar 13;143(2):691–696. doi: 10.1016/0006-291x(87)91409-4. [DOI] [PubMed] [Google Scholar]
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Randall S. K., Ruesink A. W. Orientation and integrity of plasma membrane vesicles obtained from carrot protoplasts. Plant Physiol. 1983 Oct;73(2):385–391. doi: 10.1104/pp.73.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J. J., Boss W. F. Polyphosphoinositides are present in plasma membranes isolated from fusogenic carrot cells. Plant Physiol. 1987 Oct;85(2):389–392. doi: 10.1104/pp.85.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M. J., Northcote D. H. Plasma membrane ultrastructure during plant protoplast plasmolysis, isolation and wall regeneration: a freeze-fracture study. J Cell Sci. 1980 Apr;42:401–415. doi: 10.1242/jcs.42.1.401. [DOI] [PubMed] [Google Scholar]