Abstract
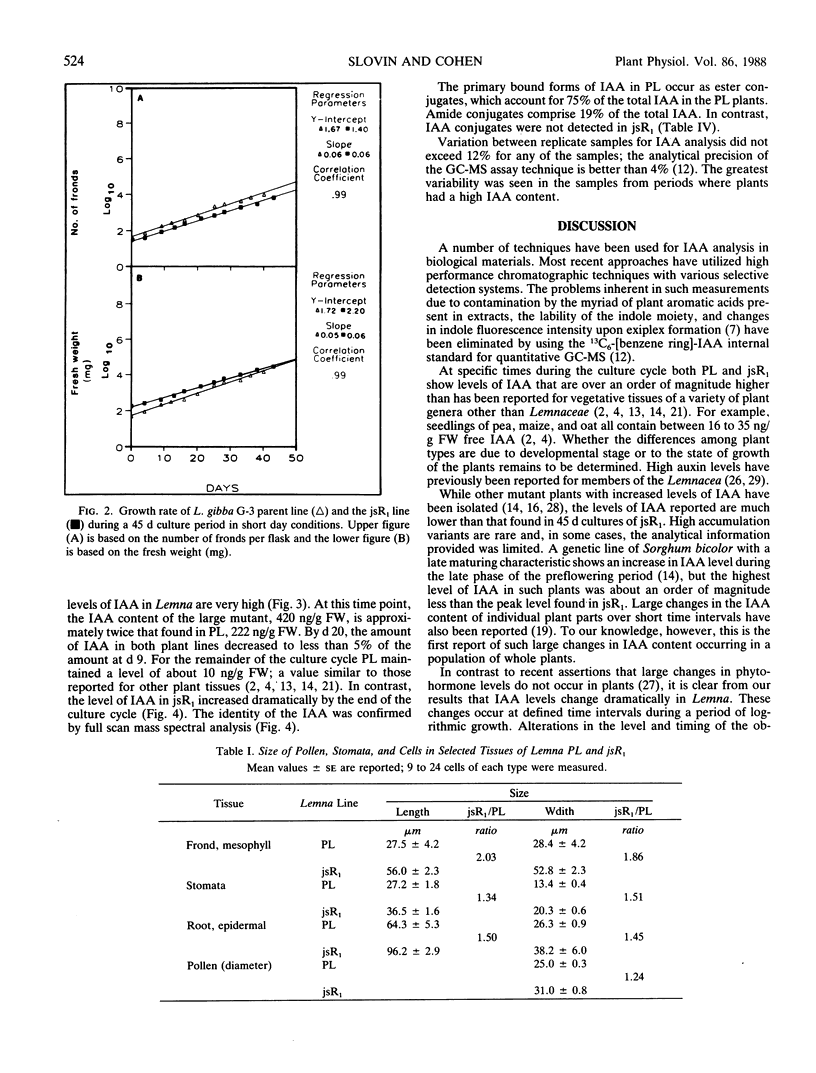
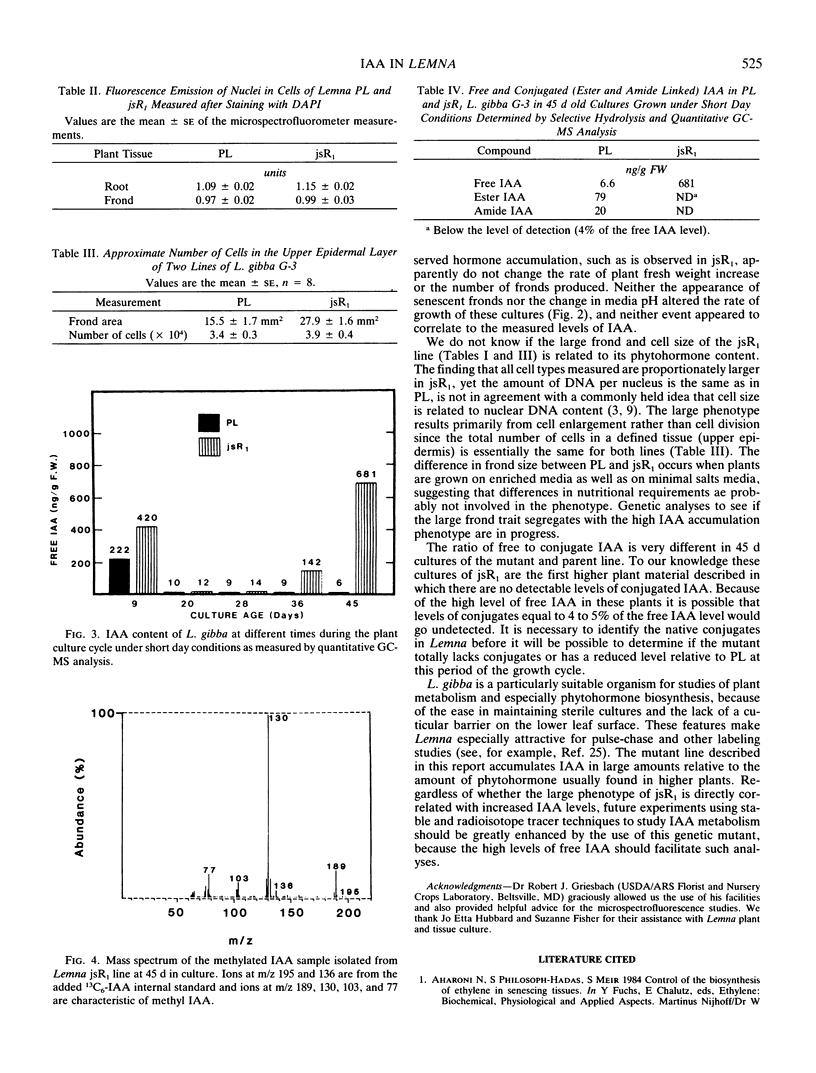
Large changes in indole-3-acetic acid (IAA) levels occur during growth of Lemna gibba G-3 in sterile culture. The levels of IAA were measured in plants during a 45 day growth cycle using HPLC and isotope dilution analysis followed by selected ion current monitoring GC-MS analysis with 13C6-IAA as the internal standard. Even though the rate of plant growth remained constant over the entire growth period, IAA levels ranged from a high of 222 to a low of 6 nanograms per gram fresh weight. A Lemna mutant (jsR1) which has a giant phenotype was obtained by regeneration from primary callus cultures. Microspectrofluorometry of diamidino-2-phenylindole stained cells showed that jsR1 has the same amount of DNA per nucleus as the parent line (PL). All jsR1 cell types measured are about 1.5 times larger than in PL. The endogenous levels of IAA per gram fresh weight were higher in jsR1 at several stages of the plant culture cycle as compared to PL. This difference ranged from 1.2 to over 100 times as much. While PL showed only one high peak at day 9, jsR1 had IAA levels of 480 and 680 nanograms per gram fresh weight at days 9 and 45, respectively. Throughout the midculture stage of the growth cycle (20-28 days) both jsR1 and PL had IAA levels in the range of 9 to 14 nanograms per gram fresh weight. In contrast to PL, at day 45, jsR1 had no detectable ester or amide conjugates of IAA. These changes in IAA levels were determined in sterile plant cultures and thus cannot be attributed to bacterial or fungal activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A., Dayanandan P., Kaufman P. B. Response to gravity by Zea mays seedlings. I. Time course of the response. Plant Physiol. 1984;74:284–288. doi: 10.1104/pp.74.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland C. F., Briggs W. R. Flowering Responses of the Long-day Plant Lemna gibba G3. Plant Physiol. 1967 Nov;42(11):1553–1561. doi: 10.1104/pp.42.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Baldi B. G., Slovin J. P. C(6)-[benzene ring]-indole-3-acetic Acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic Acid in plants. Plant Physiol. 1986 Jan;80(1):14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Magnus V., Bandurski R. S., Schulze A. Synthesis of 4,5,6,7 and 2,4,5,6,7 Deuterium-labeled Indole-3-Acetic Acid for Use in Mass Spectrometric Assays. Plant Physiol. 1980 Oct;66(4):775–781. doi: 10.1104/pp.66.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]