Abstract
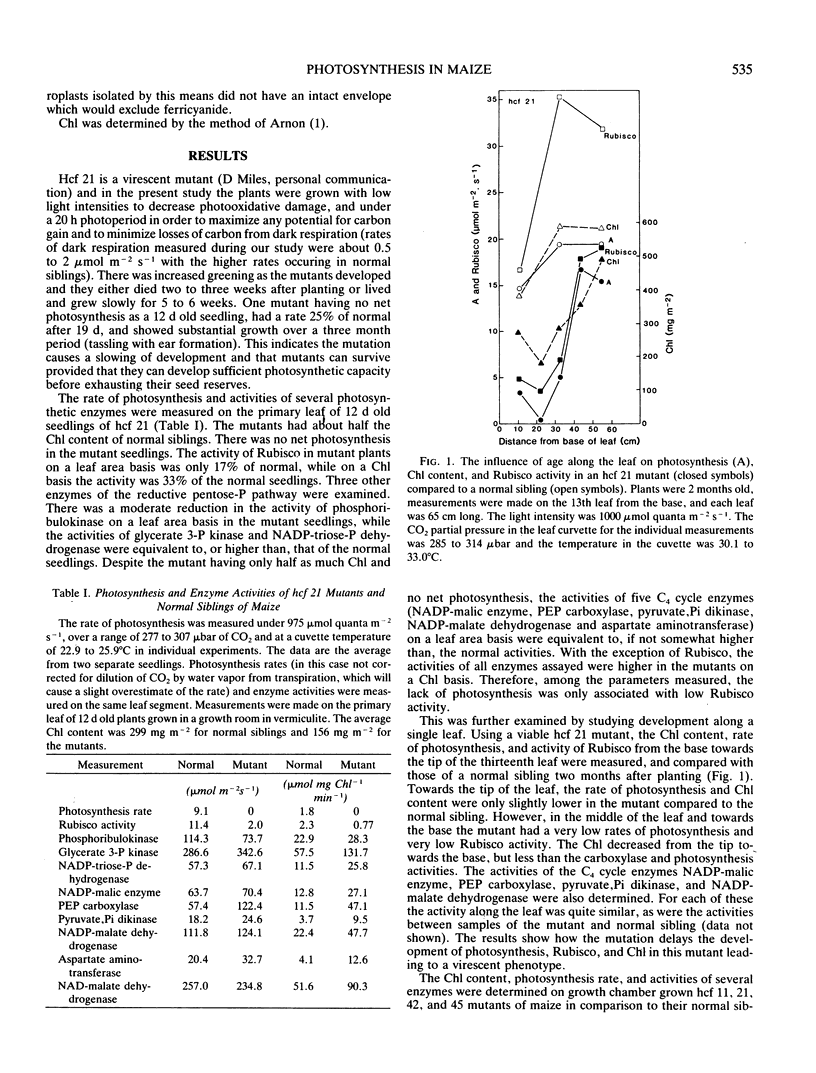
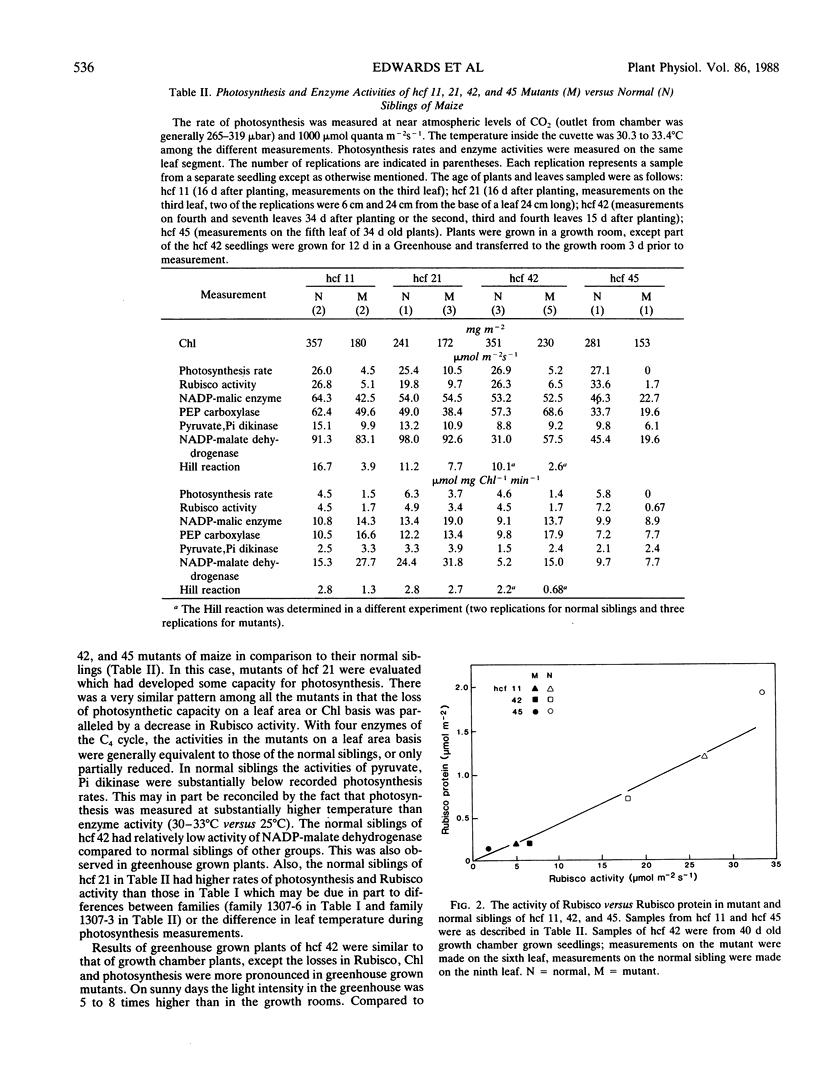
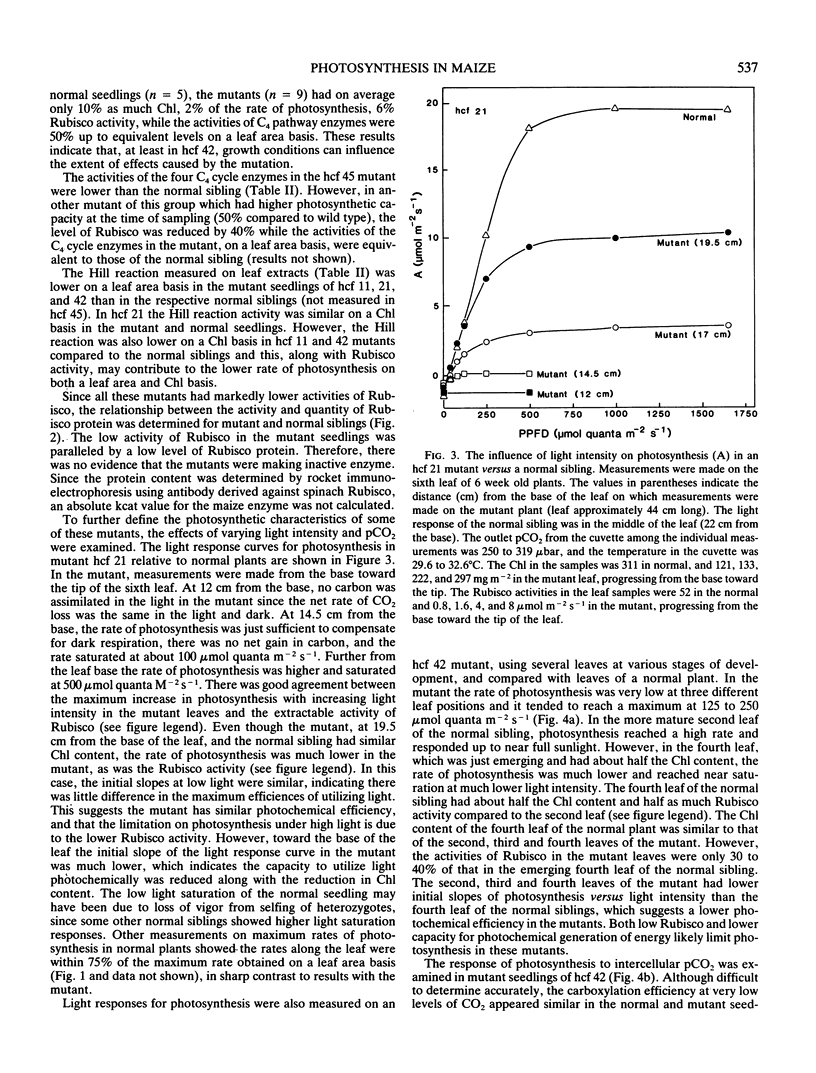
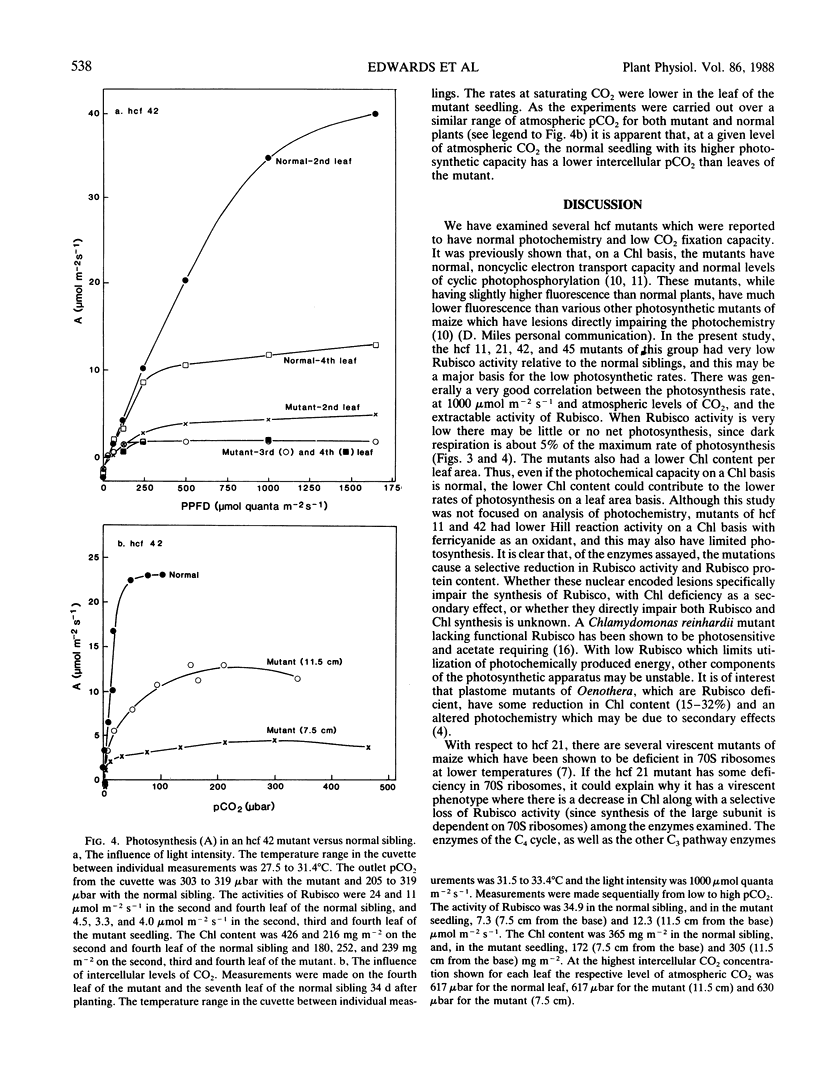
Photosynthetic properties were examined in several hcf (high chlorophyll fluorescence 11, 21, 42 and 45) nuclear recessive mutants of maize which were previously found to have normal photochemistry and low CO2 fixation. Mutants usually either died after depletion of seed reserves (about 18 days after planting), or survived with slow growth up to 7 or 8 weeks. Both the activity and quantity of ribulose 1,5-bisphosphate carboxylase (Rubisco) were low in the mutants (5-25% of the normal siblings on a leaf area basis) and the loss of Rubisco tended to parallel the reduction in photosynthetic capacity. The Rubisco content in the mutants was often marginal for photosynthetic carbon gain, with some leaves and positions along a leaf having no net photosynthesis, while other leaves had a low carbon gain. Conversely, the activities of C4 cycle enzymes, phosphoenolpyruvate carboxylase, pyruvate, Pi dikinase, NADP-malate dehydrogenase, and NADP-malic enzyme, were the same or only slightly reduced compared to the normal siblings. The mutants had about half as much chlorophyll content per leaf area as the normal green plants. However, the Rubisco activity in the mutants was low on both a leaf area and chlorophyll basis. Low Rubisco activity and lower chlorophyll content may both contribute to the low rates of photosynthesis in the mutants on a leaf area basis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag S., Jenkins C. L. The Involvement of Aspartate and Glutamate in the Decarboxylation of Malate by Isolated Bundle Sheath Chloroplasts from Zea mays. Plant Physiol. 1986 May;81(1):115–119. doi: 10.1104/pp.81.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallier U. W., Schmitt J. R., Heber U., Chaianova S. V., Volodarsky A. D. Ribulose-1,5-biphosphate carboxylase-deficient plastome mutants of Oenothera. Biochim Biophys Acta. 1978 Oct 11;504(1):67–83. doi: 10.1016/0005-2728(78)90007-5. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Activity, location, and role of asparate aminotransferase and alanine aminotransferase isoenzymes in leaves with C4 pathway photosynthesis. Arch Biochem Biophys. 1973 May;156(1):195–206. doi: 10.1016/0003-9861(73)90357-3. [DOI] [PubMed] [Google Scholar]
- Jenkins C. L., Hatch M. D. Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase. Arch Biochem Biophys. 1985 May 15;239(1):53–62. doi: 10.1016/0003-9861(85)90811-2. [DOI] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F., Hatch M. D. Regulation of NADP-malate dehydrogenase in C4 plants: relationship among enzyme activity, NADPH to NADP ratios, and thioredoxin redox states in intact maize mesophyll chloroplasts. Arch Biochem Biophys. 1986 Aug 15;249(1):171–179. doi: 10.1016/0003-9861(86)90572-2. [DOI] [PubMed] [Google Scholar]


