Abstract
The therapeutic effectiveness of water-soluble echinocandin compounds obtained from Coleophoma empetri F-11899, which has a strong inhibitory effect on the growth of fungi, was examined in nude mice with experimental Pneumocystis pneumonia. The studies demonstrated the potential usefulness of the compounds.
Recently, it has been reported that a β-1,3-d-glucan synthesis inhibitor has inhibitory effects on the growth of fungi through inhibition of the synthesis of the β-1,3-glucan which is present in fungal cell walls. The inhibitor is also effective against Pneumocystis carinii infection because the cell wall of the P. carinii cyst resembles that of the yeast Saccharomyces cerevisiae and contains high levels of β-1,3-glucan (6). Since β-1,3-glucan synthesis activity is not present in mammalian cells, it was thought that inhibition of β-1,3-glucan synthesis might be a good target for the prevention of the formation of P. carinii cysts and thus for the selective killing of P. carinii in lungs infected with this organism. Generally, however, the lipopeptide compounds so far reported as being β-1,3-d-glucan inhibitors are hardly soluble in water. This insolubility limits their potential use as parenterally administered agents and is one of the reasons why they cannot be developed for clinical use. It was reported that semisynthetic water-soluble prodrugs synthesized from naturally occurring lipopeptide products are effective for the treatment and prevention of P. carinii infection in rats treated with cortisone (9, 10). Recently, we found that a strain of Coleophoma empetri, strain F-11899, produces water-soluble echinocandin analogs which have strong inhibitory effects on the growth of fungi (5).
In the study described in this paper, we examined the therapeutic effectiveness of these water-soluble echinocandin analogs on Pneumocystis pneumonia in immunodeficient animals. The results of this study indicate that the compounds are potentially useful for the treatment of Pneumocystis pneumonia.
MATERIALS AND METHODS
Compounds.
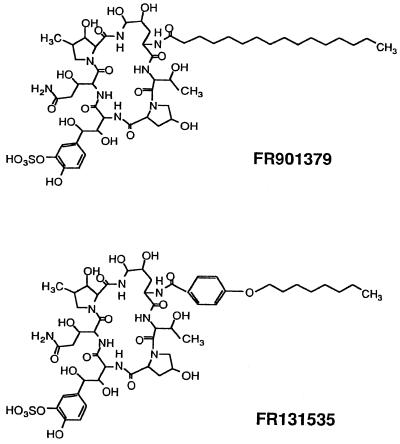
FR131535 and FR901379 (Fig. 1) were made at the Toxicology Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., as reported previously (1, 4, 5). They are novel echinocandin types of lipopeptide antibiotics produced by C. empetri F-11899, which was recently isolated from soil during a screening for antifungal agents. FR901379 is a naturally occurring lipopeptide, and FR131535 is its semisynthetic derivative. Sulfamethoxazole and trimethoprim (ST) were obtained from Sigma Chemical Co. (St. Louis, Mo.).
FIG. 1.
Animals and maintenance.
Female athymic nude mice with a BALB/c background were purchased from CLEA (Tokyo, Japan) at 4 weeks of age and were maintained in vinyl isolators placed in P 2 facilities; throughout the experiment water, pellets (CLEA), and bedding were used after autoclaving. To make sure that the lot of mice purchased was free of P. carinii, 10 mice were kept in a separate isolator, sacrificed at the end of the experiment, and examined as described for the mice in the treatment groups. None of the mice had any sign of infection.
Infection and experimental protocol.
P. carinii-infected nude mice were bred in our laboratory. An inoculum was made from the infected mouse lungs as described previously (2). Each mouse was inoculated intranasally with 104 cysts while the mouse was under anesthesia. Prior to the start of drug treatment, some of the infected mice were randomly chosen and were examined as described below. The remaining mice were separated into several groups, treated with drugs or saline (as a control), and examined according to the protocol described below.
In the first experiment, 45 mice were inoculated intranasally with P. carinii at 5 weeks of age; 5 of the animals were killed 7 weeks later, and the remaining 40 mice were divided into 4 groups of 10 mice each (see Table 1). The first group received a daily subcutaneous (s.c.) injection of 10 mg of FR131535 solution per kg of body weight for 5 days each week. The second group was similarly injected with FR901379. The third group was treated orally with ST in the drinking water (0.4 mg of sulfamethoxazole and 0.8 mg of trimethoprim per ml). The control group received saline s.c. At 3 and 8 weeks after treatment, the mice were sacrificed and examined. In the second experiment, 48 mice were infected at 5 weeks of age; 13 weeks later, 5 mice were examined, and the remaining 43 mice were divided into four groups of 10 or 11 mice. These animals were given various doses of FR131535 (0.1, 1.0, or 10.0 mg/kg per injection) or saline, as in the first experiment, and were examined 4 weeks later.
TABLE 1.
Numbers of P. carinii in lungs after drug treatment
| Time of testing and treatment | Cysts
|
No. of lungs positive for trophozoites/no. tested | No. of lungs positive for lesions/no. tested | |
|---|---|---|---|---|
| No. of lungs positive/no. tested | No. of cysts/lung (log10) | |||
| Day 0 | 5/5 | 4.7 ± 0.2 | 5/5 | 5/5 |
| 3 wk | ||||
| Saline | 5/5 | 5.4 ± 0.3 | 5/5 | 5/5 |
| FR131535 | 0/5 | <1.8a,b | 0/5 | 0/5 |
| FR901379 | 0/5 | <1.8b | 0/5 | 0/5 |
| ST | 0/5 | <1.8b | 0/5 | 0/5 |
| 8 wk | ||||
| Saline | 5/5 | 6.3 ± 0.1 | 5/5 | 5/5 |
| FR131535 | 4/5 | 2.3 ± 0.3b | 0/5 | 4/5 |
| FR901379 | 2/5 | 2.0 ± 0.3b | 0/5 | 3/5 |
| ST | 5/5 | 4.1 ± 0.3b | 0/5 | 5/5 |
Below the level of detection.
Significantly different from the values for the saline-treated control group (P < 0.01).
Examination of severity of P. carinii infection.
The numbers of P. carinii cysts in the lungs were measured as follows. A 10% lung homogenate in phosphate-buffered saline (pH 7.2) was made by using a glass homogenizer after the lung specimen was weighed, and 25 μl of the homogenate was smeared onto a slide glass. The total numbers of cysts on the smear were counted by microscopy after staining with toluidine blue O (TBO) and were expressed as the numbers of cysts per lung.
P. carinii trophozoites in the lung were also examined by Giemsa staining after the imprint of the cut surface the lung specimen was made on microscope slides.
PCR and Southern blot hybridization for the detection of P. carinii DNA.
For PCR and Southern blot hybridization, P. carinii-infected lungs were minced, and P. carinii DNA was extracted by a combination of digestion with proteinase K (500 μg/ml in the presence of 2% sodium dodecyl sulfate, 10 mM Tris-HCl, 150 mM NaCl, and 10 mM EDTA at 37°C for 16 h) and extraction with phenol-chloroform by the method reported previously (3). Oligonucleotide primers (pAZ102-E [5′-GATGGCTGTTTCCAAGCCCA-3′] and pAZ102-H [5′-GTGTACGTTGCAAACTACTC-3′]), which encode a portion of the mitochondrial large-subunit rRNA gene of P. carinii (11), were used in this study. Extracted DNA and the primer (final concentration, 1 μM) were added to an amplification mixture containing PCR buffer (Boehringer Mannheim, Mannheim, Germany) supplemented with MgCl2 to a final concentration of 2 mM, 200 μM (each) deoxynucleoside triphosphates (Boehringer Mannheim), and 2.5 U of Taq DNA polymerase (Boehringer Mannheim). The amplification conditions were denaturation at 94°C for 90 s, annealing at 50°C for 90 s, and extension at 72°C for 2 min for 40 cycles. The amplified products were subjected to electrophoresis in a 1.5% agarose gel and were visualized after ethidium bromide staining. The amplified product of mouse P. carinii was sequenced, and the sequence was compared with the sequence of the same region of mitochondrial large-subunit rRNAs of rat, simian, and human P. carinii isolates reported previously (3, 7, 11) to confirm its specificity. The amplified DNA of mouse P. carinii in the gel was Southern blotted onto nitrocellulose membranes and hybridized overnight with the 32P-labeled PCR product of rat P. carinii amplified at 46°C by using the same primers (pAZ102-E and pAZ102-H) as probes. The specificity of this probe was confirmed as described above. The membranes were subsequently washed at 42 and 52°C and were then exposed to X-ray film with intensifying screens at −80°C.
Histopathology.
The lungs were fixed in 10% buffered formalin, and paraffin sections were made and stained with hematoxylin-eosin or periodic acid-Schiff stain. Some sections were stained by the Grocott silver impregnation method and with TBO.
Statistical analysis.
For statistical analysis the Student t test was used. P values of <0.05 were considered significant.
RESULTS
Effects of FR131535 and FR901379 on P. carinii in mouse lungs.
As shown in Table 1, the number of P. carinii cysts in the lungs of the mice at the start of drug administration was very high, and characteristic Pneumocystis pneumonia lesions were confirmed histopathologically. Extensive areas of the alveolar spaces contained eosinophilic materials consisting of innumerable trophozoites that could be stained with hematoxylin-eosin and periodic acid-Schiff stains. P. carinii cysts stained with TBO and Grocott stains were scattered separately or in aggregate on the alveolar surface or within eosinophilic foamy materials. At 3 weeks after treatment, the numbers of cysts and trophozoites in the lungs of mice in the FR131535-, FR901379-, and ST-treated groups decreased to undetectable levels, while high cyst numbers and many trophozoites were found in the lungs of control mice. The areas of histopathologic lesions in groups treated with the FR compounds and ST were diminished at 3 weeks, and no trophozoite or cyst was found in the histopathologic sections. At 8 weeks, cysts were detected in some of the FR compound-treated mice and in all of the mice in the ST-treated groups; however, the numbers of cysts in the lungs of the treated groups of mice were significantly lower than the numbers in the control groups of mice.
Detection of P. carinii in the lung by PCR.
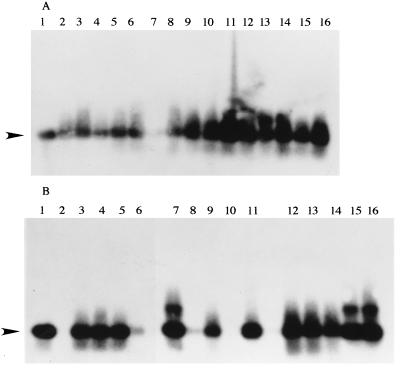
To confirm whether the P. carinii organism was completely eliminated from lungs by the administration of these drugs, homogenates of all lung samples obtained individually from the mice in the experiment whose results are presented in Table 1 were examined by Southern blot hybridization with a specific probe for P. carinii DNA after PCR. As shown in Fig. 2, at 3 weeks after drug administration, specific bands suspected of being target bands were seen in all mice treated with FR131535, FR901379, and ST, even though histopathologic changes were not found in the lungs of the treated groups of mice. At 8 weeks after treatment, although the P. carinii organisms were still detected in all of ST-treated mice having histopathologic changes in the lungs, the organisms were eliminated completely in lanes 2 and 10, which were treated with the FR compounds (Fig. 2B).
FIG. 2.
Autoradiographs obtained by Southern blot hybridization with 32P-labeled primers specific for P. carinii after DNA templates extracted from the mouse lungs were amplified with P. carinii-specific oligonucleotide primers by PCR. (A) Three weeks after drug treatment. Lane 1, positive control; lanes 2 to 6, FR131535-treated mice 1 to 5, respectively; lanes 7 to 11, FR901379-treated mice 1 to 5, respectively; lanes 12 to 16, ST-treated mice 1 to 5, respectively. (B) Eight weeks after drug treatment. Lane 1, positive control; lanes 2 to 6, FR131535-treated mice 1 to 5, respectively; lanes 7 to 11, FR901379-treated mice 1 to 5, respectively; lanes 12 to 16, ST-treated mice 1 to 5, respectively. The arrowhead indicates 367 bp.
Dose-dependent effect of FR131535 on P. carinii infection.
To determine the effective dose of FR131535, various doses of FR131535 were administered to P. carinii-infected nude mice to examine their effects on the growth of P. carinii cysts in the lungs. At 4 weeks after administration (Table 2), a significant therapeutic effect was observed with a daily dose of 10 mg of FR131535 per kg.
TABLE 2.
Effects of various doses of FR131535 on P. carinii-infected nude mice
| Time of testing and FR131535 dose (mg/kg) | No. of positive mice/total no. of mice | No. of cysts/lung (log10) |
|---|---|---|
| Before treatment | 5/5 | 6.90 ± 0.36 |
| 4 wk after treatment | ||
| 10 | 3/10 | 2.30 ± 0.17a |
| 1 | 11/11 | 4.02 ± 1.08 |
| 0.1 | 11/11 | 5.54 ± 0.48 |
| Control (saline) | 11/11 | 6.84 ± 0.25 |
Significantly different from the values for the saline-treated control group (P < 0.01).
DISCUSSION
We recently found that C. empetri F-11899 produces water-soluble echinocandin analogs which have strong inhibitory effects on the growth of fungi (1, 4, 5). In particular, FR901379 was easily soluble in water even at a concentration of 50 mg/ml. The excellent water solubilities of FR901379 and its derivative FR131535 (Fig. 1) are ascribed to the structural sulfate moiety. The high water solubility of this lipopeptide, which acts as a β-1,3-d-glucan inhibitor, was thought to be useful in the development of FR131535 for clinical application as therapy against P. carinii infection.
Prolonged s.c. administration of FR131535 or FR901379 showed that these FR compounds are highly effective in decreasing the numbers of P. carinii organisms in the lungs of infected nude mice. Several criteria were used to evaluate the effectiveness of the compounds: the numbers of P. carinii organisms in the lung suspension, detection of trophozoites in lung smears, lung histopathology, and detection of P. carinii-specific DNA sequences by Southern blot hybridization applied after PCR with individual lung specimens. All of these methods confirmed the efficacy of the drug. As shown in Fig. 2A, Southern blot hybridization combined with PCR seemed to be the most sensitive since P. carinii DNA was detected in all mice that were negative by the other three methods at 3 weeks (Table 1).
The P. carinii cysts and pulmonary lesions present at 3 weeks reappeared at 8 weeks (Table 1), although the values were still lower than those for the group receiving no drug. One possible explanation is the development of drug resistance by P. carinii. Another possibility concerning the pathology of the lungs is that the lesion seen at 8 weeks could be attributable to the inflammation resulting from the killed organisms and the large amounts of components released from P. carinii due to effective killing by the drug. Similar findings have been reported in another study with a lipopeptide (8). These points are worth future study. The effective dose of the FR compound was investigated with FR131535 (Table 2), and a dose of 10 mg/kg was shown to be needed. The reason that FR131535 was used, despite its seemingly lower efficacy than that of FR901379, is that the former has lower hemolytic activity (minimum hemolytic concentration, >500 μg/ml for FR131535 versus 62 μg/ml for FR901379); the hemolytic activity of FR901379 might cause adverse side effects if it is applied clinically. After administration of the FR compounds (Fig. 2B), P. carinii DNA was undetectable in some mice, unlike after the administration of ST. This observation encourages further investigations on the development of new drugs that can be used to treat Pneumocystis pneumonia.
ACKNOWLEDGMENTS
We thank Seiji Hashimoto (Exploratory Research Laboratories, Fujisawa Pharmaceutical Co., Ltd.) for valuable scientific contributions to this study. We also thank Katsumoto Ueda for helpful advice.
REFERENCES
- 1.Fujie A, Iwamoto T, Kasahara C, Yamashita Y, Hashimoto S, Okumura M. Abstracts of papers of the Annual Meeting of Japan Society for Bioscience, Biotechnology, and Agrochemistry. (In Japanese.) 1996. FR131535, a novel lipopeptide antifungal; p. 51. [Google Scholar]
- 2.Furuta T, Ueda K, Kyuwa K, Fujiwara K. Effect of T cell transfer on Pneumocystis carinii infection in nude mice. Jpn J Exp Med. 1984;54:59–64. [PubMed] [Google Scholar]
- 3.Furuta T, Fujita M, Mukai R, Sakakibara I, Sata T, Hayami M, Kojima S, Yoshikawa Y. Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus: its characterization by polymerase chain reaction method and failure of experimental transmission to immunodeficient animals. Parasitol Res. 1993;79:624–628. doi: 10.1007/BF00932502. [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto T, Fujie A, Sakamoto K, Tsurumi Y, Shigematsu N, Yamashita M, Hashimoto S, Okamura M, Kohsaka M. WF11899A, B, and C, novel antifungal lipopeptides. I. Taxonomy, fermentation, isolation and physico-chemical properties. J Antibiot. 1994;47:1084–1091. doi: 10.7164/antibiotics.47.1084. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto T, Fujie A, Nitta K, Hashimoto S, Okuhara M, Kohsaka M. WF11899A, B and C, novel antifungal lipopeptides. II. Biological properties. J Antibiot. 1997;47:1092–1097. doi: 10.7164/antibiotics.47.1092. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto Y, Matsuda S, Tegoshi T. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool. 1989;36:21s–22s. doi: 10.1111/j.1550-7408.1989.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters S E, Wakefield A E, Whitwell K E, Hopkin J M. Pneumocystis carinii pneumonia in thoroughbred foal: identification of a genetically distinct organism by DNA amplification. J Clin Microbiol. 1994;32:213–216. doi: 10.1128/jcm.32.1.213-216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmatz D M, Romanchek A M, Pittarelli A L, Schwartz R A, Fromtling K H, Nollstadt F V, Vanmiddlesworth K E, Wilson K E, Turner M J. Treatment of Pneumocystis carinii pneumonia with 1,3-β-glucan synthesis inhibitors. Proc Natl Acad Sci USA. 1990;87:5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmatz D M, Abruzzo G, Powles M A, McFadden D C, Balkovec J M, Black R M, Nollstadt K, Bartizal K. Pneumocandins from Zalerion arboricola. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumocystis carinii and Candida species. J Antibiot. 1992;45:1886–1891. doi: 10.7164/antibiotics.45.1886. [DOI] [PubMed] [Google Scholar]
- 10.Schmatz D M, Powles A M, Mcfadden C D, Pittarelli J, Balkovec M, Hammond R, Zambias P, Liberator P, Anderson J. Antipneumocystis activity of water-soluble lipopeptide L-693,989 in rats. Antimicrob Agents Chemother. 1992;36:1964–1970. doi: 10.1128/aac.36.9.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakefield A E, Pixley B S, Sinclair K, Miller F P, Moxon R E, Hopkin M J. Amplification of Pneumocystis carinii DNA of rat and human origin. Mol Biochem Parasitol. 1990;43:69–72. doi: 10.1016/0166-6851(90)90131-5. [DOI] [PubMed] [Google Scholar]