Abstract
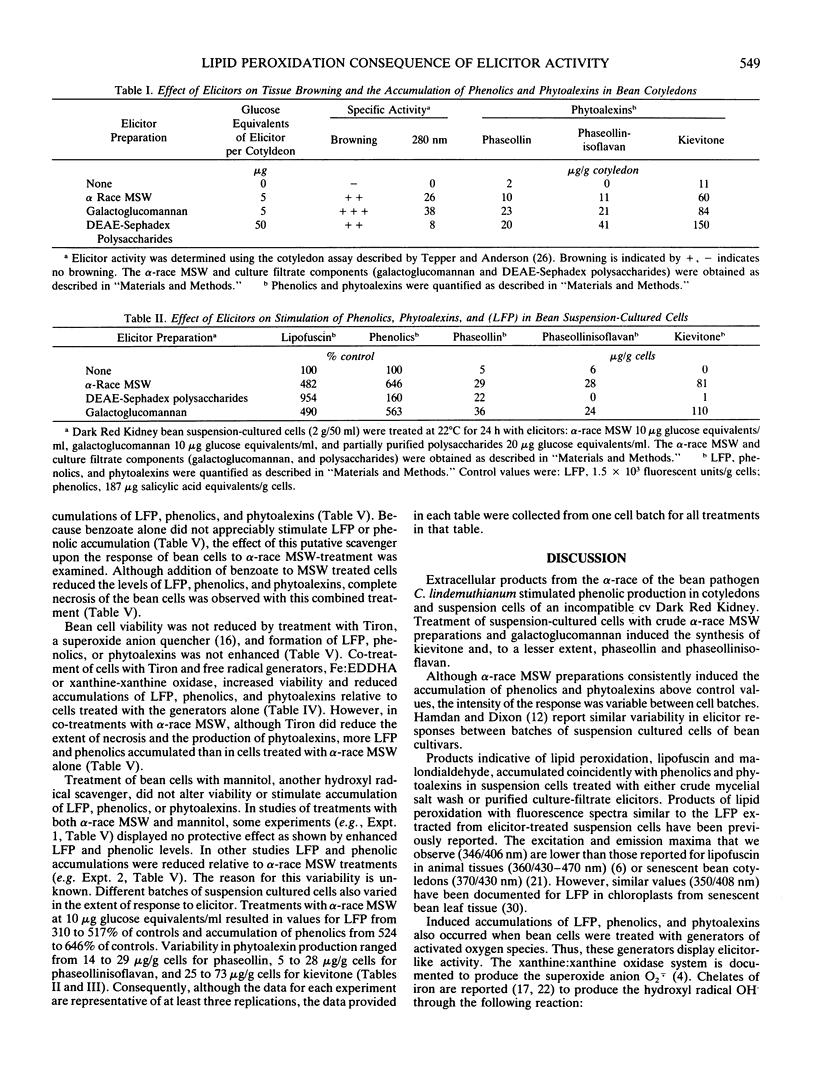
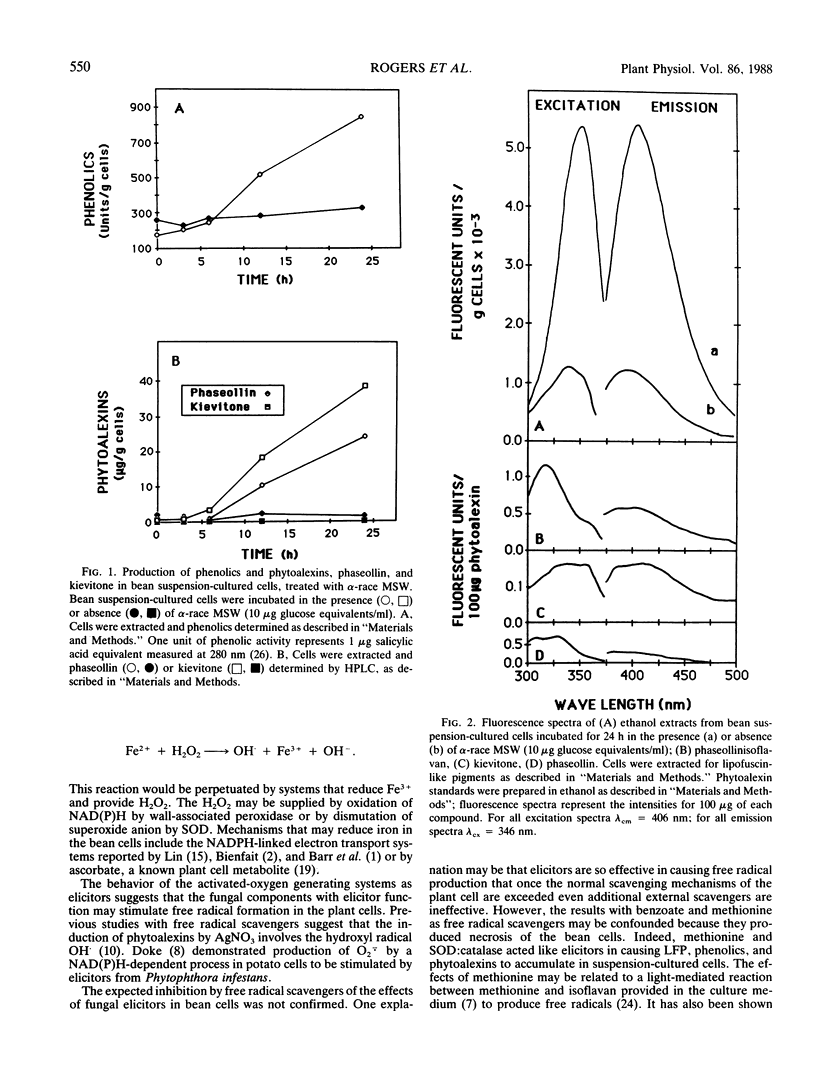
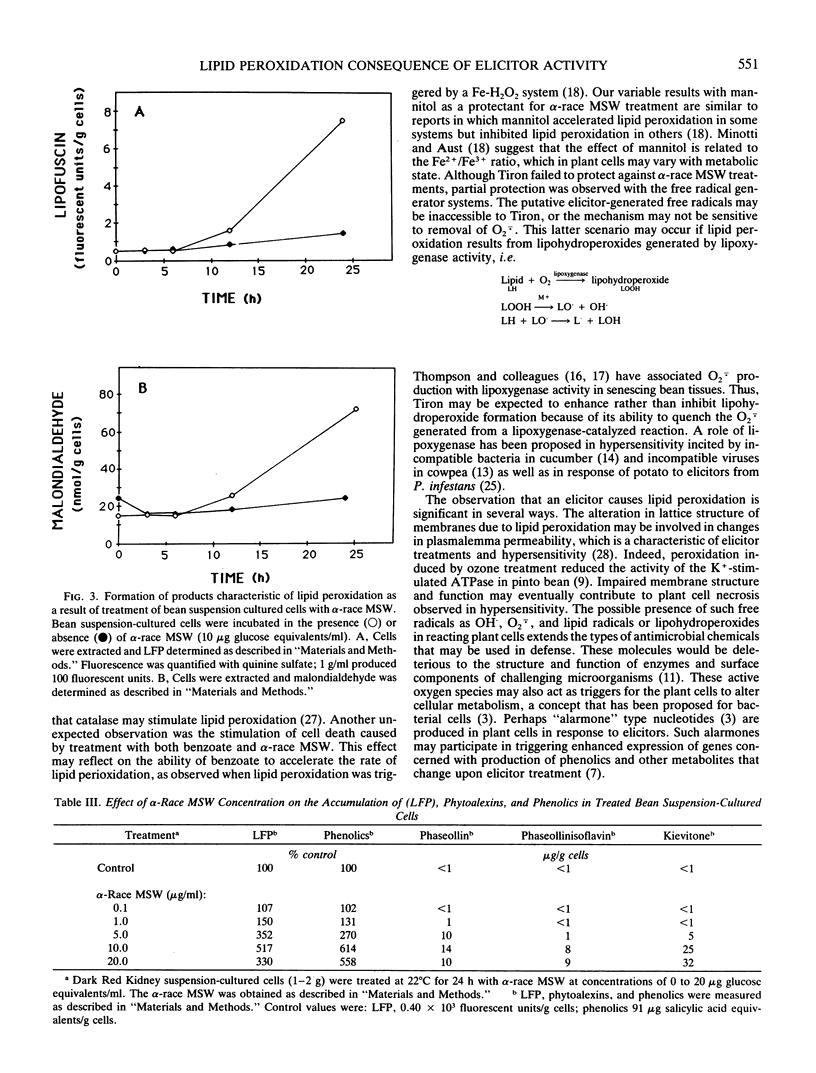
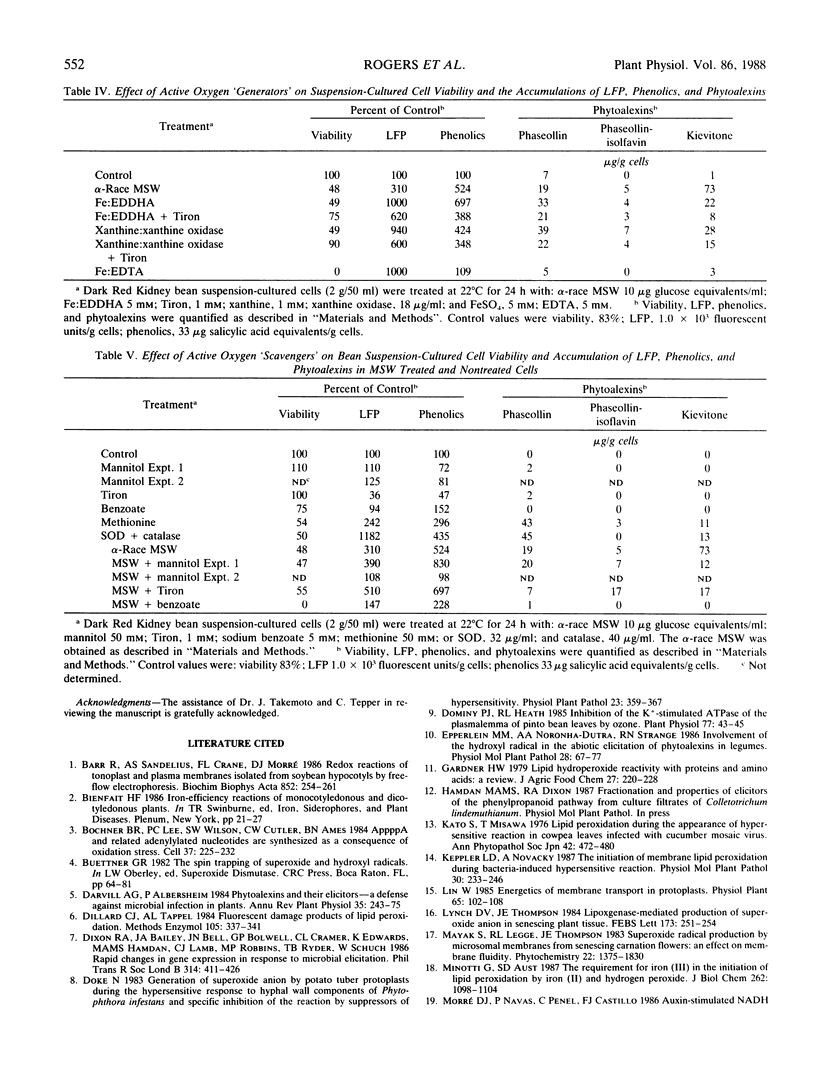
Elicitor-active preparations from the fungal pathogen of bean Colletotrichum lindemuthianum stimulated the accumulation of products characteristic of lipid peroxidation in treated bean tissues. Bean suspension cells treated with crude and purified elicitors accumulated `lipofuscin-like pigment' (LEP) and malondialdehyde. The accumulation of LFP after about 6 h of treatment coincided with the onset of visible browning and production of the bean phytoalexins kievitone, phaseollin, and phaseollinisoflavan. The induction of phytoalexins and accumulation of LFP were also triggered by treatments with generators of activated oxygen species, xanthine:xanthine oxidase and Fe:ethylenediaminedi-o-hydroxyphenylacetic acid. These data suggest that generation of active oxygen species may be involved in lipid peroxidation triggered by elicitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr R., Sandelius A. S., Crane F. L., Morré D. J. Redox reactions of tonoplast and plasma membranes isolated from soybean hypocotyls by free-flow electrophoresis. Biochim Biophys Acta. 1986 Dec 3;852(2-3):254–261. doi: 10.1016/0005-2728(86)90230-6. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent damage products of lipid peroxidation. Methods Enzymol. 1984;105:337–341. doi: 10.1016/s0076-6879(84)05044-8. [DOI] [PubMed] [Google Scholar]
- Dominy P. J., Heath R. L. Inhibition of the k-stimulated ATPase of the plasmalemma of pinto bean leaves by ozone. Plant Physiol. 1985 Jan;77(1):43–45. doi: 10.1104/pp.77.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem. 1987 Jan 25;262(3):1098–1104. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pauls K. P., Thompson J. E. Evidence for the accumulation of peroxidized lipids in membranes of senescing cotyledons. Plant Physiol. 1984 Aug;75(4):1152–1157. doi: 10.1104/pp.75.4.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K. R., Anderson A. J. The Effect of Extracellular Components from Colletotrichum lindemuthianum on Membrane Transport in Vesicles Isolated from Bean Hypocotyl. Plant Physiol. 1987 Jun;84(2):428–432. doi: 10.1104/pp.84.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzig D. A., Allen R. D., Bhatia S. K. Inhibition of phytoalexin synthesis in arachidonic Acid-stressed potato tissue by inhibitors of lipoxygenase and cyanide-resistant respiration. Plant Physiol. 1983 Jul;72(3):746–749. doi: 10.1104/pp.72.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. Role of superoxide radicals in the lipid peroxidation of intracellular membranes. FEBS Lett. 1975 Mar 1;51(1):180–183. doi: 10.1016/0014-5793(75)80882-9. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]


