Supplemental Digital Content is available in the text
Keywords: China, gender, middle-aged and elderly people, non-alcoholic fatty liver disease, type 2 diabetes mellitus
Abstract
Limited data are available regarding the association of non-alcoholic fatty liver disease (NAFLD) with the risk of type 2 diabetes mellitus (T2DM) in China. Therefore, the purpose of this study is to evaluate the gender-specific association between NAFLD and T2DM risk in a middle-aged and elderly Chinese population.
This cross-sectional study was carried out in a group of 1492 Chinese adults (60.30% males) aged between 45 and 69 years old, in Hangzhou city, Zhejiang province who were attending their annual health check-up from June 2015 to December 2016 in the Medical Center for Physical Examination, Zhejiang Hospital. Face-to-face interviews were conducted using a written questionnaire. NAFLD was divided into none, mild, moderate/severe based on ultrasound examination. Logistic regression analyses were employed to determine the relationship between NAFLD and the risk of T2DM, with adjustment of potential confounding variables.
Of the 1492 participants, 163 (10.92%) were diagnosed with T2DM. Educational level, smoking, body mass index (BMI), waist circumference (WC), waist-hip ratio (WHR), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting glucose (FG), triglycerides (TG), alanine aminotransferase (ALT), asparagine aminotransferase (AST)and the prevalence of T2DM were significantly higher in males than in females (P < .05). Besides, females had significantly higher levels of high density lipoprotein-cholesterol (HDL-C) (1.51 ± 0.37 vs 1.29 ± 0.42, P < .001) than males. Pearson bivariate correlation analysis indicated that FG was positively associated with weight, BMI, WC, WHR, SBP, DBP, TG, TC, ALT and AST in both males and females (P < .05). Besides, FG was inversely associated with HDL-C in females (P < .001). After adjusting for confounding variables, NAFLD was positively associated with the risk of T2DM, and the effect of NAFLD on T2DM was stronger in males (OR = 2.442, 95%CI: 1.003–3.757) than in females (OR = 1.814, 95%CI: 1.011–3.257).
Our data showed that NAFLD was significantly associated with the risk of T2DM in middle-aged and elderly males than in females. Further prospective cohort studies are needed to determine the causal effect of NAFLD on T2DM.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the main causes of chronic liver disease worldwide, with a global prevalence of 25.2%.[1] It represents a wide spectrum of liver diseases, from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma.[2] In the United States, NAFLD affects over 64 million people (>20% of the general population), with annual medical costs of $103 billion.[3] Meanwhile, with the increasing epidemic of overweight/obesity in China, NAFLD has also become an alarming public health issue and the prevalence is increasing year by year.[4] Anstee et al, reported that NAFLD was associated with not only hepatic complications such as liver cirrhosis and hepatocellular carcinoma but also extrahepatic complications, including metabolic syndrome, type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD).[5] Of note, recent studies also suggest that NAFLD may be a potential precursor to the development of metabolic syndrome and T2DM.[6,7]
It is well-known that T2DM is a non-communicable chronic disease mainly characterized by the disorder of glucose metabolism.[8] Based on the statistics reported by the International Diabetes Federation (IDF), 449 million people had T2DM worldwide, and this number is estimated to reach 702 million by 2045.[9] In China, a middle and low-income country, T2DM has been recognized as a major health problem and the prevalence has rapidly increased since 1980.[10] Previous studies have demonstrated that obesity and insulin resistance are key pathogenic factors for NAFLD and T2DM, and these 2 diseases have numerous common risk factors.[11] To date, a considerable number of epidemiological studies have reported the significant association of NAFLD with the risk of T2DM.[12–18] The majority of these studies, however, were conducted in Korean and Japanese populations, and evidence from Chinese population is extremely limited.[17,18] Furthermore, to the authors knowledge, no previous study has yet explored a gender difference on the relationship between NAFLD and the risk of T2DM in the Chinese population, particularly in middle-aged and elderly people. In this context, we carried out the present study to ascertain the gender difference on the association between NAFLD as diagnosed by ultrasonography and T2DM risk among a middle-aged and elderly Chinese population.
2. Subjects and methods
2.1. Study Sample
The recruitment details of this study have been published elsewhere.[19] Briefly, a total 1727 eligible participants aged 45 to 69 years, who were recruited to attend their annual health check-up from June 2015 to December 2016 in the Medical Center for Physical Examination, Zhejiang Hospital. First, 68 participants self-reported a history of usage of drugs or hereditary diseases were excluded. Second, we further excluded 106 participants who reported excessive alcohol intake (>14 drinks/week for men and 7 drinks/week for women), as well as 36 participants who provided the missing or incomplete information in their written questionnaires. Third, 25 participants who reported implausible energy intakes (<800 or >4200kcal/d for men and <600 or >3500kcal/d for women) were also excluded. Finally, 1492 participants who met the above eligibility were included in the analyses of the association between NAFLD and the risk of T2DM. A flow chart detailing the process of study selection is shown in Figure 1. This study was conducted in accordance with the guidelines described in the Declaration of Helsinki, and all procedures involving human participants were approved by the Institutional Review and Ethics committee of Zhejiang hospital. Written informed consent prior to commencing the studies was obtained from all participants.
Figure 1.
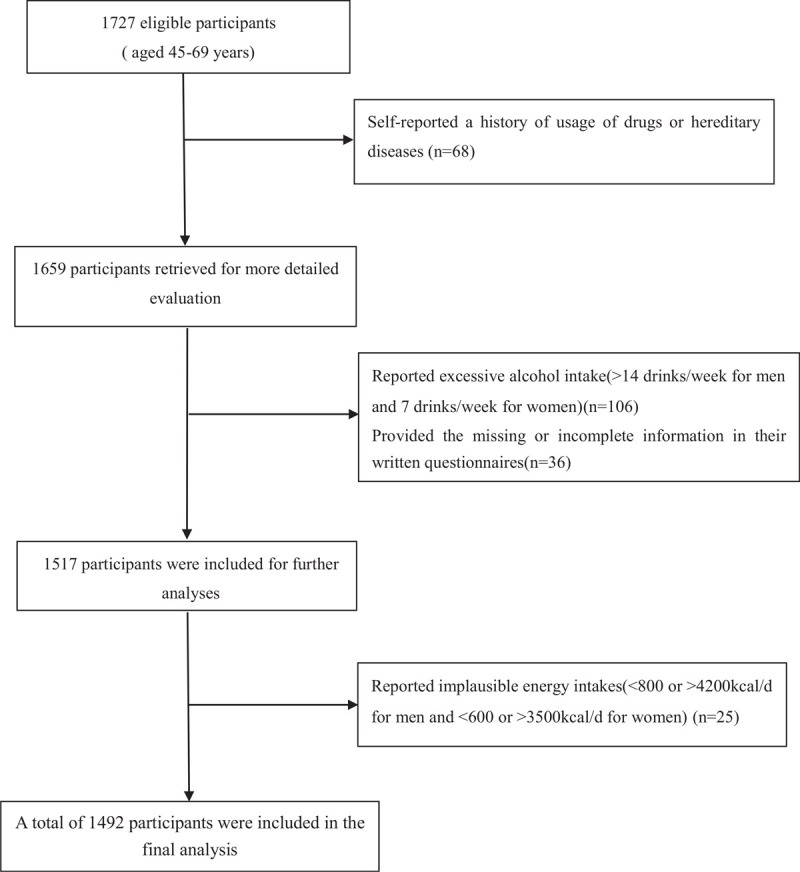
Flow chart of article screening and selection process.
2.2. Anthropometric measurements
Height was measured to the nearest 0.1 cm with participants in standing position and without shoes. Body weight was measured to the nearest 0.1 kg on digital scales (HW-700) with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms (kg) divided by the square of height in meters (kg/m2). Waist circumference (WC) was measured at the midpoint between the lower ribs and iliac crest at the end of an expiratory phase.[20] These anthropometric measurements were performed by a trained nurse using standardized procedures.
2.3. Assessment of biomarker
Participants were asked to take fast for 12 hours, and were measured using a Hitachi 911 Analyzer. All participants were invited for blood collection after fasting overnight (12 hours). The samples were analyzed in the Medical Center for Physical Examination, Zhejiang Hospital for fasting glucose (FG), glycated haemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein -cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), alanine aminotransferase (ALT) and asparagine aminotransferase (AST) using the Hitachi 7180 auto-analyzer (Hitachi, Tokyo, Japan).
2.4. Blood pressure measurement
Blood pressure was measured using a previously validated automatic monitor (Model: OMRON HEM- 780) with the participants in a quite environment and sitting position. A well-trained nurse measured the blood pressure 3 times, and thereafter an average of the last 2 measurements was considered as the participant's blood pressure. Three measurements were taken at one-minute intervals following a 5 to 10 minutes rest.[21]
2.5. Definitions
NAFLD was defined as the absence of excessive alcohol use (>30 g/d in men and 20 g/d in women), no use of steatogenic medications within the past 6 months, no exposure to hepatotoxins, no history of bariatric surgery, and the presence of moderate-severe hepatic steatosis (by B-ultrasonic examination).[22] NAFLD was classified based the severity of fatty liver.[18] Patients with NAFLD were divided into mild, moderate, and severe according to ultrasound criteria. Moderate fatty liver is defined as moderate diffuse increase in fine echoes with slightly impaired visualization of the intrahepatic vessels and diaphragm. Severe fatty liver is defined as marked increase in fine echoes with poor or no visualization of the intrahepatic vessel borders, diaphragm and posterior portion of the right lobe of the liver.T2DM was defined as the presence of any one of the following:
-
1.
FG≥7.0 mmol/L on at least 2 separate occasions, or an oral glucose tolerance test (OGTT) with a value≥11.1 mmol/L;
-
2.
current use of insulin or oral hypoglycemic agents; or
-
3.
a positive response to the question: have you ever been diagnosed with diabetes by a doctor?.[19]
Obesity was defined by BMI≥28 kg/m2 and abdominal adiposity was defined as (male: WC≥85 cm; female: WC≥80 cm).[23] Hypertension was defined as systolic blood pressure≥140 mm Hg, or/and diastolic blood pressure≥90 mm Hg, or self-reported use of antihypertensive medications.[24]
2.6. Covariates
The following variables were considered as covariates: education (middle school or below, high school, college or above), age, gender (male/female), smoking, alcohol, medication use and physical activity. Covariates were assessed using a structured questionnaire at the recruitment interview. Smoking status was defined as current smoker, ex-smoker and non-smoker. Physical activity levels were obtained from the International Physical Activity Questionnaire (IPAQ, short version) that was completed under the supervision of the trained researcher.[4] The physical activity levels were expressed as metabolic equivalent hours per week (MET-h/week), in which different MET levels were ranged on a scale from sleep/rest (0.9 METs) to high-intensity physical activities (>6 METs). The values obtained for various activities (MET-h) added together and were expressed as metabolic equivalents in hours per week (MET-h/week). Then, the different MET levels were grouped into light, moderate and heavy.
2.7. Statistical analysis
In the present study, data were generally analyzed by different gender, and presented as mean ± standard deviation (SD) or median and interquartile range (IQR), and categorical variables are presented as numbers and percentages. First, data were checked for normality (normal plots), and non-normally distributed variables were log transformed. Between-group differences in participants with or without T2DM were compared by Student t tests. The Independent-Samples t test (for normal distributed variables) or the Mann–Whitney test (if non-parametric tests were required) was used assess the significant differences in continuous variables, while the Chi-Squared tests were used to assess the significant differences in categorical variables. Pearson bivariate correlation was performed to analyze the relation between FG and other variables. The coefficient of determination (R2) is used for judging the goodness of fit in the multivariate regression models. Multivariate logistic regression analyses were performed to estimate the effect of NAFLD on the risk of T2DM in the fully adjusted model. Model 1 was unadjusted; Model 2 was adjusted for age, income (continuous), physical activity (light, moderate, heavy), educational level (middle school or below, high school, college or above), blood pressure (continuous), triglycerides, total cholesterol, high and low-density lipoprotein-cholesterol. Model 3 was further adjusted for BMI (continuous). For the all statistic analyses, SPSS 23.0 (BM Corp., Armonk, NY, USA) was used and all reported P values were two-tailed and a P value <.05 was regarded as statistically significant.
3. Results
Demographic, and clinical characteristics of participants by gender were shown in Table 1 Of the 1492 enrolled participants, 229 (157 males and 72 females) fulfilled the diagnostic criteria for NAFLD. The overall prevalence of NAFLD was 15.35%. In males it was 10.52%, and in females it was 4.83%. Our data showed that educational level, smoking, BMI, WC, WHR, SBP, DBP, FG, TG, ALT, AST and the prevalence of T2DM were significantly higher in males than in females (P < .05). Besides, females had significantly higher levels of HDL-C (1.51 ± 0.37 vs 1.29 ± 0.42, P < .001) than males.
Table 1.
Demographic and clinical characteristics of subjects by gender.
| Variables | Male | Female | P value |
| Demographic | |||
| N (%) | 900 (60.30%) | 592 (39.70%) | |
| Age (years) | 50.82 ± 4.60 | 50.87 ± 4.75 | .827 |
| Educational level (%) | <.001 | ||
| <High school | 19 (2.2) | 72 (12.2) | |
| High school | 397 (44.11) | 305 (51.5) | |
| >High school | 484 (53.78) | 215 (36.3) | |
| Smoking status (%) | <.001 | ||
| Yes | 478 (40.1) | 8 (1.4) | |
| No | 422 (59.9) | 584 (98.6) | |
| Clinical characteristics | |||
| BMI(kg/m2) | 24.95 ± 2.93 | 23.94 ± 2.90 | <.001 |
| WC (cm) | 87.84 ± 8.34 | 80.70 ± 8.15 | <.001 |
| WHR | 0.90 ± 0.05 | 0.84 ± 0.07 | <.001 |
| SBP (mm Hg) | 132.40 ± 17.15 | 128.16 ± 17.22 | <.001 |
| DBP (mm Hg) | 83.14 ± 13.48 | 77.90 ± 12.21 | <.001 |
| FG (mmol/L) | 6.28 ± 2.41 | 5.81 ± 1.06 | <.001 |
| TG (mmol/L) | 1.97 ± 1.91 | 1.34 ± 0.99 | <.001 |
| TC (mmol/L) | 5.16 ± 1.00 | 5.22 ± 0.87 | .225 |
| HDL-C (mmol/L) | 1.29 ± 0.42 | 1.51 ± 0.37 | <.001 |
| LDL-C (mmol/L) | 3.31 ± 0.86 | 3.22 ± 0.84 | .095 |
| ALT (U/L) | 32.18 ± 16.61 | 21.96 ± 13.44 | <.001 |
| AST (U/L) | 26.09 ± 8.86 | 22.52 ± 7.05 | <.001 |
| NAFLD (%) | 157 (17.44%) | 72 (12.16%) | .956 |
| T2DM (%) | 122 (13.56%) | 41 (6.93%) | <.01 |
ALT = alanine aminotransferase, AST = asparagine aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, FG = fasting glucose, HDL-C = high density lipoprotein-cholesterol, LDL-C = low density lipoprotein-cholesterol, NAFLD = nonalcoholic fatty liver disease, SBP = systolic blood pressure, SUA = serum uric acid, TC = total cholesterol, TG = triglycerides, WC = waist circumference, WHR = waist-hip ratio. All values are mean ± SD.
A total of 163 participants with T2DM were included in this population. In antidiabetic treatment, 141 patients were treated with oral medications only, e.g., medications, pioglitazone, GLP-1 agonists. Seven patients were treated with insulin only.15 patients were treated with oral medications combined insulin. The patterns of antidiabetic treatment have been summarized in Supplemental Digital Content (Appendix 1, http://links.lww.com/MD/F660).
Clinical and biochemical variables are shown and compared by the cut-off value of NAFLD (Table 2). The results showed that there were significantly higher levels of BMI, WC, WHR, SBP, DBP, HbA1c, TG, and ALT, and significantly lower level of HDL-C, in both males and females in participants with NAFLD than Non-NAFLD. Moreover, there were significantly higher levels of age, FG, TC, LDL-C and SUA in females in participants with NAFLD than Non-NAFLD.
Table 2.
The characteristics of study population according to non-alcoholic fatty liver disease (NAFLD) status and gender (means ± standard deviation).
| Male (n = 900) | Female (n = 592) | |||
| Non-NAFLD | NAFLD | Non-NAFLD | NAFLD | |
| Case count | 743 | 157 | 520 | 72 |
| Age (years) | 50.92 ± 4.66 | 50.32 ± 4.29 | 50.69 ± 4.71∗ | 52.14 ± 4.87 |
| BMI (kg/m2) | 24.74 ± 2.94∗∗ | 25.96 ± 2.69 | 23.64 ± 2.79∗∗ | 26.09 ± 2.74 |
| WC (cm) | 87.15 ± 8.45∗∗ | 91.07 ± 7.00 | 79.80 ± 7.96∗∗ | 87.27 ± 6.35 |
| WHR | 0.89 ± 0.05∗∗ | 0.91 ± 0.04 | 0.84 ± 0.07∗∗ | 0.88 ± 0.05 |
| SBP (mm Hg) | 131.65 ± 17.05∗∗ | 135.95 ± 17.25 | 127.43 ± 17.22∗∗ | 133.44 ± 16.31 |
| DBP (mm Hg) | 82.53 ± 13.27∗∗ | 86.07 ± 14.12 | 77.25 ± 12.19∗∗ | 82.59 ± 11.32 |
| FG (mmol/L) | 6.17 ± 1.90 | 6.30 ± 2.51 | 5.73 ± 0.89∗∗ | 6.47 ± 1.73 |
| HbA1c (%) | 5.26 ± 1.40∗ | 6.18 ± 0.95 | 6.83 ± 1.53∗ | 7.32 ± 1.09 |
| TG (mmol/L) | 1.88 ± 1.54∗ | 2.39 ± 3.11 | 1.25 ± 0.83∗∗ | 1.96 ± 1.64 |
| TC (mmol/L) | 5.14 ± 0.87 | 5.23 ± 1.48 | 5.17 ± 0.84∗∗ | 5.62 ± 0.95 |
| HDL-C (mmol/L) | 1.32 ± 0.44∗∗ | 1.16 ± 0.29 | 1.54 ± 0.38∗∗ | 1.33 ± 0.26 |
| LDL-C (mmol/L) | 3.28 ± 0.86 | 3.43 ± 0.87 | 3.16 ± 0.84∗∗ | 3.60 ± 0.78 |
| SUA (μmol/L) | 358.96 ± 74.66 | 369.86 ± 88.94 | 269.63 ± 56.80∗∗ | 295.40 ± 64.52 |
| ALT (U/L) | 27.09 ± 15.50∗∗ | 34.88 ± 18.21 | 20.78 ± 12.86∗∗ | 26.84 ± 14.73 |
| AST (U/L) | 24.68 ± 8.82∗ | 26.27 ± 8.56 | 23.01 ± 7.40 | 23.34 ± 7.07 |
P < .05, variable means in male or female with and without NAFLD.
P < .01, variable means in male or female with and without NAFLD.
ALT = alanine aminotransferase, AST = asparagine aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, FG = fasting glucose, HbA1c = Glycosylated haemoglobin, HDL-C = high density lipoprotein-cholesterol, LDL-C = low density lipoprotein- cholesterol, NAFLD = nonalcoholic fatty liver disease, SBP = systolic blood pressure, SUA = serum uric acid, TC = total cholesterol, TG = triglycerides, WC = waist circumference, WHR = waist-hip ratio.
Pearson bivariate correlation between FG and other variables demonstrated 4 major correlations with ALT (r = 0.201, P < .001), TG (r = 0.142, P < .001), AST (r = 0.135, P < .001), and SBP (r = 0.129, P < .001) in males, and SBP (r = 0.193, P < .001), HDL-C (r = -0.193, P < .001), ALT (r = 0.184, P < .001), and DBP (r = 0.171, P < .001) in females (Table 3). Besides, age was positively associated with FG in females (r = 0.153, P < .001).
Table 3.
Pearson bivariate correlations between fasting glucose and other variables in male and female, respectively.
| Male (n = 900) | Female (n = 592) | |||
| Factors | r | P value | r | P value |
| Age (years) | 0.031 | .351 | 0.153 | .000 |
| Height (cm) | −0.029 | .382 | −0.035 | .395 |
| Weight (kg) | 0.068 | .043 | 0.087 | .035 |
| BMI (kg/m2) | 0.094 | .005 | 0.117 | .005 |
| WC (cm) | 0.095 | .004 | 0.139 | .001 |
| WHR | 0.100 | .003 | 0.092 | .025 |
| SBP (mm Hg) | 0.129 | .000 | 0.193 | .000 |
| DBP (mm Hg) | 0.096 | .004 | 0.171 | .000 |
| TG (mmol/L) | 0.142 | .000 | 0.159 | .000 |
| TC (mmol/L) | 0.093 | .005 | 0.099 | .016 |
| HDL-C (mmol/L) | −0.034 | .416 | −0.193 | .000 |
| LDL-C (mmol/L) | 0.076 | .068 | 0.098 | .055 |
| ALT (U/L) | 0.201 | .000 | 0.184 | .000 |
| AST (U/L) | 0.135 | .000 | 0.113 | .036 |
ALT = alanine aminotransferase, AST = asparagine aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, FG = fasting glucose, HDL-C = high density lipoprotein-cholesterol, LDL-C = low density lipoprotein-cholesterol, SBP = systolic blood pressure, TC = total cholesterol, TG = triglycerides, WC = waist circumference, WHR = waist-hip ratio.
Multivariable-adjusted OR (95% CI) of NAFLD for T2DM stratified by gender were shown in Table 4. In the Model 1, males were more likely to suffer from T2DM compared to females (OR = 2.390, 95% CI (1.294–4.414); OR = 2.219, 95% CI (1.217–4.013), P < .001, respectively). In the Model 2, our results indicated that the odds of having T2DM was significantly higher in males (OR = 2.275, 95% CI: 1.198–4.323; P < .05) than in females (OR = 1.966, 95%CI: 1.105–3.543, P < .05). In addition, after further adjusting for WC, WHR and BMI, the results in Model 3 also showed that the odds of having T2DM was significantly higher in males (OR = 2.442, 95%CI: 1.003–3.757, P < .05) compared with in females (OR = 1.814,95%CI: 1.011–3.257, P < .05). Besides, in the multivariable regression analysis, the final model is significant (F = 43.152, P < .000) and the adjusted R2 = 0.512.
Table 4.
Multivariable-adjusted odds ratios (OR) and 95% confidence intervals (CI) of NAFLD for T2DM stratified by gender.
| Male | Female | |||||
| Variable | OR | 95% CI | P value | OR | 95% CI | P value |
| NAFLD | ||||||
| Model 1 | 2.390 | (1.294–4.414) | .005 | 2.219 | (1.217–4.013) | .008 |
| Model 2 | 2.275 | (1.198–4.323) | .012 | 1.966 | (1.105–3.543) | .037 |
| Model 3 | 2.442 | (1.003–3.757) | .049 | 1.814 | (1.011–3.257) | .046 |
NAFLD = nonalcoholic fatty liver disease, T2DM = type 2 diabetes mellitus; Model 1 was unadjusted; Model 2 was adjusted for age, income, physical activity level, educational level, blood pressure, triglycerides, total cholesterol, high and low-density lipoprotein-cholesterol; Model 3 was adjusted forage, income, physical activity level, educational level, blood pressure, triglycerides, total cholesterol, high and low-density lipoprotein-cholesterol and BMI.
4. Discussion
In the present study, we found that NAFLD was significantly associated with higher risk of T2DM. Simultaneously, the association between NAFLD and T2DM was stronger in middle-aged and elderly men than in women. This study is, to our knowledge, the first study analyzing the gender-specific association between NAFLD and the risk of T2DM in a middle-aged and elderly Chinese population.
The present study revealed a significant positive association between NAFLD and T2DM risk in both males and females, independent of the potential confounders. Our findings are in line with those of previous studies that have shown a detrimental effect of NAFLD against T2DM.[14,18] Similarly, a systematic review and meta-analysis of 20 prospective studies also concluded that ultrasound- diagnosed NAFLD was significantly associated with an increased risk of T2DM.[7] Moreover, in a Finnish study of hypertensive patients followed for 21 years, Käräjämäki and colleagues showed that NAFLD was associated with an increased risk of incident T2DM when coexisting with the metabolic syndrome, but not in its absence.[25] Although the exact mechanisms behind the association between NAFLD and T2DM have yet to be elucidated, several plausible explanations have been proposed to explain this positive association. First, NAFLD is characterized by a large number of hepatic triglyceride which could result in dysfunctional lipid metabolism and disordered glucose regulation.[6] Second, NAFLD could promote insulin resistance of hepatic, skeletal muscle, and adipose tissues.[26] Moreover, insulin resistance (IR) increases the transportation of free tatty acid to liver and increases hepatic fatty acid β oxidation.[27] Third, compared to those without NAFLD, participants with NAFLD have higher levels of inflammatory markers which are known risk factors for diabetes.[28] Fourth, NAFLD may contribute to T2DM development through the release of some circulating mediators, such as fetuin-A, selenoprotein P, fibroblast growth factor-21, and other hepatokines, which are involved in glucose metabolism and insulin sensitivity.[29]Finally, the study participants with the risk of NAFLD are more likely to change their lifestyles and dietary habits in health check-up. Earlier studies have shown that exercise and dietary modification could improve hepatic steatosis and normalize altered liver tests.[30,31] Besides, Knowler et al, also found that lifestyle intervention might reduce the risk of T2DM.[32]
In our analyses, another novel finding was that the relationship for incident T2DM in middle-aged and elderly men with NAFLD was significantly stronger than in women. Although the effect of gender differences on progression of NAFLD and T2DM respectively, have been reported,[33,34] little is known about gender differences in respect to the effect of NAFLD on T2DM. To our knowledge, no study has yet evaluated the effect of NAFLD on the development of T2DM in a middle-aged and elderly Chinese population, emphasizing the impact from the gender difference. It is notable that increased T2DM risk associated with NAFLD was observed in both sexes; however it was typically higher in men, mirroring the male predominance in T2DM.[35,36] Similarly, we also found a closer relationship between NAFLD and T2DM in males than in females, due to higher r values between FG and NAFLD components (Table 3) and approximately 1.3-fold as high ORs for the risk of T2DM in males (Table 4). There are several possible mechanisms underlying the gender difference on the association between NAFLD and T2DM. First, as we know, NAFLD affected men more commonly than women. Yang and colleagues in Chinese adults have demonstrated that the prevalence of NAFLD in males was higher than in females.[4] As mentioned before, emerging data have suggested that NAFLD was significantly associated with an increased risk of T2DM.[14,18] In this study, we also observed that the prevalence of NAFLD was lower in females (4.83%) than in males (10.52%).Second, an earlier study reported that estrogen was a uricosuric agent, which might promote the degradation and excretion of uric acid.[37] Thence, the gender difference in respect to NAFLD and T2DM may be, at least, in part, attributed to the properties of estrogen. Gambineri et al, has proven the inverse association between estrogen and insulin resistance, as well as a decreased risk of T2DM.[38] Thus, there is an assumption that gender could be an independent protective factor for NAFLD due to the gender differences in hormone levels and lipid levels. Third, in our analyses, there were significantly higher levels of BMI, WC and WHR in middle-aged and elderly men compare with in women. Besides, the middle-aged and elderly men with NAFLD had higher levels of BMI and WC than women. Previous studies have shown that overweight/obesity is a well-known risk factor for incident T2DM.[18] Overweight/obesity may increase fat accumulation in liver and subsequently cause fatty liver. Fat accumulation in hepatic may decrease insulin activation of glycogen synthase and increase gluconeogenesis, and subsequently lead to T2DM.[17] Fourth, in the present study, males with NAFLD had higher levels of ALT and AST than females. Previous studies have demonstrated that elevation of ALT and AST was frequently associated with obesity, insulin resistance, and hyperlipidemia.[39] Finally, exercise has been shown to improve hepatic steatosis and normalize altered liver tests.[30] Lee and Yu et al, reported that in China, middle-aged and elderly women are more prone to comply with physician's advises to keep body fitness, while middle-aged and elderly men tend to be more sedentary.[33,40]
5. Strengths and limitations
This study has several strengths. First, to the best of our knowledge, this is the first study to examine a gender difference on the relationship between NAFLD and T2DM among a middle-aged and elderly people in Hangzhou city, Zhejiang Province, East China. It provides more evidence for the prevention and control of T2DM in a middle-aged and elderly people in China. Second, the face-to-face interview ensured that the data we collected are accurate. Additionally, all laboratory tests were done in the Department of Laboratory, Zhejiang Hospital. Thus, these biochemical data are reliable. Third, we have also controlled for potential known confounders in our analyses. Nonetheless, some potential limitations of this study also merit consideration. First, because of the cross-sectional design of this study, we are unable to determine the causal relationship between NAFLD and the risk of T2DM. Thus, future prospective cohort studies are required to confirm these findings. Second, abdominal ultrasonography (Model: GE LOGIQ E9) was used to assess the presence of NAFLD instead of liver biopsy, the gold standard for the diagnosis. However, the imaging technique is not sensitive enough to detect mild steatosis. Third, information about alcohol intake was self-reported and, therefore, might have inaccracies. Fourth, although we examined and controlled for several potential confounding factors in the statistical model, residual confounding by unknown or unmeasured factors might have also been present. Fifth, because of excluding some study participants in this study, selection bias is inevitable to a certain extent. Finally, this study was conducted in a middle-aged and elderly Chinese population, thus the results might not be generalized to other populations.
6. Conclusions
In conclusion, our findings showed that NAFLD significantly increased the risk of T2DM, and the NAFLD-T2DM association was stronger in middle-aged and elderly men than in women. Our findings provide evidence to support the hypothesis that NAFLD is a predictor of T2DM. However, additional prospective studies are needed to further clarify the causal association between NAFLD and T2DM.
Acknowledgments
The authors thank all participants from the Department of Endocrinology, Zhejiang Hospital because without their assistance and support, the authors could not have accomplished this study. Besides, the authors also thank the Medical Center for Physical Examination, Zhejiang Hospital for their important contributions to analysis of biochemical data in this study.
Author contributions
Ni LP and Jin FB conceived and designed the experiments. Ni LP, Yu D, and Wu TF conducted research. Yu D and Jin FB analyzed data and wrote the paper. All authors read and approved the final manuscript.
Conceptualization: Liping Ni, Fubi Jin.
Data curation: Liping Ni.
Formal analysis: Liping Ni, Dan Yu.
Funding acquisition: Tianfeng Wu, Fubi Jin.
Investigation: Dan Yu, Tianfeng Wu.
Methodology: Dan Yu.
Writing – original draft: Liping Ni.
Writing – review & editing: Fubi Jin.
Supplementary Material
Glossary
Abbreviations: ALT = alanine aminotransferase, AST = asparagine aminotransferase, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, FG = fasting glucose, HDL-C = high density lipoprotein-cholesterol, LDL-C = low density lipoprotein-cholesterol, NAFLD = nonalcoholic fatty liver disease, OR = odds ratio, SBP = systolic blood pressure, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TG = triglycerides, WC = waist circumference, WHR = waist-hip ratio.
References
- [1].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [2].Liu X, Peng Y, Chen S, et al. An observational study on the association between major dietary patterns and non-alcoholic fatty liver disease in Chinese adolescents. Medicine (Baltimore) 2018;97:e0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577–86. [DOI] [PubMed] [Google Scholar]
- [4].Yang CQ, Shu L, Wang S, et al. Dietary patterns modulate the risk of non-alcoholic fatty liver disease in Chinese adults. Nutrients 2015;7:4778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- [6].Lonardo A, Ballestri S, Marchesini G, et al. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 2015;47:181–90. [DOI] [PubMed] [Google Scholar]
- [7].Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:936–44. [DOI] [PubMed] [Google Scholar]
- [8].Pcsolyar NS, De Jonghe BC. Examining the use of dietary fiber in reducing the risk of type 2 diabetes mellitus in latino youth. J Transcult Nurs 2014;25:249–55. [DOI] [PubMed] [Google Scholar]
- [9].International Diabetes Federation (IDF). Diabetes Atlas. 8th edition, International Diabetes Federation, Brussels. http://diabetesatlas.org/resources/2017-atlas.html (2017). [Google Scholar]
- [10].Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with country-years and 2.7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- [11].Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- [12].Park SK, Seo MH, Shin HC, et al. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology 2013;57:1378–83. [DOI] [PubMed] [Google Scholar]
- [13].Kasturiratne A, Weerasinghe S, Dassanayake AS, et al. Influence of non-alcoholic fatty liver disease on the development of diabetes mellitus. J Gastroenterol Hepatol 2013;28:142–7. [DOI] [PubMed] [Google Scholar]
- [14].Shibata M, Kihara Y, Taguchi M, et al. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 2007;30:2940–4. [DOI] [PubMed] [Google Scholar]
- [15].Kim CH, Park JY, Lee KU, et al. Fatty liver is an independent risk factor for the development of type 2 diabetes in Korean adults. Diabet Med 2008;25:476–81. [DOI] [PubMed] [Google Scholar]
- [16].Chang Y, Jung HS, Yun KE, et al. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol 2013;108:1861–8. [DOI] [PubMed] [Google Scholar]
- [17].Ma YB, Cheng N, Lu YB, et al. Association between fatty liver and type 2 diabetes in the baseline population of Jinchang cohort. Zhonghua Liu Xing Bing Xue Za Zhi 2018;39:760–4. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Wang J, Tang Y, et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS One 2017;12:e0174291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shu L, Shen XM, Li C, et al. Dietary patterns are associated with type 2 diabetes mellitus among middle-aged adults in Zhejiang Province, China. Nutr J 2017;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Esmaillzadeh A, Kimiaqar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 2007;85:910–8. [DOI] [PubMed] [Google Scholar]
- [21].Margerison C, Riddell LJ, McNaughton SA, et al. Associations between dietary patterns and blood pressure in a sample of Australian adults. Nutr J 2020;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. [Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update]. Zhonghua Gan Zang Bing Za Zhi. 2018; 26(3):195-203. [DOI] [PubMed] [Google Scholar]
- [23].Wang HJ, Wang ZH, Yu WT, et al. Changes of waist circumference distribution and the prevalence of adiposity among Chinese adults from 1993 to 2006. Zhonghua Liu Xing Bing Xue Za Zhi 2008;29:953–8. [PubMed] [Google Scholar]
- [24].Zheng PF, Shu L, Zhang XY, et al. Association between dietary patterns and the risk of hypertension among Chinese: a cross-sectional study. Nutrients 2016;8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Käräjämäki AJ, Bloigu R, Kauma H, et al. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metab Clin Exp 2017;66:55–63. [DOI] [PubMed] [Google Scholar]
- [26].Ortiz-Lopez C, Lomonaco R, Orsak B, et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetescare 2012;35:873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–92. [DOI] [PubMed] [Google Scholar]
- [28].Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes the atherosclerosis risk in communities study. Diabetes 2003;52:1799–805. [DOI] [PubMed] [Google Scholar]
- [29].Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62: (1 Suppl): S47–64. [DOI] [PubMed] [Google Scholar]
- [30].Jin YJ, Kim KM, Hwang S, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol 2012;27:1341–7. [DOI] [PubMed] [Google Scholar]
- [31].Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 2005;43:1060–6. [DOI] [PubMed] [Google Scholar]
- [32].Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yu XL, Shu L, Shen XM, et al. Gender difference on the relationship between hyperuricemia and nonalcoholic fatty liver disease among Chinese: an observational study. Medicine (Baltimore) 2017;96:e8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tran AT, Berg TJ, Gjelsvik B, et al. Ethnic and gender differences in the management of type 2 diabetes: a cross-sectional study from Norwegian general practice. BMC Health Serv Res 2019;19:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamada T, Fukatsu M, Suzuki S, et al. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol 2010;25:352–6. [DOI] [PubMed] [Google Scholar]
- [36].Yamazaki H, Tsuboya T, Tsuji K, et al. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care 2015;38:1673–9. [DOI] [PubMed] [Google Scholar]
- [37].Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab 2008;93:2230–3. [DOI] [PubMed] [Google Scholar]
- [38].Gambineri A, Pelusi C. Sex hormones, obesity and type 2 diabetes: is there a link? Endocr Connect 2019;8:R1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960–7. [DOI] [PubMed] [Google Scholar]
- [40].Lee SA, Xu WH, Zheng W, et al. Physical activity patterns and their correlates among Chinese men in Shanghai. Med Sci Sports Exerc 2007;39:1700–7. [DOI] [PubMed] [Google Scholar]


