Abstract
To detect the molecular characterization of hemoglobinopathies and thalassemias in Northern Guangdong Province of China.
We recruited 10,285 subjects who were screened for hemoglobin (Hb) variants and thalassaemia genotypes in the outpatient department of Yuebei People's Hospital from January 2018 to December 2020. The subjects collected venous blood samples for blood cell parameter analysis and Hb electrophoresis analysis. When the average red blood cell volume is <82 fL, or the average red blood cell Hb is <27 pg, or HbA2 > 3.5%, or HbA2 < 2.5%, or HbF > 2.0%, the screening is positive if one of them is satisfied. All subjects who were screened positive were tested for the thalassaemia gene by gap-polymerase chain reaction, PCR-based reverse dot blot, and DNA sequencing.
Among all subjects screened, the overall prevalence of hemoglobinopathies and thalassemias were 0.46% (47/10,285) and 21.02% (2162/10,285) in Northern Guangdong Province. We found that Hb Q-Thailand is the most common, and other types of hemoglobinopathies are followed by Hb E, Hb New York, Hb G-Chinese, Hb G-Coushatta, Hb J-Bangkok, Hb J-Broussais, Hb Ottawa, and Hb G-Taipei. We identified 1340 cases (13.03%) of α-thalassemia, mainly includes --SEA deletion (71.64%), –α3.7 deletion (12.01%), –α4.2 deletion (4.78%). And identified 652 cases (6.34%) of β-thalassemia, the most prevalent being CD 41/42(-TTCT) (35.89%), IVS-II-654 (C > T) (33.44%), CD 17 (A > T) (10.28%) and –28(A > G) (9.66%). Furthermore, there are 170 cases (1.65%) of α combined β thalassaemia. In addition, we found a rare case with –80 (T > A) of β-thalassemia. The results of this study found a high prevalence of hemoglobinopathies and thalassemias in Northern Guangdong Province, China. There were some differences molecular characterizations of thalassemia in different areas of China.
Our results enriched the related information of hemoglobinopathies and thalassemias in the region, which provided valuable references for the prevention and control of thalassemia.
Keywords: genotype, hemoglobinopathy, molecular characterization, thalassemia
1. Introduction
Hemoglobinopathy and thalassemia are genetic disorders caused by aberrant hemoglobin (Hb). Hemoglobinopathy is caused by an alteration of the globin peptide chain conformation, whereas thalassemia is caused by reduced synthesis of globin peptide chains.[1] More than 600 hemoglobinopathy and 470 thalassemia mutations have been reported and recorded in databases (http://globin.bx.psu.edu/hbvar) until now.[2] Worldwide, thalassemia mainly occurs in malaria-prone parts of the world including Far East Africa, the Mediterranean, the Middle East, the Indian subcontinent, and Southeast Asia. In China, this inherited blood disorder is highly prevalent in Guangxi, Guangdong, and Yunnan provinces.[3] Globally, more than 1.0% of couples are at risk of having children with severe hemoglobinopathy, with more than 330,000 affected babies born each year. It constitutes a major health problem in areas with high carrier frequency, most of which occur in developing countries.[4–7]
Guangdong province, which is located in the southern part of China, has a high prevalence of thalassemia. Shaoguan, a northern city in Guangdong province, is the border area between Guangdong, Hunan, and Jiangxi provinces of China. It is also an important gateway to the north and south of China. The Hakka people account for about 70% of the 3 million residents in Shaoguan. A previous survey showed that Hakka populations living in Guangxi,[8] Guangdong,[9] and Jiangxi provinces had a higher incidence of hemoglobinopathies and thalassemias, thalassemia major is a fatal and disabling single-gene genetic disease, leading to serious public health problems in these areas.[10] Currently, detection of carriers and prenatal diagnosis are the only effective interventions to prevent the birth of babies with thalassemia major, due to lack of effective treatments for thalassemia. However, the scarcity of studies on thalassemia genotypes and hemoglobinopathy in Northern Guangdong Province greatly influences genetic counseling and prenatal diagnosis in this area. This is the first study that seeks to identify the hemoglobinopathy and thalassemia gene mutations in Northern Guangdong Province, which will contribute to find out the cause of anemia, treat it symptomatically and provide a scientific basis for genetic counseling and prenatal diagnosis, so as to prevent the birth of babies with thalassemia major and improve the quality of the birth population.
2. Methods
We recruited 10,285 subjects that were screened for Hb variants and thalassemia genotype at the Outpatient Department of Yuebei People's Hospital from January 2018 to December 2020. The subjects had a mean age of 32 years (range, 7 months-83 years). The Ethics Committee of Yuebei People's Hospital reviewed and approved this study. Signed informed consent was obtained from all participants. All experiments were performed in accordance with relevant guidelines and regulations.
2.1. Hemoglobinopathy analysis
Venous blood samples were collected from each subject and anticoagulated with ethylenediaminetetraacetic acid-K2. Approximately 2 mL of the anticoagulated blood samples were used for analysis of blood cell parameters on Sysmex XT-2000i instrument (Japan East Asia Medical Electronics Co., Ltd.), and the Hb components were analyzed with Capillary electrophoresis system (Sebia Electrophoresis System, France). Positive thalassemia screening was defined as a mean corpuscular volume < 82 fL, or mean corpuscular Hb < 27 pg, or HbA2 > 3.5%, or HbA2 < 2.5%, HbF > 2.0%. All patients positive for thalassemia screening were subjected to genetic testing.
2.2. Analysis of thalassemias
Genomic DNA was extracted from venous blood samples using a genomic DNA extraction kit (Qiagen, Germany). The 3 common deletional α-thalassemias were detected by gap-polymerase chain reaction; detection of the 3 non-deletional α-thalassemia and 17 genotypes of β-thalassemia were done using reverse dot-blot hybridization with the thalassemia gene detection kit (Shenzhen Yaneng Biotechnology Co., Ltd. China). The assay was carried out following the manufacturer's instructions.
For suspected rare types of hemoglobinopathy, the full-length α1-, α2- and β-globin genes were amplified using PCR assay. The purified PCR products were sequenced by ABI 3700 Sequencer (Applied Bio systems, USA).
2.3. Statistical analysis
Statistical analysis was conducted with SPSS 22.0 software (IBM SPSS22.0). Data were reported with the descriptive statistics method and showed as the means ± SD. The chi-square test was used to compare the distribution of various alleles of Hb variation in Shaoguan and other areas of China. A value of P < .05 was considered as statistically significant.
3. Results
3.1. Hemoglobinopathy results
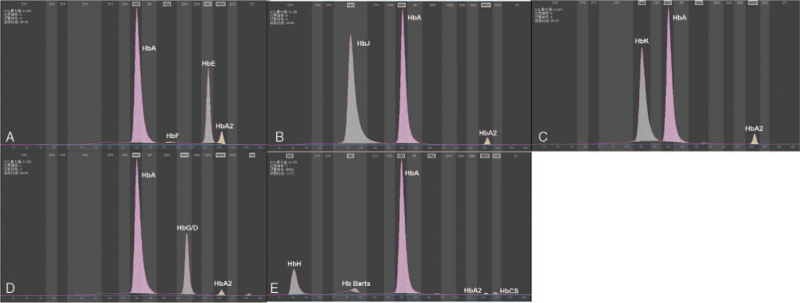
Among the 10,285 subjects screened for hemoglobinopathy, there were 47 cases positive, include 26 cases α-globin gene mutations and 21 cases β-globin mutations, the prevalence of hemoglobinopathy was 0.46%. Nine different Hb variants were identified, Hb Q-Thailand (HBA1: c.223 G > C) (17/47) was the main Hb variant, followed by Hb E (HBB: c.79 G > A) (7/47), Hb New York (HBB: c.341 T > A) (6/47), Hb G-Chinese (HBA2: c.91 G > C) (6/47), Hb G-Coushatta (HBB: c.68 A > C) (4/47), Hb J-Bangkok (HBB: c.170 G > A) (3/47), Hb J-Broussais (HBA1:c.273G > C) (2/47), Hb Ottawa (HBA1: c.46 G > C) (1/47), and Hb G-Taipei (HBB:c.68A > G) (1/47) (Table 1 and Fig. 1).
Table 1.
Prevalence of hemoglobinopathy in Northern Guangdong Province (n = 10,285).
| Hemoglobin variant | No. patients detected | Constituent ratio (%) | Prevalence ratio (%) |
| Hb Q-Thailand (HBA1: c.223 G > C) | 17 | 36.17 | 0.17 |
| Hb E (HBB: c.79 G > A) | 7 | 14.89 | 0.07 |
| Hb New York (HBB: c.341 T > A) | 6 | 12.77 | 0.06 |
| Hb G-Chinese (HBA2: c.91 G > C) | 6 | 12.77 | 0.06 |
| Hb G-Coushatta (HBB: c.68 A > C) | 4 | 8.51 | 0.04 |
| Hb J-Bangkok (HBB: c.170 G > A) | 3 | 6.38 | 0.03 |
| Hb J-Broussais (HBA1:c.273G > C) | 2 | 4.26 | 0.02 |
| Hb Ottawa (HBA1: c.46 G > C) | 1 | 2.13 | 0.01 |
| Hb G-Taipei (HBB:c.68A > G) | 1 | 2.13 | 0.01 |
| Total | 47 | 100.00 | 0.46 |
Figure 1.
Hemoglobinopathy electrophoresis. (A) Hb E. (B) Hb J. (C) Hb K. (D) HbG/D. (E) Hb H, Hb Barts, Hb CS.
3.2. Thalassemia results
Of the 10,285 cases who were screened for thalassemia, 1340 cases were diagnosed as α-thalassemia, the prevalence of α-thalassemia was 13.03% (1340/10,285). The most frequent mutations were seen in the genotype --SEA/αα (71.64%), followed by -α3.7/αα (12.01%), and -α4.2/αα (4.78%), and the 3 most frequent non-deletional mutation detected were αCSα/αα (2.54%), αWSα/αα (1.87%), and αQSα/αα (0.75%) (Table 2).
Table 2.
Prevalence of α-thalassemia in Northern Guangdong Province (n = 10,285).
| Genotypes | Phenotype | Case number | Constituent ratio (%) | Prevalence ratio (%) |
| --SEA/αα | α0/α | 960 | 71.64 | 9.33 |
| –α3.7/αα | α+/α | 161 | 12.01 | 1.57 |
| –α4.2/αα | α+/α | 64 | 4.78 | 0.62 |
| –α3.7/--SEA | α+/α0 | 52 | 3.88 | 0.51 |
| αCSα/αα | α+/α | 34 | 2.54 | 0.33 |
| αWSα/αα | α+/α | 25 | 1.87 | 0.24 |
| –α4.2/--SEA | α+/α0 | 16 | 1.19 | 0.16 |
| αQSα/αα | α+/α | 10 | 0.75 | 0.10 |
| αCSα/--SEA | α+/α0 | 9 | 0.67 | 0.09 |
| αQSα/--SEA | α+/α0 | 4 | 0.30 | 0.04 |
| αWSα/--SEA | α+/α0 | 3 | 0.22 | 0.03 |
| –α3.7/–α4.2 | α+/α+ | 2 | 0.15 | 0.02 |
| Total | 1340 | 100.00 | 13.03 |
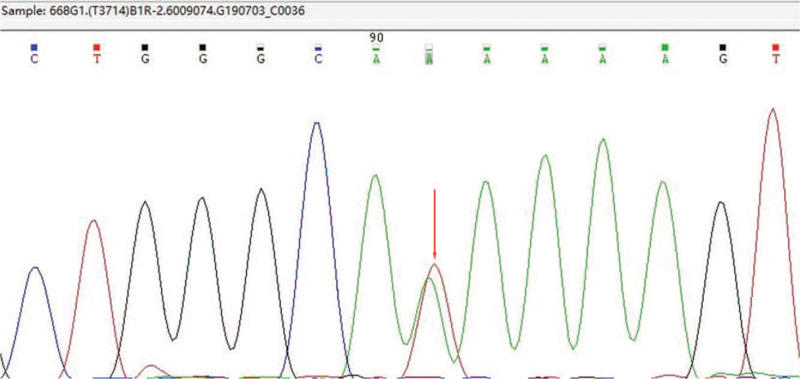
Furthermore, 652 cases were diagnosed as β-thalassemia. The prevalence of β-thalassemia was 6.34% (652/10,285). The 4 most common mutation genotypes were βCD41–42(-TCTT)/βN (35.89%), βIVS-II-654(C>T)/βN (33.44%), βCD17(A>T)/βN (10.28%) and β−28(A>G)/βN (9.66%) (Table 3). Finally, there are 170 cases of α combined β thalassaemia (Table 4). In addition, A case of rare type with -80(T > A) homozygous mutation of β-thalassemia was found (Fig. 2).
Table 3.
Prevalence of β-thalassemia in Northern Guangdong Province (n = 10,285).
| Genotypes | Phenotype | Case number | Constituent ratio (%) | Prevalence ratio (%) |
| CD 41/42 (–TTCT)/N | β0/βN | 234 | 35.89 | 2.28 |
| IVS-II-654 (C > T)/N | β+/βN | 218 | 33.44 | 2.12 |
| CD 17 (A > T)/N | β0/βN | 67 | 10.28 | 0.65 |
| –28 (A > G)/N | β+/βN | 63 | 9.66 | 0.61 |
| CD 71/72 (+A)/N | β0/βN | 24 | 3.68 | 0.23 |
| CD 27/28 (+C)/N | β0/βN | 14 | 2.15 | 0.14 |
| βE(G > A)/N | β+/βN | 13 | 1.99 | 0.13 |
| CD 14/15 (+G)/N | β0/βN | 5 | 0.77 | 0.05 |
| –29 (A > G)/N | β+/βN | 4 | 0.61 | 0.04 |
| CAP +1 (A > C)/N | β+/βN | 4 | 0.61 | 0.04 |
| IVS-I-1 (G > T/A)/N | β0/βN | 3 | 0.46 | 0.03 |
| CD43 (G > T)/N | β0/βN | 2 | 0.31 | 0.02 |
| –80 (T > A)/N∗ | β+/βN | 1 | 0.15 | 0.01 |
| Total | 652 | 100.00 | 6.34 |
A rare case with –80(T > A) homozygous mutation of β-thalassemia by Sanger sequencing.
Table 4.
Prevalence of α+β-thalassemia in Northern Guangdong Province (n = 10,285).
| Genotypes | Case number | Constituent ratio (%) | Prevalence ratio (%) |
| --SEA/αα; CD 41/42 (–TTCT)/N | 40 | 23.53 | 0.39 |
| --SEA/αα; IVS-II-654 (C > T)/N | 33 | 19.41 | 0.32 |
| --SEA/αα; –28 (A > G)/N | 18 | 10.59 | 0.18 |
| --SEA/αα; CD 17 (A > T)/N | 4 | 2.35 | 0.04 |
| --SEA/αα; CD 71/72 (+A)/N | 3 | 1.76 | 0.03 |
| --SEA/αα; βE(G > A)/N | 3 | 1.76 | 0.03 |
| --SEA/αα; CD 41/42 (–TTCT)/–28 (A > G) | 3 | 1.76 | 0.03 |
| --SEA/αα; IVS-II-654 (C > T)/–28 (A > G) | 3 | 1.76 | 0.03 |
| --SEA/αα; –28 (A > G)/CD 17 (A > T) | 1 | 0.59 | 0.01 |
| –α3.7/αα; CD 41/42 (–TTCT)/N | 22 | 12.94 | 0.21 |
| –α3.7/αα; IVS-II-654 (C > T)/N | 15 | 8.82 | 0.15 |
| –α3.7/αα; –28 (A > G)/N | 4 | 2.35 | 0.04 |
| –α3.7/αα; CD 27/28 (+C)/N | 1 | 0.59 | 0.01 |
| –α3.7/αα; CD 41/42 (–TTCT)/–28 (A > G) | 2 | 1.18 | 0.02 |
| –α4.2/αα; CD 17 (A > T)/N | 1 | 0.59 | 0.01 |
| –α4.2/αα; βE(G > A)/N | 1 | 0.59 | 0.01 |
| –α3.7/--SEA; CD 41/42 (–TTCT)/N | 2 | 1.18 | 0.02 |
| –α3.7/--SEA; IVS-II-654 (C > T)/N | 2 | 1.18 | 0.02 |
| –α4.2/--SEA; CD 41/42 (–TTCT)/N | 10 | 5.88 | 0.10 |
| αQSα/--SEA; IVS-II-654 (C > T)/N | 1 | 0.59 | 0.01 |
| αCSα/αα; IVS-II-654 (C > T)/N | 1 | 0.59 | 0.01 |
| Total | 170 | 100.00 | 1.65 |
Figure 2.
A case with –80(T > A) homozygous mutation of β-thalassemia by Sanger sequencing.
4. Discussion
Epidemiological data have shown that thalassemia is highly prevalent in Guangdong province of China,[11] however; there are no studies on the hemoglobinopathy and thalassemia genotypes in Northern Guangdong Province until now. The present study detected for the first time, a prevalence 0.46% of hemoglobinopathy, and 19.37% overall prevalence of thalassemia in Northern Guangdong Province. Our findings confirm that hemoglobinopathy and thalassemia are highly prevalent in Guangdong province, and suggest that screening of thalassemia should be performed to prevent the birth of babies with thalassemia major.
The prevalence of hemoglobinopathy was similar to that of the neighboring Meizhou (0.47%). There was no significant statistical difference between the 2 regions (P = .13). However, the frequency is higher than that of the average level of Guangdong province (0.36%, P < .05), that of the average level of the 7 provinces in southern region of the Yangtze River (0.33%, P < .05), and that of the average level of the 7 provinces in northern region of the Yangtze River (0.17%, P < .05).[11]
In this study, Hb Q-Thailand (HBA1: c.223 G > C) was the most frequent Hb variant (0.17%, 17/10,285) in Shaoguan. All Hb Q-Thailand cases were associated with -α4.2 thalassemia and showed slight microcytosis. Although this structural Hb variant was initially identified from a Chinese family, data on its origin and spread remains to be elucidated.[12] Previous studies have shown that Hb Q-Thailand is mainly distributed in the Hakka population of Meizhou, Guangdong province.[9] It is also widely distributed in Southeast Asia, with the Hakka migration model, which has led to populations in Taiwan, Thailand, and Singapore.[13,14] Our data supports previous speculations about Hb Q-Thailand originating from Chinese Hakkas.
In the present study, we detected α-thalassemias genotypes, including 93.66% deletional mutations and 6.34% non-deletional mutations. The most common deletional mutations were --SEA/αα (71.64%), −α3.7/αα (12.01%) and −α4.2/αα (4.78%). The prevalence of β-thalassemias was 6.34%, and the most common genotypes were βCD41–42(-TCTT)/βN (35.89%), followed by βIVS-II-654(C>T)/βN (33.44%), βCD17(A>T)/βN (10.28%), β−28(A>G)/βN (9.66%) and these 4 genotypes consisted 89.27% of all β-thalassemias. Our data are consistent with the report of Guangdong,[15] Hainan,[16] Hunan,[17] and Jiangxi.[18] Our findings differ from that of the report in Guangxi and Chongqing,[19,20] where βCD17(A>T)/βN was the most common genotype in these areas, indicating region-specific prevalence of thalassemia genotypes. It is important to first obtain reliable epidemiological data on indigenous populations in order to propose effective public health strategies for prevention and control of hemoglobinopathies. Therefore, special attention should be paid to screening for hemoglobinopathies and thalassemia.[21]
The present study confirmed the high prevalence of hemoglobinopathies and thalassemias in Northern Guangdong Province of China. There were some differences molecular characterizations of thalassemia in different areas of China. Our results enriched the related information of hemoglobinopathies and thalassemias in the region, which provided valuable references for the prevention and control of thalassemia.
Acknowledgments
We would like to thank all the patients who volunteered to participate in this study and the research assistants for their contribution throughout this study.
Author contributions
Conceptualization: Zhanzhong Ma.
Data curation: Shushu Fan, Jun Liu.
Formal analysis: Jun Liu.
Investigation: Jun Liu, Yulan Liu, Yanle Guo.
Resources: Shushu Fan.
Writing – original draft: Zhanzhong Ma.
Writing – review & editing: Wenbo Huang.
Footnotes
Abbreviation: Hb = hemoglobin.
How to cite this article: Ma Z, Fan S, Liu J, Liu Y, Guo Y, Huang W. Molecular characterization of hemoglobinopathies and thalassemias in Northern Guangdong Province, China. Medicine. 2021;100:45(e27713).
This work was supported by the grants from Shaoguan Science and Technology Plan Project (No. 2017CX/016) and the Guangdong Province Science and Technology Innovation Strategy Project (No. 201803011).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Vrettou C, Kakourou G, Mamas T, Traeger-Synodinos J. Prenatal and preimplantation diagnosis of hemoglobinopathies. Int J Lab Hematol 2018;40 Suppl 1:74–82. [DOI] [PubMed] [Google Scholar]
- [2].Kim Y, Park J, Kim M. Diagnostic approaches for inherited hemolytic anemia in the genetic era. Blood Res 2017;52:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang H, Xu L, Chen M, et al. Molecular characterization of thalassemia and hemoglobinopathy in Southeastern China. Sci Rep 2019;9:3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maji SK, Dolai TK, Pradhan S, et al. Implications of population screening for thalassemias and hemoglobinopathies in rural areas of West Bengal, India: report of a 10-year study of 287,258 cases. Hemoglobin 2020;44:432–7. [DOI] [PubMed] [Google Scholar]
- [5].Shrestha RM, Pandit R, Yadav UK, Das R, Yadav BK, Upreti HC. Distribution of hemoglobinopathy in Nepalese population. J Nepal Health Res Counc 2020;18:52–8. [DOI] [PubMed] [Google Scholar]
- [6].Anh TM, Sanchaisuriya K, Kieu GN, et al. Thalassemia and hemoglobinopathies in an ethnic minority group in Northern Vietnam. Hemoglobin 2019;43:249–53. [DOI] [PubMed] [Google Scholar]
- [7].Mankhemthong K, Phusua A, Suanta S, Srisittipoj P, Charoenkwan P, Sanguansermsri T. Molecular characteristics of thalassemia and hemoglobin variants in prenatal diagnosis program in northern Thailand. Int J Hematol 2019;110:474–81. [DOI] [PubMed] [Google Scholar]
- [8].Zheng CG, Liu M, Du J, Chen K, Yang Y, Yang Z. Molecular spectrum of α- and β-globin gene mutations detected in the population of Guangxi Zhuang Autonomous Region, People's Republic of China. Hemoglobin 2011;35:28–39. [DOI] [PubMed] [Google Scholar]
- [9].Lin M, Wen YF, Wu JR, et al. Hemoglobinopathy: molecular epidemiological characteristics and health effects on Hakka people in the Meizhou region, southern China. PLoS One 2013;8:e55024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin M, Zhong TY, Chen YG, et al. Molecular epidemiological characterization and health burden of thalassemia in Jiangxi Province, P.R. China. PLoS One 2014;9:e101505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao P, Wu H, Weng R. Molecular analysis of hemoglobinopathies in a large ethnic Hakka population in southern China. Medicine (Baltimore) 2018;97:e13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jiang F, Zhou JY, Yan JM, Lu YC, Li DZ. First report of a Chinese family carrying a double heterozygosity for Hb Q-Thailand and Hb J-Bangkok. Hemoglobin 2016;40:425–7. [DOI] [PubMed] [Google Scholar]
- [13].Lin M, Wang Q, Zheng L, et al. Prevalence and molecular characterization of abnormal hemoglobin in eastern Guangdong of southern China. Clin Genet 2012;81:165–71. [DOI] [PubMed] [Google Scholar]
- [14].Zheng L, Li Y, Lu S, et al. Physical characteristics of Chinese Hakka. Sci China Life Sci 2013;56:541–51. [DOI] [PubMed] [Google Scholar]
- [15].Peng Q, Zhang Z, Li S, et al. Molecular epidemiological and hematological profile of thalassemia in the Dongguan Region of Guangdong Province, Southern China. J Clin Lab Anal 2021;35:e23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li M, Xiang SH, Ding Y, Liu WW, Xu YY, Bo J. Genotype analysis of patients with thalassemia in Sanya area of Hainan Province in China. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018;26:1146–50. [DOI] [PubMed] [Google Scholar]
- [17].Liu Q, Jia ZJ, Xi H, Liu J, Peng Y, Wang H. Analysis on the genotype of 5018 cases of thalassemia in Hunan area. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019;27:1938–42. [DOI] [PubMed] [Google Scholar]
- [18].Gan J, Ding F, Liu H. Analysis of thalassemia-related mutations in Pingxiang area of Jiangxi. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2020;37:1101–3. [DOI] [PubMed] [Google Scholar]
- [19].Xiong F, Sun M, Zhang X, et al. Molecular epidemiological survey of haemoglobinopathies in the Guangxi Zhuang Autonomous Region of southern China. Clin Genet 2010;78:139–48. [DOI] [PubMed] [Google Scholar]
- [20].Qin M, Gu HY, Zhang HY, Ren J, Peng R, Zhu X. Prevalence and genotypes analysis of thalassemia among people of reproductive age in Yuzhong District of Chongqing based on next-generation sequencing. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020;28:932–6. [DOI] [PubMed] [Google Scholar]
- [21].Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet 2018;391:155–67. [DOI] [PubMed] [Google Scholar]