Abstract
Self-regulation denotes the processes by which people initiate, maintain, and control their own thoughts, behaviors, or emotions to produce a desired outcome or avoid an undesired outcome. Self-regulation brings the influence of distal factors such as biology, temperament, and socialization history onto cognition, motivation, and behavior. Dysfunction in self-regulation represents a contributory causal factor for psychopathology. Accordingly, we previously proposed a risk phenotype model for depression drawing from regulatory focus theory and traditional task-based fMRI studies. In this article, we revise and expand our risk phenotype model using insights from new methodologies allowing quantification of individual differences in task-free macroscale brain organization. We offer a set of hypotheses as examples of how examination of intrinsic macroscale brain organization can extend and enrich investigations of self-regulation and depression. In doing so, we hope to promote a useful heuristic for model development and for identifying transdiagnostic risk phenotypes in psychopathology.
Self-Regulation and Psychopathology
Human behavior is both organized and motivated by goals, which are representations of desired end-states (Austin & Vancouver, 1996). Goal pursuit is among the most important of all psychological activities; goals – as knowledge structures – provide both logical and temporal organization to complex behaviors (Miller et al., 1960). Mechanisms supporting goal-directed behavior are distributed throughout the brain, involving multiple levels of organization and types of information processing, all subserving goal attainment within a complex social environment (Depue & Collins, 1999).
In psychology, the term self-regulation is used to denote the processes by which people initiate, maintain, and control their own thoughts, behaviors, or emotions, with the intention of producing a desired outcome or avoiding an undesired outcome (Hoyle & Gallagher, 2015). Self-regulation is a critical locus for the influence of factors such as biology, temperament, and socialization history on behavior. Brain correlates of self-regulatory capacity can be found at multiple levels of organization. Dysfunction in self-regulation increases risk for psychopathology, and a number of etiological pathways by which self-regulatory dysfunction might lead to disorders such as depression, generalized anxiety disorder, and eating disorders have been identified (Karoly, 2010).
Two decades ago (Strauman, 2002), we proposed a model for vulnerability to depression in relation to unsuccessful personal goal pursuit using regulatory focus theory (Higgins, 1998), which distinguishes between goal pursuit via promotion (making good things happen) vs. prevention (keeping bad things from happening). At the time, the model was proposed as a guide for translational research, and consisted mainly of hypotheses yet to be tested. Subsequently, we have been able to refine the model based on empirical findings, postulating a self-regulation risk phenotype for depression (Strauman, 2017). The phenotype postulates that depression can result from hypoactivation of the promotion system (associated with anhedonia and dysphoria) due to chronic perseverative goal pursuit failure as well as hyperactivation of the prevention system (associated with anxiety) due to failure of normal reciprocal inhibition between promotion and prevention.
While this risk phenotype model is consistent with a range of findings linking self-regulatory dysfunction with depression, including treatment data suggesting a specific link between self-regulatory cognition and response to psychotherapy (Strauman et al., 2006), it is instantiated exclusively at the level of individual brain regions or circuits identified from task-based functional magnetic resonance imaging (fMRI). If self-regulation is as ubiquitous as behavioral scientists and neuroscientists have postulated, then reliably detectable individual differences in self-regulatory capacity should manifest at multiple levels of organization within the brain. The model as it stands is too limited in scope to fully account for how altered information processing in the brain may mediate the effects of chronic failure in goal pursuit on the initiation and maintenance of depressive episodes. If self-regulation is essential to our understanding of transdiagnostic vulnerability to psychopathology, then it should account for significant variance in such vulnerability not just at a local level (e.g., an isolated brain network) but across the brain as a whole.
The goals of this article are twofold. First, in the spirit of ongoing refinement of the current model, we take this opportunity to “pre-register” a revised set of hypotheses regarding the risk phenotype that incorporate new methodological developments, offering a priori predictions that will need to be tested. Second, we believe this approach itself is a useful heuristic for model development and for identifying transdiagnostic risk phenotypes in psychopathology and wish to have our work considered as an example of systematic approaches to modeling emotional vulnerability.
Macroscale Brain Organization and Individual Differences in Behavior
How can a model such as ours be expanded to create a more comprehensive understanding of self-regulatory dysfunction, which in turn could enhance treatment selection and preventive interventions? Recently a valuable organizing principle has emerged for the functional architecture of the human brain, creating an important opportunity to revisit, refine, and deepen the self-regulation construct through patterns of divergence in macroscale functional brain organization.
In 2016, Margulies and colleagues provided the first description of a macroscale pattern of brain organization by analyzing task-free (i.e., intrinsic) functional connectivity data collected through the Human Connectome Project (Margulies et al., 2016). Specifically, their analyses revealed the presence of a hierarchical gradient of intrinsic functional connectivity patterns across the human cortex that is anchored on one end by unimodal sensory and motor networks (roughly construed as bottom-up) and on the other end by heteromodal association networks (roughly construed as top-down). Subsequent studies across multiple, independent datasets have demonstrated that this macroscale hierarchical gradient is a highly replicable and reliable feature of the human cortex (Hong et al., 2020) (Bernhardt et al., 2022). Moreover, this macroscale hierarchical gradient recapitulates a wide array of foundational features of the human brain including patterns of myelination and synaptic connectivity, the balance of excitatory and inhibitory neurotransmission, as well as the developmental progression and evolutionary expansion of the cortex (Mesulam, 1998) (Wang, 2020) (H. M. Dong et al., 2021). Thus, estimating the macroscale hierarchical gradient from intrinsic functional connectivity data offers a remarkable opportunity for both empirically driven and theory-based exploration into the brain underpinnings of human behavior in general and psychopathology in particular.
What would be required in order to use this macroscale perspective on functional brain organization to refine and expand risk phenotypes for psychopathology? In addition to demonstrating reproducible patterns across different samples, it is necessary to demonstrate sensitivity to reliable individual differences potentially associated with vulnerability. Nonetheless, the macroscale hierarchical gradient not only captures the topography and spatial characteristics of functional brain networks, but also offers a structure to quantify the organized divergence of brain function from perceptions and actions (grounded in unimodal networks) to abstract cognitions and self-referential processes (grounded in heteromodal networks).
We see this emerging perspective on macroscale brain organization as both a challenge and an opportunity. It will require re-examination of existing models like ours which are expressed at a limited number of levels of organization and analysis, and we take first steps in this direction below. By doing so, we can use the hierarchical macroscale gradient as a foundational architecture for a more comprehensive understanding of how people respond to and interact with the external world during everyday goal pursuit, as well as how we process information about those interactions in reference to internalized knowledge structures (such as goals and standards).
Figure 1 illustrates a hypothetical conceptual mapping along this macroscale hierarchical gradient depicting the simultaneous influence of bottom-up signaling from unimodal sensorimotor networks and top-down signaling from heteromodal association networks. While presumably these macroscale patterns emerge as adaptive processes that reflect an optimized balance between intrinsic states and extrinsic forces, neurodevelopmental insights into psychopathology suggest that dysfunctions can become instantiated through altered patterns of intrinsic brain functional organization (Sydnor et al., 2021). Under those circumstances, our model predicts that in the face of consistent goal pursuit failure, effective self-regulation becomes increasingly difficult and perseverative. That phenomenon (perseveration in response to continued goal pursuit failure) is the core of the model, and so we would need to look for evidence that certain features of the macroscale hierarchical gradient map onto perseverative goal pursuit behavior under specific conditions (above and beyond patterns already observed in task-based fMRI studies).
Figure 1.
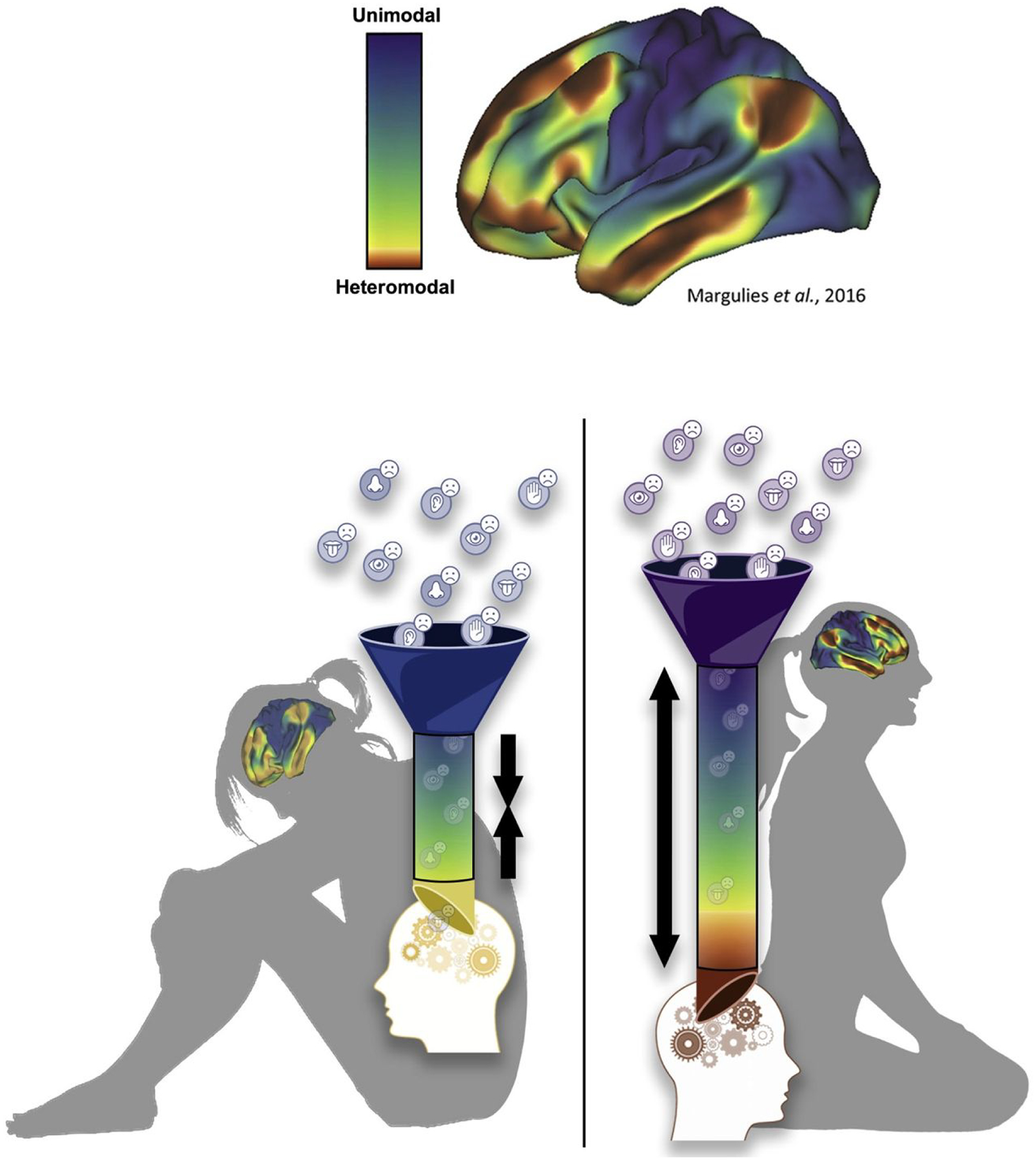
Depiction of hypothesized mapping of macroscale hierarchical gradients onto the individual’s experience in ongoing goal pursuit. Top panel depicts the distribution of functional cortical networks (left) across the macroscale hierarchical gradient (right) following from Margulies et al. (2016). Bottom panel depicts how hypothesized individual differences in the macroscale gradient influence goal pursuit based on perceptions of goal attainment success as well as associated affective and motivational feedback. As detailed in the text, a relatively compressed gradient (left) is maladaptive because negative experiences of goal pursuit failure, being functionally closer to our internal representations of goals and intrinsic motivations through a shorter gradient, unduly influence them and lead to perseveration over past failures and a self-regulatory bias toward preventing negative outcomes. A relatively expanded gradient (right) is adaptive because our negative experiences would be functionally more distant from our internal representations of goals and intrinsic motivations through a longer gradient, affording the opportunity to further parse the experience of goal pursuit failure before it can influence subsequent goal pursuit strategies thereby cultivating greater resilience.
If our intuition that broader perspectives on functional brain organization could allow us to elaborate on hypothesized mechanisms of vulnerability is correct, then variability in the manifestation of this gradient has the potential to explain differences in how sensitive we may be to goal-relevant feedback, including our propensity for promotion or prevention oriented self-regulation. In turn, providing a more comprehensive account of self-regulation provides a broader framework for developing and testing hypotheses about vulnerability to depression and other forms of psychopathology. As noted, the macroscale gradient challenges our model by pointing out its limitations but also invites revision, expansion, and refinement, and we offer a revision here primarily to set a research agenda and to articulate falsifiable predictions.
Macroscale Brain Organization and Self-Regulatory Dysfunction: Revised Hypotheses
A rapidly increasing number of studies are reporting alterations of the macroscale hierarchical gradient across different categorical psychiatric diagnoses (D. Dong et al., 2021) (Hong et al., 2019). This trend is consistent with existing evidence that dysfunction in self-regulation is a transdiagnostic feature of psychopathology (Stanton et al., 2020). Additionally, ongoing research in our laboratories on the transdiagnostic general psychopathology or p factor supports the potential value of bridging research on self-regulation and the macroscale hierarchical gradient. For example, we have reported that alterations in cortical thickness associated with general psychopathology are overrepresented in networks at the heteromodal end of the gradient (Romer, Elliott, et al., 2021). In turn, we have reported that general psychopathology is associated with promotion and prevention system dysfunction (Romer, Hariri, et al., 2021). These convergent patterns associated with transdiagnostic psychopathology further suggest that dysfunction in self-regulation may similarly reflect alterations in the macroscale hierarchical gradient, aspects of which have been identified at local levels of analysis but which have yet to be comprehensively articulated at the macroscale level. Moving towards this articulation, we recently found that variability in prevention success can be predicted by patterns of distributed cortical intrinsic functional connectivity using a data-driven strategy for developing predictive models of brain-behavior relationships through cross-validation (manuscript in preparation).
How might consideration of macroscale brain organization both augment and refine our understanding of the links between self-regulatory dysfunction and psychopathology? We begin to address this question by revising our original hypotheses about individual differences along this macroscale hierarchical gradient that incorporate what has been learned about regulatory focus and vulnerability to psychopathology but significantly expand our conceptions of how those vulnerabilities might be reflected across the brain. To illustrate how a macroscale perspective can both validate and expand our current understanding of self-regulation and psychopathology, Table 1 summarizes our current risk phenotype model and then offers a set of revised hypotheses generated from the perspective of a macroscale hierarchical gradient within which self-regulatory cognition operates. These revised hypotheses represent additional necessary requirements for a valid and reliable self-regulation-based depression risk phenotype, over and above the predictions included in the original model. They are, in effect, a research agenda we set for ourselves and the field.
Table 1.
Revising the Original Self-Regulation Risk Phenotype with Hypotheses Generated From a Macroscale Brain Organization Perspective
| Regulatory Focus and Depression: Hypotheses for the Original Risk Phenotype |
|---|
|
| Revised Hypotheses from a Macroscale Brain Organization Perspective |
|
What features of the risk phenotype, reflecting the specific hypothesized premorbid characteristics of an individual’s promotion and prevention system, should be identifiable at the hierarchical gradient level? First, we hypothesize that the three postulated features of the risk phenotype model pertaining to individual differences in promotion system function – genetically driven tendencies for perseverative approach goal pursuit, a socialization history dominated by promotion (i.e., a world view reflecting the priority of “making good things happen”), and a reinforcement history of consistent promotion goal attainment that in effect creates a standard for subsequent self-regulation that is difficult to maintain – will interact with individual differences in the macroscale hierarchical gradient to ultimately shape the emergence of depression in response to promotion failure.
Second, we hypothesize that tendencies to pursue personal goals dominated by preventing unwanted or negative outcomes reflects a macroscale hierarchical gradient wherein the distance between unimodal networks on one end and heteromodal networks on the other end is shorter than average (i.e., compressed). If this were indeed observed, it would suggest that the tendency for an individual to engage in prevention-oriented self-regulation may reflect a hypersensitivity to both the perception of and actions associated with unsuccessful efforts to achieve goals using this overarching strategy. In other words, it would mean that negative experiences of goal pursuit failure, being functionally closer to our internal representations of goals and intrinsic motivations through a shorter gradient, unduly influence them and lead to perseveration over past failures (characteristic of many individuals in depressed states) and a self-regulatory bias toward preventing negative outcomes that ultimately was both ruminative and self-defeating. In contrast, a longer gradient pushing unimodal and heteromodal networks further apart (i.e., expanded) could support more adaptive promotion-oriented self-regulation. In this scenario, our negative experiences would be functionally more distant from our internal representations of goals and intrinsic motivations through a longer gradient, affording the opportunity to further parse the experience of goal pursuit failure before it can influence subsequent goal pursuit strategies thereby cultivating greater resilience.
These divergent hypotheses are supported by recent studies reporting relative compression of the macroscale hierarchical gradient across categorical psychiatric disorders including autism, depression, and schizophrenia (Hong et al., 2019; Dong et al. 2021, Xia et al., 2022). They are further consistent with emerging research from our labs revealing that higher cognitive functioning is associated with an expanded macroscale gradient while aging, which is characterized by cognitive decline, is associated with a more compressed gradient (manuscript in preparation). Moreover, these plausible phenotypes of relative risk and resiliency could be shaped through an individual’s personal history of goal pursuit and models of self-regulation in childhood (Manian et al., 1998). For example, in a prospective longitudinal study we observed that maternal parenting behaviors discriminantly predicted children’s subsequent preferences for pursuing goals via promotion vs. prevention (Manian et al., 2006). This observation suggests what macroscale gradient patterns might be associated with individual differences in goal pursuit socialization (e.g., based on parenting styles).
The current risk phenotype model, summarized in the top panel of Table 1, is based on data obtained primarily from task-based fMRI and makes assumptions about how individual mechanisms and variables (e.g., individual differences in dopamine signaling, socialization, and reinforcement history as noted in H1) influence other mechanisms and variables (e.g., down-regulation of promotion goal pursuit that inevitably also leads to excessive reliance on prevention-based goal pursuit strategies and motivational states) leading to precursors of a depressive state (anhedonia and dysphoria with high likelihood of comorbid anxiety, as noted in H2). At this traditional local level of analysis, it is difficult to test the model as a whole. For example, we observed that depressed individuals manifest a reliable and specific attenuated BOLD signal response in left prefrontal cortex (particularly left middle frontal gyrus) in response to their own promotion goals (Eddington et al., 2009). While our model implies that such a down-regulation of promotion goal pursuit would have broader repercussions for self-regulation (as we tried to capture in H3), our task-based investigations have been limited in their ability to identify downstream, brain-wide consequences of promotion goal pursuit failure (immediate or subsequent).
The bottom panel of Table 1 lists hypotheses that reflect our findings to date but reformulates them within a macroscale brain organization framework as was just proposed above. For example, H1* predicts identification of a macroscale gradient pattern, prior to the onset of a first episode of depression, associated with covariation of the hypothesized individual difference components of the risk phenotype (dopamine signaling, socialization history, reinforcement history), and postulates discriminant features of that pattern corresponding to promotion-linked anhedonia and dysphoria as well as prevention-linked comorbid anxiety when it occurs. H2* predicts that this reliably identifiable macroscale gradient pattern is characteristic of depressive episodes associated with promotion system hypoactivation and, in cases of depressive/anxious comorbidity, corresponding prevention system hyperactivation. Similarly, H3* and H4* are attempts to expand and reformulate what the original model predicted, specifically by postulating that at the macroscale level specific patterns will be discriminantly correlated with the clinical features of self-regulation-based depression/comorbid anxiety (H3*) and that with each additional episode, those patterns become increasingly resistant to change (H4*). These hypotheses represent a radical restatement of the risk phenotype itself and hopefully offer implications for studying etiology, onset, prevention, and treatment.
How can the revised hypotheses be integrated into a testable flowchart or algorithm that can guide subsequent research examining predicted causal associations between elements within the overall model? Figure 2 depicts a revised and expanded version of the current self-regulation risk phenotype model suggesting key points at which a broader perspective on functional brain organization could illuminate critical aspects of self-regulatory dysfunction. Our intent is to identify a priori which macroscale-level characteristics of functional brain organization will be associated with each of the causal links in the model. As another example, self-system therapy (Strauman et al., 2006) was designed to target self-regulatory deficits among depressed individuals fitting the risk profile, and there is substantial behavioral evidence that it leads to the predicted mechanistic changes (Eddington et al., 2015). Building on these treatment studies, evidence that such interventions lead to predictable and robust changes in heteromodal networks associated with self-regulation would have both theoretical and therapeutic implications (e.g., treatment matching, preventive intervention, relapse prevention).
Figure 2.
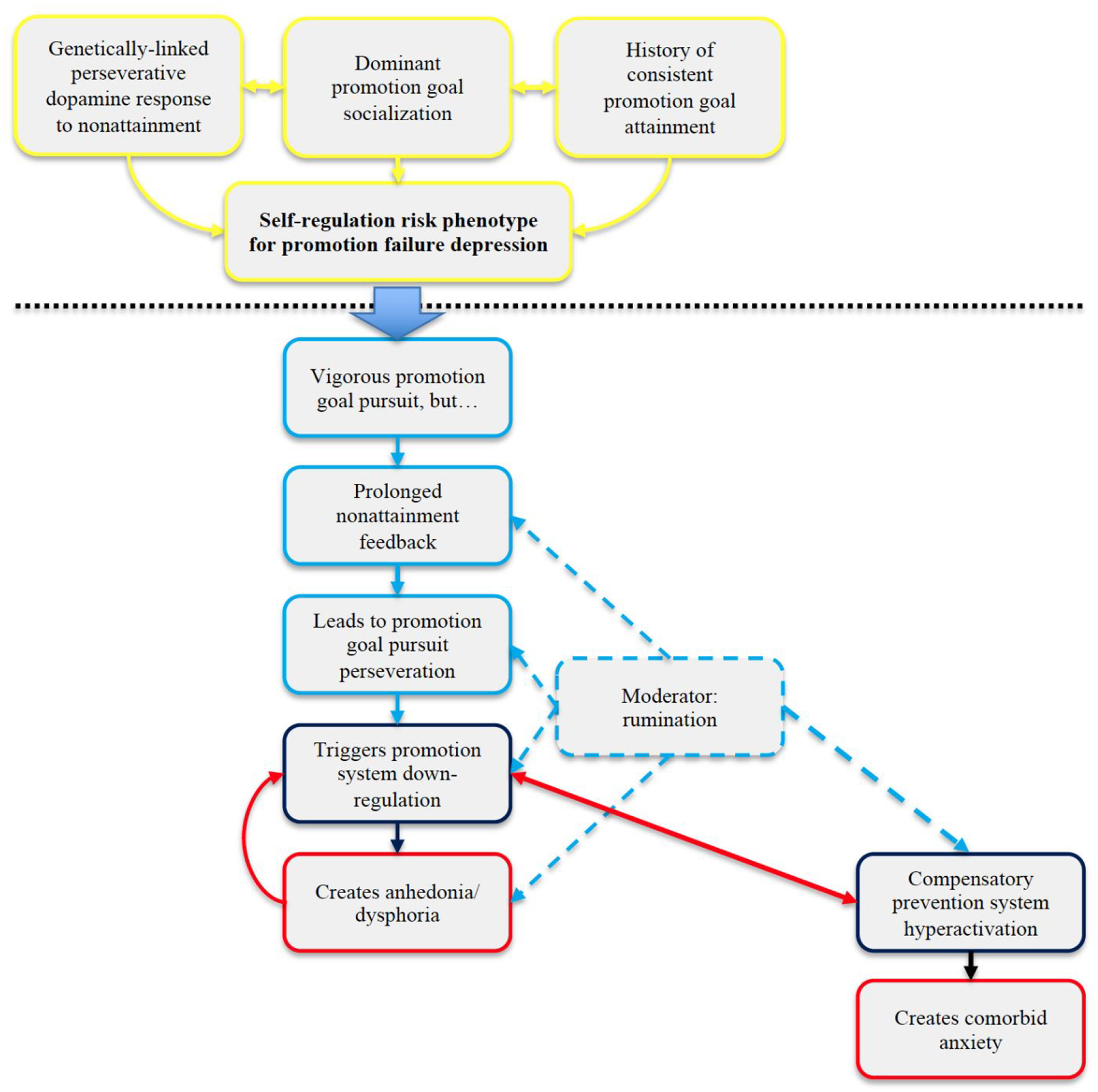
The current self-regulation risk phenotype model for depression, which is operationalized both behaviorally and via task-based fMRI studies (Strauman, 2017). Yellow panels represent predicted premorbid features of the phenotype. Blue panels represent a hypothetical sequence of events from engagement of promotion goal pursuit through receipt of consistent failure feedback through perseveration on ineffective goal pursuit behaviors, which are the events hypothesized to trigger and maintain a depressive episode. Black panels depict promotion system dysfunction (hypoactivation) and corresponding prevention system dysfunction (compensatory hyperactivation). Red panels illustrate the symptom clusters hypothesized to be associated with promotion vs. prevention system dysfunction.
What we find particularly exciting is the opportunity to revise our model with a priori new hypotheses and then empirically test them and, in doing so, deepen our understanding of the nature of differences in self-regulation tendencies as well as illuminate potential novel strategies for shifting away from more harmful toward more helpful self-regulation strategies that can include new, integrative approaches to intervention. For example, it is possible that psychedelic compounds including psilocybin, which have emerged as a potential breakthrough complementary therapy for a number of psychiatric disorders characterized by impoverished self-regulation such as addiction and depression, alter the organization of the macroscale hierarchical gradient (Girn et al., 2021). Of course, evaluating such competing hypotheses requires normative maps for the distribution of the macroscale hierarchical gradient in population representative samples across different developmental windows. Fortunately, an increasing number of publicly accessible large studies (e.g., ABCD) and consortium-driven data repositories (e.g., ENIGMA) are poised to generate exactly such maps. A more comprehensive mapping out to behavior will likely also require the systematic integration of the macroscale hierarchical gradient with subcortical structures (e.g., amygdala, ventral striatum, hippocampus, thalamus, basal ganglia) critical for experience-dependent learning and cerebellar networks critical for feed-forward monitoring of both actions and thoughts (Guell, 2021) (Tian et al., 2020).
In summary, we and others have argued that the construct of self-regulation is central to our understanding of vulnerability to psychopathology, across the spectrum of diagnostic categories. Our goal here is to once again, following the original proposal of the model twenty years ago, make a priori predictions that in effect pre-register the claims that must be tested in order for the model to be properly evaluated. Our work using regulatory focus theory to generate and test hypotheses about the psychological consequences of perceived failure in personal goal pursuit has led to formulation of a risk phenotype for depression. The findings to date are consistent with the hypothesized structure of the phenotype, but there remain critical unanswered questions about its validity, our ability to reliably identify individuals already on that depressogenic neurodevelopmental trajectory, and the impact of treatments on the hypothesized mechanisms that are responsible for vulnerability to depression. In this article, we have used our own work to illustrate how new developments in identifying and interrogating macroscale functional brain organization has the potential to revolutionize the study of transdiagnostic risk phenotypes for psychopathology. We look forward to participating in this new chapter of research alongside our fellow investigators and clinicians.
Acknowledgements:
This work has been supported by NIH grants DA031579, DA022569, MH039429, DA023026 and AG049789, as well as funding from Bass Connections, the Social Science Research Institute, and the Office of the Vice Provost for Interdisciplinary Research at Duke University.
Footnotes
Conflict of Interest Statement: Nothing declared.
References
- Austin JT, & Vancouver JB (1996). Goal constructs in psychology: Structure, process, and content. Psychological Bulletin, 120(3), 338–375. 10.1037/0033-2909.120.3.338 [DOI] [Google Scholar]
- Bernhardt BC, Smallwood J, Keilholz S, & Margulies DS (2022). Gradients in brain organization. Neuroimage, 251, 118987. 10.1016/j.neuroimage.2022.118987 [DOI] [PubMed] [Google Scholar]
- Depue RA, & Collins PF (1999). Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci, 22(3), 491–517. [DOI] [PubMed] [Google Scholar]
- Dong D, Yao D, Wang Y, Hong SJ, Genon S, Xin F, Jung K, He H, Chang X, Duan M, Bernhardt BC, Margulies DS, Sepulcre J, Eickhoff SB, & Luo C (2021). Compressed sensorimotor-to-transmodal hierarchical organization in schizophrenia. Psychol Med, 1–14. 10.1017/S0033291721002129 [DOI] [PubMed] [Google Scholar]; Example of exploratory research using macroscale gradient features to better characterize aspects of vulnerability to schizophrenia.
- Dong HM, Margulies DS, Zuo XN, & Holmes AJ (2021). Shifting gradients of macroscale cortical organization mark the transition from childhood to adolescence. Proc Natl Acad Sci U S A, 118(28). 10.1073/pnas.2024448118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington KM, Dolcos F, McLean AN, Krishnan KR, Cabeza R, & Strauman TJ (2009). Neural correlates of idiographic goal priming in depression: goal-specific dysfunctions in the orbitofrontal cortex. Soc Cogn Affect Neurosci, 4(3), 238–246. 10.1093/scan/nsp016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington KM, Silvia PJ, Foxworth TE, Hoet A, & Kwapil TR (2015). Motivational deficits differentially predict improvement in a randomized trial of self-system therapy for depression. Journal of Consulting and Clinical Psychology, 83(3), 602–616. 10.1037/a0039058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girn M, Roseman L, Bernhardt B, Smallwood J, Carhart-Harris R, & Spreng RN (2021). Serotonergic psychedelic drugs LSD and psilocybin reduce the hierarchical differentiation of unimodal and transmodal cortex. bioRxiv, 2020.2005.2001.072314. 10.1101/2020.05.01.072314 [DOI] [PubMed] [Google Scholar]
- Guell X (2021). Functional Gradients of the Cerebellum: a Review of Practical Applications. Cerebellum. 10.1007/s12311-021-01342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ET (1987). Self-discrepancy: a theory relating self and affect. Psychological Review, 94(3), 319–340. http://www.ncbi.nlm.nih.gov/pubmed/3615707 [PubMed] [Google Scholar]
- Higgins ET (1998). Promotion and prevention: Regulatory focus as a motivational principle. Advances in Experimental Social Psychology, 30, 1–46. [Google Scholar]
- Hong SJ, Vos de Wael R, Bethlehem RAI, Lariviere S, Paquola C, Valk SL, Milham MP, Di Martino A, Margulies DS, Smallwood J, & Bernhardt BC (2019). Atypical functional connectome hierarchy in autism. Nat Commun, 10(1), 1022. 10.1038/s41467-019-08944-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; Example of exploratory research using macroscale gradient features to better characterize aspects of vulnerability to autism.
- Hong SJ, Xu T, Nikolaidis A, Smallwood J, Margulies DS, Bernhardt B, Vogelstein J, & Milham MP (2020). Toward a connectivity gradient-based framework for reproducible biomarker discovery. Neuroimage, 223, 117322. 10.1016/j.neuroimage.2020.117322 [DOI] [PubMed] [Google Scholar]
- Hoyle RH, & Gallagher P (2015). The interplay of personality and self-regulation. In Mikulincer M, Shaver PR, Cooper ML, & Larsen RJ (Eds.), APA handbook of personality and social psychology: Vol. 4, personality processes and individual differences (pp. 189–207). American Psychological Association. [Google Scholar]
- Manian N, Papadakis AA, Strauman TJ, & Essex MJ (2006). The development of children’s ideal and ought self-guides: parenting, temperament, and individual differences in guide strength. J Pers, 74(6), 1619–1645. 10.1111/j.1467-6494.2006.00422.x [DOI] [PubMed] [Google Scholar]
- Manian N, Strauman TJ, & Denney N (1998). Temperament, recalled parenting styles, and self-regulation: testing the developmental postulates of self-discrepancy theory. J Pers Soc Psychol, 75(5), 1321–1332. 10.1037//0022-3514.75.5.1321 [DOI] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, Jefferies E, & Smallwood J (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A, 113(44), 12574–12579. 10.1073/pnas.1608282113 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article operationalizes how features of a gradient of macroscale cortical organization can be used to interrogate psychologically relevant individual differences.
- Mesulam MM (1998). From sensation to cognition. Brain, 121 ( Pt 6), 1013–1052. 10.1093/brain/121.6.1013 [DOI] [PubMed] [Google Scholar]
- Miller GA, Galanter E, & Pribram KH (1960). Plans and the structure of behavior. Henry Holt and Co. 10.1037/10039-000 [DOI] [Google Scholar]
- Romer AL, Elliott ML, Knodt AR, Sison ML, Ireland D, Houts R, Ramrakha S, Poulton R, Keenan R, Melzer TR, Moffitt TE, Caspi A, & Hariri AR (2021). Pervasively Thinner Neocortex as a Transdiagnostic Feature of General Psychopathology. Am J Psychiatry, 178(2), 174–182. 10.1176/appi.ajp.2020.19090934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AL, Hariri AR, & Strauman TJ (2021). Regulatory focus and the p factor: Evidence for self-regulatory dysfunction as a transdiagnostic feature of general psychopathology. J Psychiatr Res, 137, 178–185. 10.1016/j.jpsychires.2021.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton K, McDonnell CG, Hayden EP, & Watson D (2020). Transdiagnostic approaches to psychopathology measurement: Recommendations for measure selection, data analysis, and participant recruitment. J Abnorm Psychol, 129(1), 21–28. 10.1037/abn0000464 [DOI] [PubMed] [Google Scholar]
- Strauman TJ (2002). Self-Regulation and Depression. Self and Identity, 1, 151–157. [Google Scholar]
- Strauman TJ (2017). Self-Regulation and Psychopathology: Toward an Integrative Translational Research Paradigm. Annu Rev Clin Psychol, 13, 497–523. 10.1146/annurev-clinpsy-032816-045012 [DOI] [PubMed] [Google Scholar]; This article presents the current version of our self-regulation risk phenotype for depression along with a broader discussion of self-regulation as a contributory factor in transdiagnostic psychopathology.
- Strauman TJ (2021). Modeling the onset of a depressive episode: A self-regulation perspective. Curr Opin Psychol, 41, 100–106. 10.1016/j.copsyc.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauman TJ, Vieth AZ, Merrill KA, Kolden GG, Woods TE, Klein MH, Papadakis AA, Schneider KL, & Kwapil L (2006). Self-system therapy as an intervention for self-regulatory dysfunction in depression: a randomized comparison with cognitive therapy. J Consult Clin Psychol, 74(2), 367–376. 10.1037/0022-006X.74.2.367 [DOI] [PubMed] [Google Scholar]
- Sydnor VJ, Larsen B, Bassett DS, Alexander-Bloch A, Fair DA, Liston C, Mackey AP, Milham MP, Pines A, Roalf DR, Seidlitz J, Xu T, Raznahan A, Satterthwaite TD. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron. 2021. Sep 15;109(18):2820–2846. doi: 10.1016/j.neuron.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Margulies DS, Breakspear M, & Zalesky A (2020). Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci, 23(11), 1421–1432. 10.1038/s41593-020-00711-6 [DOI] [PubMed] [Google Scholar]
- Wang XJ (2020). Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat Rev Neurosci, 21(3), 169–178. 10.1038/s41583-020-0262-x [DOI] [PMC free article] [PubMed] [Google Scholar]


