Abstract
Bone metastasis seriously affects the survival of breast cancer. Therefore, the study aimed to explore the independent prognostic factors in bone metastatic breast cancer (BMBC) and to construct a prognostic nomogram that can accurately predict the survival of BMBC and strictly divide the patients into different risk stratification.
Four thousand three hundred seventy six patients with BMBC from the surveillance, epidemiology, and end results database in 2010 to 2015 were collected and randomly divided into training and validation cohort. Multivariate Cox regression identified the independent prognostic factors of BMBC. A nomogram for predicting cancer-specific survival (CSS) in BMBC was created using R software. The predictive performance of the nomogram was evaluated by plotting receiver operating characteristic (ROC) curves and calibration curves.
Marital status, race, age, T stage, tumor grade, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, brain metastasis, liver metastasis, lung metastasis, chemotherapy, and breast surgery were identified as independent prognostic factors for CSS of BMBC. The area under the ROC curve at 1-, 3-, and 5-year of the nomogram were 0.775, 0.756, and 0.717 in the internal validation and 0.785, 0.737, and 0.735 in the external validation, respectively. Calibration curves further confirmed the unbiased prediction of the model. Kaplan-Meier analysis verified the excellent risk stratification of our model.
The first prognostic nomogram for BMBC constructed in our study can accurately predict the survival of BMBC, which may provide a practical tool to help clinicians evaluate prognosis and stratify the prognostic risk for BMBC, thereby determining which patients should be given intensive treatment and optimizing individual treatment strategies for BMBC.
Keywords: bone, breast cancer, metastasis, nomogram, prognosis
1. Introduction
Metastatic breast cancer accounts for 30% of breast cancer and is the leading cause of death in breast cancer.[1] Bone metastasis occurs in about 70% of metastatic breast cancer and contributes to significant death due to severe bone pain, pathologic fractures, and hypercalcemia.[2,3] Moreover, the 3-year overall mortality rate of women with bone metastasis was significantly higher than that of those without bone metastasis (69% vs 22%).[3] Several studies have shown that the prognosis of bone metastatic breast cancer (BMBC) is clearly different from that of those with visceral metastatic breast cancer,[4–6] with median overall survival times of 26 to 50 months for those with bone metastasis whereas 13 to 18 months for visceral metastasis.[7,8] Therefore, existing prognostic models for metastatic breast cancer may not be suitable for predicting the survival of BMBC,[9,10] rendering it critical to building up a risk stratification model for the heterogeneous cohort.
At present, the independent factors of BMBC remain discordant. Li et al found that breast surgery can reduce the risk of death by 40% in BMBC.[11] However, a prospective trial indicated that breast surgery cannot significantly improve the survival of BMBC patients.[12] In addition, human epidermal growth factor receptor (HER)-positive has been regarded as a risk factor of breast cancer and metastatic breast cancer;[13,14] however, the latest studies of metastatic breast cancer concluded that human epidermal growth factor receptor 2 (HER2)-positive is the protective factor for metastatic breast cancer.[15,16] Hence, it is necessary to explore the definite function of controversial factors for BMBC.
An accurate prediction for the prognosis in breast cancer with distant metastasis is an essential basis for individualized treatment strategies.[17] Reliable predictive survival of patients with BMBC can provide a critical foundation for making appropriate decisions on treatment selection. Moreover, for patients with BMBC, most of them subject that reliable prediction of survival time can help them to manage their remaining days reasonably.[18] Nomogram, a graphical computational model, is constructed using known prognostic factors, which can be utilized to predict the prognosis of patients with cancer. In recent years, existing prognostic models for predicting brain metastatic breast cancer have been widely used in clinical practice.[19–21] Therefore, it is necessary to build up a valid model that especially predict the prognosis for patients with BMBC.
In the current study, we aimed to utilized surveillance, epidemiology, and end results (SEER)-based data to explore the independent prognostic factors for BMBC, for the purpose of determining the function of the controversial factors in BMBC and constructing a prognostic nomogram for BMBC.
2. Materials and methods
2.1. Study population
Data on patients with BMBC from the SEER database were obtained to select female patients aged between 20 and 80 years with BMBC as their only and primary cancer diagnosis from January 1, 2010 to December 31, 2015.
The exclusion criteria for this study are as follows:
-
(1)
unknown marital status and/or race;
-
(2)
T stage and/or clinical nodal status is missing;
-
(3)
all histopathology subtypes other than invasive lobular or invasive ductal carcinoma;
-
(4)
grade, estrogen receptor (ER) status, progesterone receptor (PR) status and/or HER2 status were missing;
-
(5)
unknown brain or visceral metastasis status;
-
(6)
unknown treatment data, such as breast surgery type or radiotherapy status were not available; and
-
(7)
follow-up for less than one month.
Finally, a cohort of 4376 patients with BMBC were included in our study. We randomized 3:2 and divided it into a training cohort (nomogram construction and internal validation) and a validation cohort (external validation for nomogram), as is presented in Figure 1.
Figure 1.
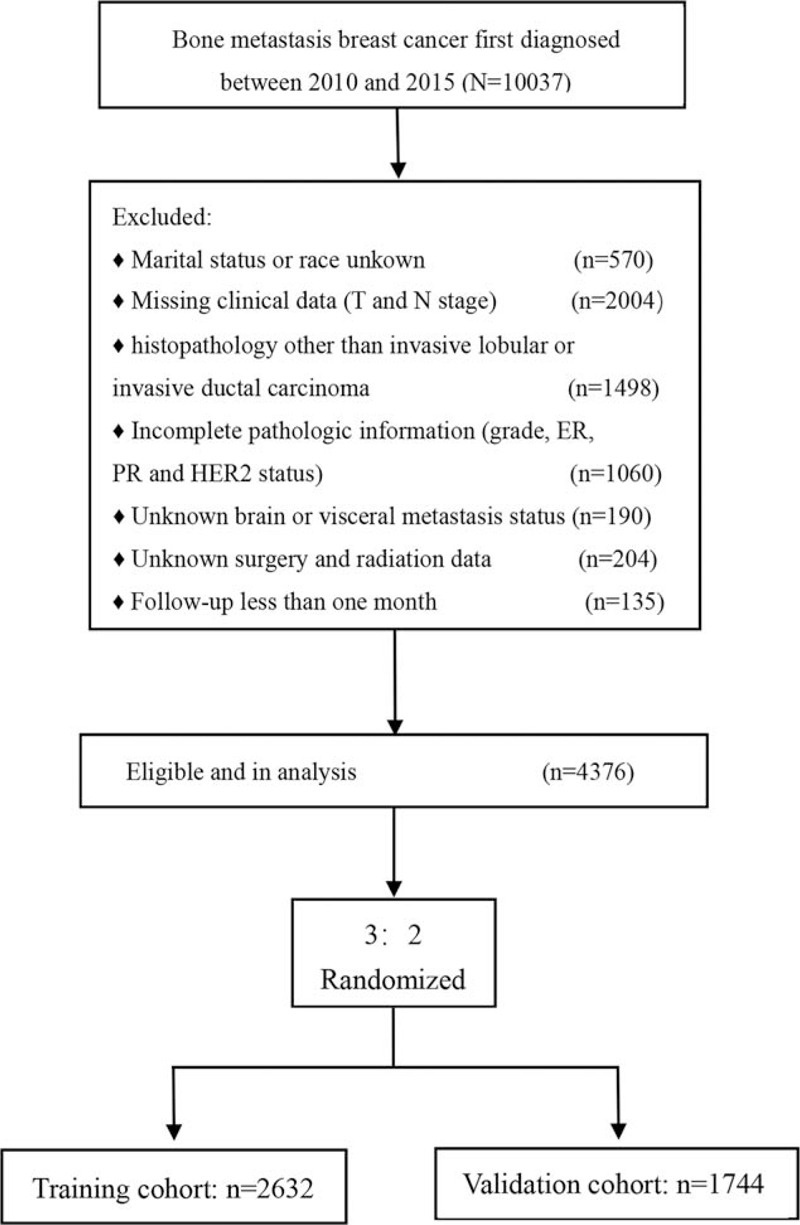
Flowchart of the study design. A total of 4376 patients with bone metastatic breast cancer were involved in our study.
2.2. Covariates
The following clinical and pathological data were collected as study variables: marital status, race, age at diagnosis, T stage, clinical lymph node status, tumor grade, ER status, PR status, HER2 status, brain metastasis, visceral metastasis, clinical treatment, and follow-up. Data on chemotherapy, endocrine therapy, and HER2-targeted therapy were not available from the SEER database. Cancer-specific survival (CSS) was defined by patient survival time, survival status, and the specific cause of death classification.
2.3. Statistical analysis
SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA) was used to conduct statistical analysis. Chi-square test or Fisher exact test was used to compare the baseline characteristics between the PMRT cohort and control cohort, whereas quantitative variables were listed as median with interquartile range (IQR) and compared by Student t test or non-parametric Mann-Whitney U test. Variables that showed P < 0.10 in univariate Cox regression analysis were included in multivariate analysis using the Cox proportional hazard model. Backward step-down selection was used to determine variables significantly associated with the survival outcome.
Based on the independent prognostic factors identified by multivariate Cox regression analysis, we used the rms package in R software, version 3.6.3 (http://www.r-project.org/), to create a nomogram for predicting CSS in BMBC. The value of the 1-, 3-, and 5-year area under the receiver operating characteristic (ROC) curve (AUC) were used to measure the discrimination of the nomogram and single independent factors. AUC = 0.5 indicates poor discrimination, and AUC = 1 indicates perfect discrimination of the nomogram to distinguish between patients with different survival probabilities. Higher the AUC value between 0.5 and 1, the more excellent the discriminative ability of the nomogram. Besides, calibration curves were plotted to evaluate the association between the 1-, 3-, and 5-year model-predicted CSS probabilities and the observed CSS probabilities. In external validation, the total points of each patient in the validation cohort were calculated based on the established nomogram. The ROC curves and calibration curves were plotted using the total points as a factor to verify the predictive performance of the nomogram in the validation set. Bootstraps with 200 times were used for these activities.
Furthermore, risk stratification was developed to divide patients into four prognostic groups on the basis of the patient's total points. Then, we used the Kaplan-Meier method to compare the CSS probabilities of different risk groups, validating the risk stratification performance of the model. In this study, P < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the study cohort
Four thousand three hundred seventy six female patients with BMBC diagnosed in 2010 to 2015 were included in analysis, of which 2632 were incorporated into the training cohort and 1744 were included into the validation cohort. The median follow-up time was 24 months (IQR, 13–40 months). Baseline characteristics of patients with BMBC in the training and validation cohort in Table 1. There was no statistical difference in the distribution of all the variables between the training and the validation cohort (P > 0.05).
Table 1.
Baseline characteristics of patients with BMBC in the training and validation cohort.
| Characteristic | Training cohort (n = 2632) | Validation cohort (n = 1744) | P∗ value | ||
| Number of patients | % | Number of patients | % | ||
| Age (years) | 0.232 | ||||
| Median (IQR) | 58 (49–66) | 58 (48–66) | |||
| Race | 0.496 | ||||
| White | 1984 | 75.4 | 1306 | 74.9 | |
| Black | 450 | 17.1 | 290 | 16.6 | |
| Other | 198 | 7.5 | 148 | 8.5 | |
| Marital status | 0.863 | ||||
| Married | 1315 | 50.0 | 876 | 50.2 | |
| USDW | 1317 | 50.0 | 868 | 49.8 | |
| T stage | 0.872 | ||||
| T0-T1 | 319 | 12.1 | 223 | 12.8 | |
| T2 | 932 | 35.4 | 600 | 34.4 | |
| T3 | 506 | 19.2 | 338 | 19.4 | |
| T4 | 875 | 33.2 | 583 | 33.4 | |
| Clinical nodal status | 0.919 | ||||
| Negative | 549 | 20.9 | 366 | 21.0 | |
| Positive | 2083 | 79.1 | 1378 | 79.0 | |
| Histology | 0.779 | ||||
| IDC | 2306 | 87.6 | 1523 | 87.3 | |
| ILC | 326 | 12.4 | 221 | 12.7 | |
| Grade | 0.139 | ||||
| I | 205 | 7.8 | 163 | 9.3 | |
| II | 1254 | 47.6 | 796 | 45.6 | |
| III | 1159 | 44.0 | 780 | 44.7 | |
| IV | 14 | 0.5 | 5 | 0.3 | |
| ER | 0.407 | ||||
| Negative | 477 | 18.1 | 299 | 17.1 | |
| Positive | 2155 | 81.9 | 1445 | 82.9 | |
| PR | 0.709 | ||||
| Negative | 829 | 31.5 | 540 | 31.0 | |
| Positive | 1803 | 68.5 | 1204 | 69.0 | |
| HER2 | 0.593 | ||||
| Negative | 1981 | 75.3 | 1325 | 76.0 | |
| Positive | 651 | 24.7 | 419 | 24.0 | |
| Brain metastasis | 0.763 | ||||
| No | 2460 | 93.5 | 1626 | 93.2 | |
| Yes | 172 | 6.5 | 118 | 6.8 | |
| Liver metastasis | 0.553 | ||||
| No | 2044 | 77.7 | 1341 | 76.9 | |
| Yes | 588 | 22.3 | 403 | 23.1 | |
| Lung metastasis | 0.273 | ||||
| No | 1975 | 75.0 | 1334 | 76.5 | |
| Yes | 657 | 25.0 | 410 | 23.5 | |
| Breast surgery | 0.631 | ||||
| No | 1658 | 63.0 | 1123 | 64.4 | |
| Breast-conserving surgery | 296 | 11.2 | 186 | 10.7 | |
| Mastectomy | 678 | 25.8 | 435 | 24.9 | |
| Distant surgery | 0.699 | ||||
| No | 2550 | 96.9 | 1686 | 96.7 | |
| Yes | 82 | 3.1 | 58 | 3.3 | |
| Chemotherapy | 0.003 | ||||
| No | 970 | 36.9 | 720 | 41.3 | |
| Yes | 1662 | 63.1 | 1024 | 58.7 | |
| Radiation therapy | 0.963 | ||||
| No | 1508 | 57.3 | 998 | 57.2 | |
| Yes | 1124 | 42.7 | 746 | 42.8 | |
ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, IDC = infiltrating ductal carcinoma, ILC = infiltrating lobular carcinoma, IQR = interquartile range, Other = American Indian/AK Native, Asian/Pacific Islander, P∗ = difference between the training and validation cohort, PR = progesterone receptor, Radiation therapy = beam radiation for breast or anterior chest wall, Tumor grade I = well differentiated, Tumor grade II = moderately differentiated, Tumor grade III = poorly differentiated, Tumor grade IV = undifferentiated, USDW = unmarried/separated/divorced/widowed.
3.2. Outcome and independent prognostic factors for CSS in the training cohort
In the training cohort, the median follow-up time was 24 months (IQR, 13–41 months). The median CSS was 40 months (95%CI, 38–42 months). The 1-, 3-, and 5-year OS rates were 79%, 50%, and 30%, respectively, with the 1-, 3-, and 5-year CSS rates being 81%, 53%, and 34%, respectively.
The results of the univariate analysis were presented in Table 2. Multivariate analysis found that marital status, race, age, T stage, tumor grade, ER, PR, HER2, brain metastasis, liver metastasis, lung metastasis, breast surgery, and chemotherapy were independent prognostic factors for CSS of BMBC (Table 2).
Table 2.
Univariate and multivariate analysis cancer-specific survival (CSS) of bone metastasis breast cancer (BMBC) in the training cohort.
| Characteristic | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, years | ||||||
| +1 year | 1.011 | 1.007–1.016 | <0.001 | 1.010 | 1.006–1.015 | <0.001 |
| Race | <0.001 | 0.013 | ||||
| White | 1(Ref) | 1(Ref) | ||||
| Black | 1.397 | 1.216–1.604 | <0.001 | 1.212 | 1.049–1.399 | 0.009 |
| Other | 0.977 | 0.790–1.209 | 0.601 | 0.893 | 0.720–1.108 | 0.305 |
| Marital status | ||||||
| Married | 1(Ref) | 1(Ref) | ||||
| USDW | 1.299 | 1.166–1.448 | <0.001 | 1.173 | 1.048–1.313 | 0.006 |
| T stage | <0.001 | 0.005 | ||||
| T0-T1 | 1(Ref) | 1(Ref) | ||||
| T2 | 0.937 | 0.775–1.133 | 0.503 | 0.972 | 0.802–1.179 | 0.776 |
| T3 | 1.333 | 1.088–1.633 | 0.005 | 1.217 | 0.990–1.497 | 0.062 |
| T4 | 1.544 | 1.282–1.859 | <0.001 | 1.200 | 0.990–1.453 | 0.063 |
| Clinical nodal status | ||||||
| Negative | 1(Ref) | |||||
| Positive | 1.055 | 0.922–1.207 | 0.439 | |||
| Histology | ||||||
| IDC | 1(Ref) | |||||
| ILC | 1.019 | 0.867–1.199 | 0.817 | |||
| Tumor grade | <0.001 | <0.001 | ||||
| I | 1(Ref) | 1(Ref) | ||||
| II | 1.361 | 1.073–1.726 | 0.011 | 1.392 | 1.094–1.770 | 0.007 |
| III | 1.929 | 1.522–2.444 | <0.001 | 1.905 | 1.488–2.438 | <0.001 |
| IV | 4.050 | 2.248–7.293 | <0.001 | 2.741 | 1.506–4.989 | 0.001 |
| ER | ||||||
| Negative | 1(Ref) | 1(Ref) | ||||
| Positive | 0.523 | 0.460–0.595 | <0.001 | 0.709 | 0.592–0.849 | <0.001 |
| PR | ||||||
| Negative | 1(Ref) | 1(Ref) | ||||
| Positive | 0.552 | 0.494–0.617 | <0.001 | 0.640 | 0.550–0.744 | <0.001 |
| HER2 | ||||||
| Negative | 1(Ref) | 1(Ref) | ||||
| Positive | 0.778 | 0.681–0.888 | <0.001 | 0.502 | 0.432–0.583 | <0.001 |
| Brain metastasis | ||||||
| No | 1(Ref) | 1(Ref) | ||||
| Yes | 2.437 | 2.028–2.927 | <0.001 | 1.806 | 1.492–2.187 | <0.001 |
| Liver metastasis | ||||||
| No | 1(Ref) | 1(Ref) | ||||
| Yes | 2.105 | 1.868–2.373 | <0.001 | 1.814 | 1.591–2.068 | <0.001 |
| Lung metastasis | ||||||
| No | 1(Ref) | 1(Ref) | ||||
| Yes | 1.799 | 1.601–2.022 | <0.001 | 1.355 | 1.195–1.536 | <0.001 |
| Breast surgery | <0.001 | <0.001 | ||||
| No | 1(Ref) | 1(Ref) | ||||
| Breast-conserving surgery | 0.385 | 0.313–0.474 | 0.001 | 0.458 | 0.370–0.567 | <0.001 |
| Mastectomy | 0.565 | 0.496–0.644 | <0.001 | 0.613 | 0.534–0.704 | <0.001 |
| Distant surgery | ||||||
| No | 1(Ref) | |||||
| Yes | 0.941 | 0.699–1.267 | 0.688 | |||
| Chemotherapy | ||||||
| No | 1(Ref) | 1(Ref) | ||||
| Yes | 0.897 | 0.803–1.003 | 0.055 | 0.845 | 0.745–0.959 | 0.009 |
| Radiation therapy | ||||||
| No | 1(Ref) | |||||
| Yes | 0.922 | 0.826–1.028 | 0.143 | |||
CI = confidence interval, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, IDC = infiltrating ductal carcinoma, ILC = infiltrating lobular carcinoma, Other = American Indian/AK Native, Asian/Pacific Islander, PR = progesterone receptor, Radiation therapy = beam radiation for breast or anterior chest wall, Tumor grade I = well differentiated, Tumor grade II = moderately differentiated, Tumor grade III = poorly differentiated, Tumor grade IV = undifferentiated, USDW = unmarried/separated/divorced/widowed.
3.3. Prognostic nomogram for CSS
A nomogram was developed by integrating all significant independent predictors for BMBC in the training cohort (Figure 2). The corresponding line length of each variable in the nomogram indicates the contribution of predictors to survival outcomes. We summed the points of all variables to calculate total points. Furthermore, the probability of 1-, 3-, and 5-year CSS for a patient can be shown by drawing a straight line from the location of total points on the “Total points” scale.
Figure 2.
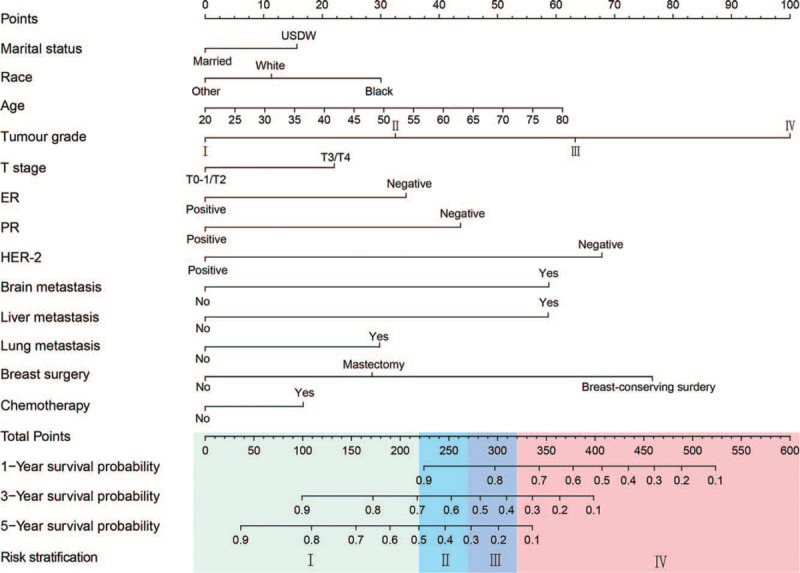
Nomogram for predicting cancer-specific survival for bone metastatic breast cancer.
The 1-, 3-, and 5-year predictive accuracy of our model (AUC values) in the internal validation were 0.775, 0.756, and 0.717, respectively, as indicated by ROC curves (Figure 3A-C). Additionally, the 1-, 3-, and 5-year CSS prediction curves of the nomogram were very close to the perfect curves (perfect curves mean that nomogram-predicted CSS is identical to the observed CSS). Therefore, the values of nomogram-predicted 1-, 3-, and 5-year of CSS were highly consistent with the actual values of 1-, 3-, and 5-year of CSS, further confirming the reliability of our model (Figure 4A-C).
Figure 3.
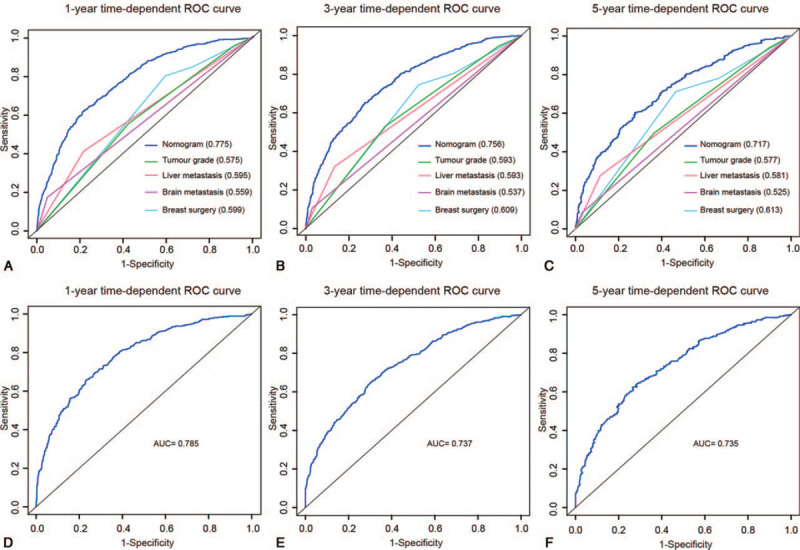
ROC curves for predicting (A) 1-year, (B) 3-year, and (C) 5-year CSS between the nomogram-predicted and single independent factors in the training cohort, and (D) 1-year, (E) 3-year, and (F) 5-year CSS in the validation cohort. The values in brackets of A-C represent the area under the ROC curves (AUC).
Figure 4.
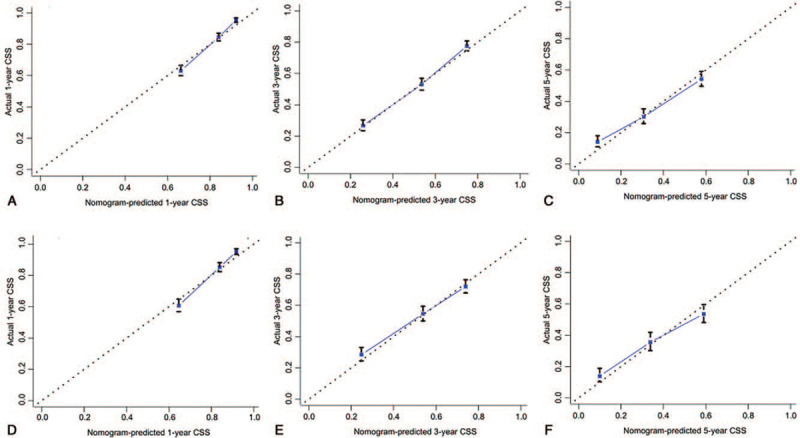
Calibration curves for predicting cancer-specific survival of BMBC. (A) 1-year, (B) 3-year, and (C) 5-year cancer-specific survival in the training cohort, and (D) 1-year, (E) 3-year, and (F) 5-year CSS in the validation cohort. Nomogram-predicted CSS is plotted on the x-axis and actual CSS is plotted on the y-axis.
3.4. Comparison of predictive performance between the nomogram and single independent factors
The hazard ratios of tumor grade, breast surgery, brain metastasis, and liver metastasis were higher than those of other predictors, as indicated in Table 2. The predictive performance for CSS of BMBC between the established model and tumor grade, breast surgery, brain metastasis, and liver metastasis was compared. As is presented in Figure 3A-C, the 1-, 3-, and 5-year predictive ability of single predictors was significantly lower than the model (P < 0.001).
3.5. External validation of predictive accuracy of the nomogram for CSS
The median follow-up time was 24 months (IQR, 13–40 months) in the validation cohort. The median CSS was 39 months (95%CI, 36–42 months). The 1-, 3-, and 5-year OS rates were 79%, 49%, and 32%, respectively, with the 1-, 3-, and 5-year CSS rates being 81%, 53%, and 34%, respectively.
The AUC values for predicting 1-, 3-, and 5-year CSS were 0.785, 0.737, and 0.735, respectively (Figure 3D-F). Moreover, calibration curves indicated good agreement between prediction and observation in the validation set (Figure 4D-F).
3.6. Risk stratification of the nomogram
All the patients were divided into four risk levels (Figure 2): I, low-risk group (total points, 0–220.00); II, intermediate-risk group (total points, 220.01–270.00); III, high-risk group (total points, 270.01–320.00); IV, very high-risk group (total points, 320.01–600.00). The median CSS time of the low-, intermediate-, high-, and very high-risk groups was 70, 46, 34, and 15 months in the training cohort (Figure 5A), respectively, >83, 44, 34, and 14 months in the validation cohort (Figure 5B), respectively. The Kaplan-Meier analysis showed that risk stratification based on nomogram could accurately differentiate BMBC with distinct CSS.
Figure 5.
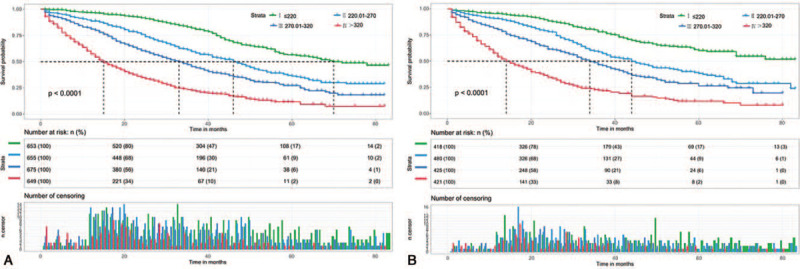
Kaplan-Meier curves of low-(I), intermediate-(II), high-(III), and very high-risk-(IV) groups in the (A) training cohort and (B) validation cohort.
4. Discussion
BMBC is the most common metastatic breast cancer, which extremely affects both qualities of life and survival of patients. The treatment decision-making for these patients was highly dependent on the expected survival.[17,18] Independent prognostic predictors for BMBC were identified for BMBC in our study. Based on the multivariate Cox analysis, we created a nomogram to predict exclusively the CSS of BMBC. External validation was performed in an independent cohort supports the excellent reliability of the model. In addition, risk stratification generated by our nomogram can accurately divide BMBC into different prognostic risk groups.
Multivariate analysis concluded that marital status, race, age, T stage, tumor grade, ER, PR, HER2, brain metastasis, liver metastasis, lung metastasis, and breast surgery were independent factors for BMBC. The study identified that married in marital status and HER2 positive were independent protective factors of BMBC for the first time. Marital status has been suggested to be an independent factor for predicting survival of cancer and breast cancer.[22,23] Our result substantiates such findings and shows that BMBC with unmarried/separated/divorced/widowed (USDW) have a higher risk of cancer-related death (hazard ratio = 1.299; 95% CI, 1.166 to 1.448). Aizer et al proposed that married patients with cancer had a better prognosis due to their greater willingness to receive definitive treatment.[22] In the past years, HER2-positive was inversely associated with the survival of breast cancer.[13,14] However, HER2-positive was found to be directly associated with the prognosis for BMBC in our study, which is consistent with the latest studies of metastatic breast cancer.[15,16] For this controversy, our team believe that the widespread and standardized use of HER2-targeted drugs in recent years has significantly improved the prognosis of HER2-positive breast cancer. Badwe et al proposed in a randomized controlled trial based on 350 patients that breast surgery cannot improve the prognosis of metastatic breast cancer at initial presentation who have responded to front-line chemotherapy.[12] Nevertheless, several retrospective studies showed that breast surgery could significantly prolong the survival of breast cancer with single metastasis.[24–27] Our study found that BMBC received with breast surgery did have longer survival than those who did not and breast-conserving surgery seem to benefit the patient most. This might result from the fact that patients who received breast surgery had a significantly lower proportion of brain and/or visceral metastasis and a higher proportion of single metastasis. Whether breast surgery can improve the prognosis of metastatic breast cancer need to be further explored in large sample clinical trials. Additionally, the other variables, such as Black in race,[28,29] older,[6,30,31] advanced T stage,[5,9,10] poorly differentiated (III) or undifferentiated (IV) in tumor grade,[6,9] hormone-negative,[6,15,16] brain metastasis, and visceral metastasis[6,7,9] are independent risk factors of CSS in BMBC, which are in agreement with those reported in breast cancer and/or metastatic breast cancer.
Taking the complexity of the multivariate prognostic factors predicting survival in BMBC into consideration, we developed a prognostic nomogram to identify the predicted survival for BMBC, stratify prognostic risk for BMBC, and validated the performance of our model in an external validation cohort. The AUC of 0.7 to 0.8 is comparable to that of widely accepted nomograms, supporting a sufficient level of accuracy.[9,10,19–21] Li et al and Xiong et al constructed a nomogram for general metastatic breast cancer patients using Sun Yat-sen University Cancer Center database and the National Cancer Database, respectively.[9,10] Metastasis-free interval and CD_Score was determined as an independent factor in Li et al study and Xiong et al study, respectively. Due to the discrepancy of including factors among the databases and models, our team could not compare the predictive performance of the two models. However, a great heterogeneity was observed in BMBC with distinct median overall survival times.[7,8] Therefore, it is critical to create a practical tool to predict the individual prognosis especially for BMBC. To the best of our knowledge, we built up the first prognostic model for BMBC and accurately stratifying the prognostic risk of BMBC. In principle, our nomogram can be used to predict survival in all patients with BMBC.
Although many advantages in the current study, including a large population, nomogram with excellent performance of prediction and risk stratification, and verification of nomogram in the external cohort, there are still several limitations: (1) some treatment data are unavailable in SEER database, so there are some unmeasured confounding factors in our study. For instance, chemotherapy is an important treatment for metastatic breast cancer, but we can only obtain chemotherapy status. (2) Detailed data on bone metastasis and bone pain are not documented in SEER database. The prognosis of BMBC may be related to the these factors. (3) Our model has not been verified in other centers or databases. In spite of these limitations, we have, in a way, clarified the function of some controversial predictors in the prognosis of BMBC. Furthermore, our nomogram predicts excellently for BMBC in both internal and external validation. Calibration curves showed that the nomogram-predicted 1-, 3-, and 5-year CSS were perfectly consistent with the observed 1-, 3-, and 5-year CSS.
5. Conclusion
Marital status, race, age, T stage, tumor grade, ER status, PR status, HER2 status, brain metastasis, liver metastasis, lung metastasis, chemotherapy, and breast surgery were identified as independent prognostic predictors for BMBC. The first prognostic nomogram created for BMBC can excellently predict individual survival and stratify prognostic risk for BMBC. This novel model can give patients a more reliable prediction of survival and aid clinicians in optimizing the individual treatment decision making in BMBC based on the risk stratification. Further verification in patients of different races remains urgently needed.
Author contributions
Conceptualization: Rui Ling.
Data curation: Niuniu Hou, Jun Yi.
Formal analysis: Niuniu Hou, Zhe Wang, Lu Yang.
Methodology: Lu Yang, Rui Ling.
Software: Niuniu Hou, Guangdong Hou.
Supervision: Zhe Wang.
Validation: Ying Wu.
Writing – original draft: Niuniu Hou.
Writing – review & editing: Meiling Huang, Rui Ling.
Glossary
Abbreviations: AUC = area under the ROC curve, BMBC = bone metastatic breast cancer, CI = confidence interval, CSS = cancer-specific survival, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, OS = overall survival, Other = American Indian/AK Native, Asian/Pacific Islander, PR = progesterone receptor, ROC curves = receiver operating characteristic curves, SEER = surveillance, epidemiology, and end results, Tumor grade I = well differentiated, Tumor grade II = moderately differentiated, Tumor grade III = poorly differentiated, Tumor grade IV = undifferentiated, USDW = unmarried/separated/divorced/widowed.
References
- [1].DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52–62. 10.3322/caac.21203 [DOI] [PubMed] [Google Scholar]
- [2].Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s–9s. 10.1158/1078-0432.CCR-06-0931 [DOI] [PubMed] [Google Scholar]
- [3].Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Breast Cancer Res Treat 2012;131:231–8. 10.1007/s10549-011-1721-x [DOI] [PubMed] [Google Scholar]
- [4].Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–7. 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- [5].Lee ES, Jung SY, Kim JY, et al. Identifying the potential long-term survivors among breast cancer patients with distant metastasis. Ann Oncol 2016;27:828–33. 10.1093/annonc/mdw036 [DOI] [PubMed] [Google Scholar]
- [6].Colleoni M, Bagnardi V, Rotmensz N, et al. A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol 2009;20:1178–84. 10.1093/annonc/mdn747 [DOI] [PubMed] [Google Scholar]
- [7].Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer implications for management. Eur J Cancer 2000;36:476–82. 10.1016/s0959-8049(99)00331-7 [DOI] [PubMed] [Google Scholar]
- [8].Domchek SM, Younger J, Finkelstein DM, et al. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 2000;89:363–8. 10.1002/1097-0142 10.1002/1097-0142(20000715)89:2<363::aid-cncr22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [9].Li S, Zhao J, Zhu L, et al. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med 2017;6:2586–94. 10.1002/cam4.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiong Z, Deng G, Huang X, et al. Score for the survival probability in metastasis breast cancer: a nomogram-based risk assessment model. Cancer Res Treat 2018;50:1260–9. 10.4143/crt.2017.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li X, Huang R, Ma L, et al. Locoregional surgical treatment improves the prognosis in primary metastatic breast cancer patients with a single distant metastasis except for brain metastasis. Breast 2019;45:104–12. 10.1016/j.breast.2019.03.006 [DOI] [PubMed] [Google Scholar]
- [12].Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380–8. 10.1016/S1470-2045 10.1016/S1470-2045(15)00135-7. Epub 2015 Sep 9. [DOI] [PubMed] [Google Scholar]
- [13].O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 2010;16:6100–10. 10.1158/1078-0432.CCR-10-1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502. 10.1001/jama.295.21.2492 [DOI] [PubMed] [Google Scholar]
- [15].Fouad TM, Kogawa T, Liu DD, et al. Overall survival differences between patients with inflammatory and noninflammatory breast cancer presenting with distant metastasis at diagnosis. Breast Cancer Res Treat 2015;152:407–16. 10.1007/s10549-015-3436-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jung SY, Rosenzweig M, Sereika SM, et al. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control 2012;23:103–12. 10.1007/s10552-011-9859-8 [DOI] [PubMed] [Google Scholar]
- [17].Hagerty RG, Butow PN, Ellis PA, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 2004;22:1721–30. 10.1200/JCO.2004.04.095 [DOI] [PubMed] [Google Scholar]
- [18].Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: “to help them live their lives the way they want to”. JAMA 2003;290:98–104. 10.1001/jama.290.15.2056 [DOI] [PubMed] [Google Scholar]
- [19].Ahn HK, Lee S, Park YH, et al. Prediction of outcomes for patients with brain parenchymal metastases from breast cancer (BC): a new BC-specific prognostic model and a nomogram. Neuro Oncol 2012;14:1105–13. 10.1093/neuonc/nos137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marko NF, Xu Z, Gao T, et al. Predicting survival in women with breast cancer and brain metastasis: a nomogram outperforms current survival prediction models. Cancer 2012;118:3749–57. 10.1002/cncr.26716 [DOI] [PubMed] [Google Scholar]
- [21].Huang Z, Sun B, Wu S, et al. A nomogram for predicting survival in patients with breast cancer brain metastasis. Oncol Lett 2018;15:7090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol 2013;31:3869–76. 10.1200/JCO.2013.49.6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ji P, Gong Y, Jiang CC, et al. Association between socioeconomic factors at diagnosis and survival in breast cancer: a population-based study. Cancer Med 2020;9:1922–36. 10.1002/cam4.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol 2007;14:3345–51. 10.1245/s10434-007-9527-0 [DOI] [PubMed] [Google Scholar]
- [25].Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol 2006;24:2743–9. 10.1200/JCO.2005.04.2226 [DOI] [PubMed] [Google Scholar]
- [26].Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg 2008;247:732–8. 10.1097/SLA.0b013e3181656d32 [DOI] [PubMed] [Google Scholar]
- [27].Pathy NB, Verkooijen HM, Taib NA, et al. Impact of breast surgery on survival in women presenting with metastatic breast cancer. Br J Surg 2011;98:1566–72. 10.1002/bjs.7650 [DOI] [PubMed] [Google Scholar]
- [28].Parkes A, Warneke CL, Clifton K, et al. Prognostic factors in patients with metastatic breast cancer with bone-only metastases. Oncologist 2018;23:1282–8. 10.1634/theoncologist.2018-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Polite BN, Cirrincione C, Fleming GF, et al. Racial differences in clinical outcomes from metastatic breast cancer: a pooled analysis of CALGB 9342 and 9840 – Cancer and Leukemia Group B. J Clin Oncol 2008;26:2659–65. 10.1200/JCO.2007.13.9782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Derks M, Bastiaannet E, van de Water W, et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Cancer 2018;99:1–8. 10.1016/j.ejca.2018.04.009 [DOI] [PubMed] [Google Scholar]
- [31].Freedman RA, Keating NL, Lin NU, et al. Breast cancer-specific survival by age: worse outcomes for the oldest patients. Cancer 2018;124:2184–91. 10.1002/cncr.31308 [DOI] [PMC free article] [PubMed] [Google Scholar]


