Abstract
The clinical characteristics of the rebound phenomenon after antiviral therapy in patients with Coronavirus disease-2019 (COVID-19) are largely unknown. There are few data comparing the rebound phenomenon after molnupiravir therapy to that after nirmatrelvir-ritonavir therapy. We investigated the incidence and risk factors associated with COVID-19 rebound after nirmatrelvir-ritonavir or molnupiravir therapy during the Omicron era. This prospective cohort study enrolled patients with mild-to-moderate COVID-19 who received nirmatrelvir-ritonavir or molnupiravir. We conducted weekly questionnaires of symptom scores from day 0 to day 28, with an additional day when patients experienced reappearing symptoms. We defined COVID-19 rebound as when patients experienced a 50% increase in symptom scores compared to the lowest symptom score between days 0 and 14. Among the 150 patients, 93 (62%) and 57 (38%) received nirmatrelvir-ritonavir therapy and molnupiravir, respectively. Of these, 11 patients (7.3%; 95% CI, 3.1–11.5) experienced COVID-19 rebound. The median duration from antiviral therapy to rebound was 12 days. Patients with clinical rebound had a higher symptom score at antiviral therapy initiation than those without (median, 5 vs 4; P = .02). There was no significant difference in the clinical rebounds associated with nirmatrelvir-ritonavir and molnupiravir therapy (5.4% vs 10.5%; P = .39). Approximately one-tenth of patients with mild-to-moderate COVID-19 who received antiviral therapy experienced rebound phenomena after treatment. Regardless of antiviral therapy type, high initial symptom scores were associated with a more frequent rebound phenomenon.
Keywords: COVID-19 rebound, molnupiravir, nirmatrelvir-ritonavir, Omicron variant
Key points:
During the Omicron era of COVID-19, approximately one-tenth of patients experienced symptom recurrence after treatment with nirmatrelvir-ritonavir or molnupiravir. Repeated assessment of symptoms, even after therapy, can assist in finding and diagnosing the phenomena of COVID-19 rebound.
1. Introduction
Nirmatrelvir-ritonavir and molnupiravir are oral antiviral agents that prevent severe disease progression in patients with mild-to-moderate cases of coronavirus disease 2019 (COVID-19).[1,2] Several reports of clinical symptoms associated with COVID-19 rebounding after antiviral treatment emerged during the Omicron era.[3,4] On May 24, 2022, the US Centers for Disease Control and Prevention issued a health advisory to inform patients of the rebound phenomenon and recurring COVID-19 symptoms after discontinuation of a 5-day course of oral antiviral agents.[5] However, data regarding the incidence of clinical rebound after oral antiviral therapy are limited. In addition, whether this phenomenon is limited to nirmatrelvir-ritonavir remains uncertain.[6] Thus, we investigated the incidence and clinical risk factors for the clinical rebound of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after a 5-day course of nirmatrelvir-ritonavir or molnupiravir in the subvariant-dominant era of Omicron BA.5.
2. Materials and Methods
2.1. Study design and patients
This prospective cohort study was conducted at a tertiary teaching hospital in Korea between August and November 2022. The predominant variant of concern was Omicron subvariant BA.5. Nirmatrelvir-ritonavir was approved for use on December 27, 2021, and molnupiravir was approved on March 23, 2022. Patients with applicable indications were treated with oral antiviral medications within 5 days of symptom onset according to the Infectious Diseases Society of America Guidelines.
This cohort comprised of patients with mild-to-moderate COVID-19 diagnosed with positive nasopharyngeal SARS-CoV-2-specific reverse transcription-polymerase chain reaction (RT-PCR) or rapid antigen test results. Patients with positive rapid antigen test results received an additional SARS-CoV-2 specific RT-PCR test for confirmation. Positive RT-PCR results were required for antiviral prescriptions supported by the National Health Insurance Service in the Republic of Korea. On enrollment day (D) of the first visit, clinical information on COVID-19 symptoms, the dates of the current and previous COVID-19 diagnoses, and the patient vaccination history against COVID-19 were collected. Information on comorbidities, medication histories, and SARS-CoV-2 RT-PCR results was also obtained. Patients were prescribed nirmatrelvir-ritonavir or molnupiravir based on their medical history and concomitant medications with potential drug-drug interactions.[7]
From D0 to D28, a single infectious disease specialist used weekly telephone surveys to obtain weekly symptom scores and information on lingering symptoms to maintain consistency. When patients experienced a previously alleviated symptom that reappeared after antiviral therapy, they were able to contact us freely to report changes in their symptoms. This day was designated as day of rebound (Dreb) if clinical rebound was suspected.
2.2. Patient consent and ethical review
Written informed consent was obtained from all participants to conduct regular telephone consultations to monitor their symptoms before enrollment in this study. No samples were collected from the participants. This study was approved by the Institutional Review Board of Asan Medical Center in Seoul, Korea (Number 2020-0297).
2.3. Symptom score assessment
Symptoms reported by patients were given points and added as a total of symptom scores for each week. We gathered symptom scores on D0, D7, D14, D28, and Dreb by using a questionnaire of 24 symptoms. The symptoms included: generalized symptoms such as fever, chills, myalgia, fatigue, and weight loss; cardiopulmonary symptoms such as chest pain, dyspnea, cough, and sputum; neurological symptoms such as headache and dizziness; gastrointestinal symptoms such as nausea, dyspepsia, vomiting, diarrhea, constipation, and abdominal pain; otorhinolaryngological symptoms such as rhinorrhea, nasal stiffness, hyposmia, hypogeusia, and sore throat; and other symptoms such as conjunctival injection and skin rash.
We used the modified symptom checklist that was used in the previous study.[8] Patients responded to the symptom survey with 24 symptoms, depending on the severity of the symptoms and interference of daily activity. Symptoms were scored using a scale from 0 to 2. Patients answered 0 if irrelevant, 1 if symptoms were mild with no required medical attention, and 2 if the symptoms were severe and interrupted daily activities requiring immediate treatment (Supplementary Material 1, http://links.lww.com/MD/J781).
2.4. Definition of COVID-19 rebound
Our previous study showed that symptom scores usually decrease as the disease progresses. After being diagnosed with symptomatic COVID-19, patients experienced a relative decrease of 25% on D7 and a 50% or more increase on D14 in their symptom scores.[9] Based on these results, we clinically defined COVID-19 rebound as a 50% increase in symptom score following the completion of 5-day antiviral therapy.
2.5. Statistical analysis and graphics
Fisher exact test or Chi-square test was conducted to compare proportions in the categorical variables, and Mann–Whitney U test or Student t test was performed to compare differences in the continuous variables between 2 groups, as appropriate. In all analyses, a 2-tailed statistical significance level of 0.05 was set a priori. The data were analyzed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). Graphical representations of the figures were designed using GraphPad Prism version 9.5 (GraphPad Software, San Diego, CA).
3. Results
3.1. Patient characteristics and symptom scores
Between August 1, 2022, and November 30, 2022, 161 patients diagnosed with COVID-19 visited our outpatient clinic. Seven patients did not respond to the telephone survey or visit the outpatient clinic on Day 14. Two patients experienced adverse effects such as nausea and fatigue after taking nirmatrelvir-ritonavir and 1 patient experienced rash after taking molnupiravir. These 3 patients arbitrarily discontinued oral antiviral therapy before completing the 5-day course of oral antiviral therapy. There was no COVID-19 rebound in them. Also, 1 patient, who was treated with molnupiravir, died on the twelfth day after enrollment. This patient death was due to an arrhythmia-related cardiac arrest and was unrelated to COVID-19 or the administration of molnupiravir. After excluding 11 patients, 150 patients were included in the final analysis. Of the 150 patients, 11 (7.3%, 95% confidence interval, 3.1–11.5) patients experienced COVID-19 rebound.
The median age of the enrolled patients was 62 years (interquartile range [IQR], 53–73 years), and 46% were male (Table 1). Of the 150 patients, 117 (78.0%) were administered 1 or more doses of booster monovalent Comirnaty (Pfizer Inc., Manhattan, NY) or Spikevax (Moderna Inc., Cambridge, Massachusetts). Out of the remaining 33 patients, 17 received 2 doses of the primary vaccine series, 1 received only a single dose of monovalent Comirnaty, Spikevax, or Vaxzevria, and 15 patients were not vaccinated. Patients were diagnosed with COVID-19 at a median of 264 days (IQR, 177–321) after their last vaccination. Six patients had been previously diagnosed with COVID-19 and all were diagnosed with a second infection during the study period. The median interval between the first and second COVID-19 cases was 206 days (IQR 205–237; not shown in Table 1). The most prevalent comorbidities were hypertension (45.3%), dyslipidemia (38.7%), obesity (27.3%), and diabetes mellitus (26.7%).
Table 1.
Baseline characteristics of patients with or without COVID-19 rebound.
| Characteristics | Total (n = 150) | COVID-19 rebound (n = 11) | No rebound (n = 139) | P value |
|---|---|---|---|---|
| Age, yr | 62.0 (53.0–73.0) | 60.0 (48.5–63.5) | 62.0 (54.0–75.0) | .09* |
| Sex | ||||
| Male | 69 (46.0) | 4 (36.4) | 65 (46.8) | .55† |
| Female | 81 (54.0) | 7 (63.6) | 74 (53.2) | |
| Vaccination status | ||||
| No vaccination | 15 (10.0) | 1 (9.1) | 14 (10.1) | .99† |
| 1 or 2 doses | 18 (12.0) | 2 (18.2) | 16 (11.5) | |
| ≥3 doses | 117 (78.0) | 8 (72.7) | 109 (78.4) | |
| D from symptom onset to antiviral therapy | 2.0 (1.0–2.0) | 2.0 (1.0–2.5) | 2.0 (1.0–2.0) | .67* |
| D from last vaccination until diagnosis | 264 (177–321) | 252 (176–315) | 266 (178–321) | .73* |
| Cycle threshold of PCR at diagnosis | 19.8 (16.6–24.8) | 18.9 (17.1–19.5) | 20.0 (16.6–24.8) | .40* |
| COVID-19 history | 6 (4.0) | 1 (9.1) | 5 (3.6) | .37† |
| Comorbidities | ||||
| Obesity (BMI > 25) | 41 (27.3) | 3 (27.3) | 38 (27.3) | .99† |
| Hypertension | 68 (45.3) | 4 (36.4) | 64 (46.0) | .76† |
| Diabetes mellitus | 40 (26.7) | 1 (9.1) | 39 (28.1) | .29† |
| Dyslipidemia | 58 (38.7) | 3 (27.3) | 55 (39.6) | .53† |
| Cardiovascular disease | 23 (15.3) | 1 (9.1) | 22 (15.8) | .99† |
| Chronic kidney disease | 18 (12.0) | 2 (18.2) | 16 (11.5) | .62† |
| Chronic pulmonary disease | 12 (8.0) | 2 (18.2) | 10 (7.2) | .22† |
| Liver cirrhosis | 9 (6.0) | 2 (18.2) | 7 (5.0) | .13† |
| Stroke | 7 (4.7) | 0 (0) | 7 (5.0) | .99† |
| Rheumatic disease | 9 (6.0) | 1 (9.1) | 8 (5.8) | .51† |
| Malignant tumor | 34 (22.7) | 3 (27.3) | 31 (22.3) | .71† |
| Hematologic malignancy | 12 (8.0) | 1 (9.1) | 11 (7.9) | .99† |
| Organ transplant recipient | 19 (12.7) | 3 (27.3) | 16 (11.5) | .15† |
| Antiviral therapy | ||||
| Nirmatrelvir-ritonavir | 93 (62.0) | 5 (45.5) | 88 (63.3) | .39† |
| Molnupiravir | 57 (38.0) | 6 (54.5) | 51 (36.7) | |
| Symptom score | ||||
| D 0 | 4.0 (2.0–5.0) | 5.0 (4.5–7.0) | 4.0 (2.0–5.0) | .02* |
| D 7 | 1.0 (0.0–2.0) | 2.0 (1.5–3.0) | 1.0 (0.0–2.0) | .01* |
| D 14 | 1.0 (0.0–2.0) | 4.0 (2.0–4.5) | 1.0 (0.0–2.0) | <.01* |
| D 28 | 0.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.0 (0.0–1.0) | .01* |
| Dreb | 1.0 (0.0–2.0) | 4.0 (3.0–5.5) | 1.0 (0.0–2.0) | <.01* |
Data are presented as median (interquartile range) or number (%).
BMI = body mass index, COVID-19 = coronavirus disease 2019, Dreb = day of rebound, PCR = polymerase chain reaction.
P value from Student t test or Mann–Whitney U test.
P value from Chi-square (χ2) test or Fisher exact test.
The median age of the patients with or without rebound was comparable (60 vs 62 years; P = .09). Patients who were diagnosed with COVID-19 were directly connected to our outpatient clinic. Therefore, the median time from diagnosis of COVID-19 until initiation of antiviral therapy was 2 days in both groups without a significant difference. Sex, vaccination status, days between the last vaccination and diagnosis, cycle threshold of PCR at diagnosis, number of patients with previous COVID-19 infection, and comorbidities were not significantly different between the 2 groups. The median symptom scores for patients with COVID-19 rebound at the start of antiviral therapy were higher than those without rebound (5 vs 4; P = .02). The median symptom scores for rebound patients were significantly higher than those for non-rebounders on D7 (2 vs 1; P = .01), D14 (4 vs 1; P < .01), and D28 (1 vs 0; P = .01).
When comparing the incidence of COVID-19 rebound among patients taking either nirmatrelvir-ritonavir or molnupiravir, the rates were 5.4% (5/93) and 10.5% (6/57) respectively, with a statistically insignificant difference (P = .39). Patients taking molnupiravir were more likely to have diabetes, chronic kidney disease, and solid organ transplants than those taking nirmatrelvir-ritonavir (Table 2). Nirmatrelvir-ritonavir interacts with various medications used for these diseases, so patients receiving those medications due to underlying diseases should take molnupiravir instead of nirmatrelvir-ritonavir. Differences in the presence of comorbidities between the 2 groups might lead to a higher incidence of COVID-19 rebound in the molnupiravir group, although there was no significant difference in the incidence rates between them.
Table 2.
Comparison of patients taking nirmatrelvir-ritonavir and molnupiravir.
| Characteristics | Nirmatrelvir-ritonavir (n = 93) | Molnupiravir (n = 57) | P value |
|---|---|---|---|
| Age, yr | 61.0 (53.0–72.0) | 66.0 (56.0–77.0) | .13* |
| Sex | |||
| Male | 42 (45.2) | 27 (47.4) | .87† |
| Female | 51 (54.8) | 30 (52.6) | |
| Vaccination status | |||
| No vaccination | 7 (7.5) | 8 (14.0) | .41† |
| 1 or 2 doses | 8 (8.6) | 10 (17.5) | |
| ≥3 doses | 78 (83.9) | 39 (68.4) | |
| D from symptom onset to antiviral therapy | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | .37* |
| D from last vaccination until diagnosis | 260 (176–321) | 268 (179–333) | .43* |
| Cycle threshold of PCR at diagnosis | 20.3 (17.0–25.0) | 18.5 (16.3–22.4) | .25* |
| COVID-19 history | 4 (4.3) | 2 (3.5) | .99† |
| Comorbidities | |||
| Obesity (BMI > 25) | 28 (30.1) | 13 (22.8) | .35† |
| Hypertension | 42 (45.2) | 26 (45.6) | .99† |
| Diabetes mellitus | 19 (20.4) | 21 (36.8) | .04† |
| Dyslipidemia | 41 (44.1) | 17 (29.8) | .09† |
| Cardiovascular disease | 12 (12.9) | 11 (19.3) | .35† |
| Chronic kidney disease | 4 (4.3) | 14 (24.6) | <.01† |
| Chronic pulmonary disease | 4 (4.3) | 8 (14.0) | .06† |
| Liver cirrhosis | 4 (4.3) | 5 (8.8) | .30† |
| Stroke | 2 (2.2) | 5 (5.0) | .11† |
| Rheumatic disease | 5 (5.4) | 4 (7.0) | .73† |
| Malignant tumor | 20 (21.5) | 14 (24.6) | .69† |
| Hematologic malignancy | 6 (6.5) | 6 (10.5) | .37† |
| Organ transplant recipient | 0 (0.0) | 19 (33.3) | <.01† |
| Symptom score | |||
| D 0 | 4.0 (3.0–5.0) | 4.0 (2.0–6.0) | .82* |
| D 7 | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | .51* |
| D 14 | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | .10* |
| D 28 | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | .64* |
Data are presented as median (interquartile range) or number (%).
BMI = body mass index, COVID-19 = coronavirus disease 2019, PCR = polymerase chain reaction.
P value from Student t test or Mann–Whitney U test.
P value from Chi-square (χ2) test or Fisher exact test.
3.2. Symptom score changes with COVID-19 rebound
Initially, patients complained of symptoms of fever (60%; 90/150), cough (49.3%; 74/150), sore throat (49.3%; 74/150), and myalgia (48.7%; 73/150). Common symptoms experienced by COVID-19 rebound patients in Dreb were sputum (63.6%, 7/11), cough (45.4%, 5/11), and sore throat (27.2%, 3/11).
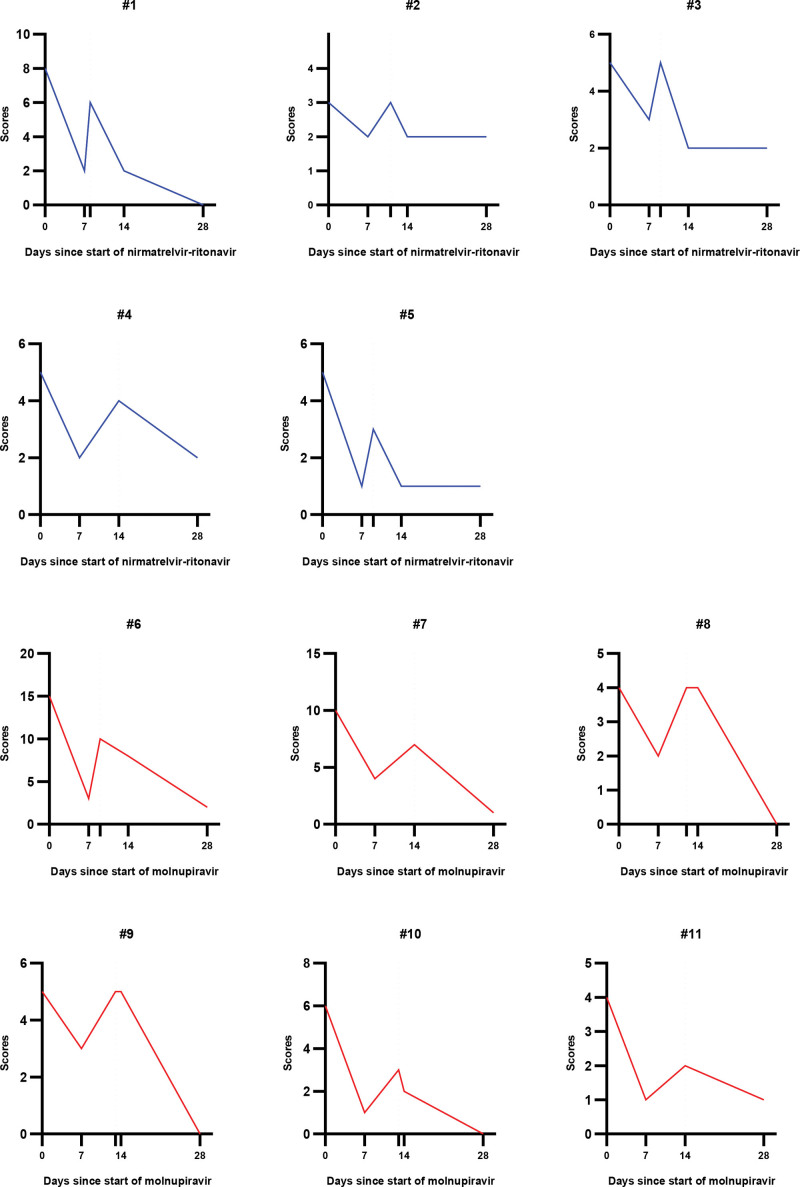
The symptom scores of each patient with COVID-19 rebound are shown in Figure 1. Of the 11 patients with rebound, 5 were treated with nirmatrelvir-ritonavir, and 6 were treated with molnupiravir. In general, symptom scores appeared to be higher in patients treated with molnupiravir. Due to drug interactions between immunosuppressants and nirmatrelvir-ritonavir, patients with a history of organ transplantation were treated with molnupiravir. However, there were no significant differences in comorbidities and symptom scores between the 2 antiviral groups with COVID-19 rebound (Supplementary Material 2, http://links.lww.com/MD/J784).
Figure 1.
Changes in symptom scores in each coronavirus disease 2019 (COVID-19) rebound patient. Changes in symptom scores in patients with COVID-19 rebound over time after antiviral therapy initiation. Each number represents a patient with COVID-19 rebound. The blue graphs show symptom scores for patients treated with nirmatrelvir-ritonavir. The red graphs show the symptom scores of patients treated with molnupiravir. The y-axis denotes the symptom scores, and the x-axis denotes the number of d since the start of the antiviral therapy. Additional tick between d 7 and 14 without a number on the x-axis indicates the d of clinical rebound for each patient. For patients #4, #7, and #11, the d of clinical rebound is d 14.
The median duration from the start of therapy to rebound was 12 days (IQR, 9.0–13.5). After antiviral therapy, the duration was 5 days (IQR, 4–7 days). Except for mild cough or sputum, all the patients with COVID-19 rebound spontaneously recovered by D28 without additional medical treatment. Scores were evaluated until D28, so the incidence of post-COVID conditions was not investigated in our study.
4. Discussion
We demonstrated that approximately 7% of patients with mild-to-moderate COVID-19 who received nirmatrelvir-ritonavir or molnupiravir experienced a rebound phenomenon 1 to 2 weeks after antiviral therapy. In addition, these rebound phenomena occurred regardless of antiviral therapy type. We also found that a higher initial symptom score at the start of antiviral therapy was associated with a greater rebound phenomenon. These data provide important information regarding the differential diagnosis of patients with COVID-19-related symptoms following symptom improvement and antiviral therapy.
Recent studies have analyzed the incidence of rebound phenomenon in patients with COVID-19 using definitions based on viral shedding kinetics.[10] A cohort study from Hong Kong involving hospitalized patients used a cycle threshold of <36 without symptom correlation to define viral rebound during the Omicron BA.2 wave.[10] A study using placebo arms of monoclonal antibodies defined viral load rebound as an increase of at least 0.5 log in viral RNA copies.[11] Another study based on the Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients trial included only unvaccinated, non-hospitalized participants during the delta wave and utilized a 0.5 log increase in viral load at day 10 or 14 as viral load rebound.[1] These studies reported incidences of COVID-19 rebound after antiviral therapy of 4.6% and 7%, respectively, which is similar to our study results (H. Soares, unpublished data, June 2022).[10] However, previous studies have determined the time of the rebound through increasing levels of viral load on a fixed date and not on the symptomatic changes of the individual patient throughout the course of infection. In contrast, we monitored symptom changes throughout the disease course and used objective symptom score changes to define COVID-19 rebound. In this context, our data reflects the incidence in a real-world clinical setting. We demonstrated a more practical way to identify the clinical rebound of non-hospitalized patients with mild-to-moderate COVID-19 when SARS-CoV-2 PCR tests cannot be performed regularly.
Notably, the rebound phenomenon occurred more frequently in patients who received molnupiravir than in those who received nirmatrelvir-ritonavir, although there was no significant difference in the incidence between the 2 groups. In a previous study, patients treated with molnupiravir had a rebound rate of 8.6% and those treated with nirmatrelvir-ritonavir had a rate of 5.4% on day 30 (L. Wang, unpublished data, June 2022). Although they assessed rebound with 3 outcomes (COVID-19-related symptoms, hospitalizations, and re-infections) at a fixed date, their definition of the rebound phenomenon itself was obscured in the literature (L. Wang, unpublished data, June 2022). In another study, the rebound rate in the patients receiving molnupiravir or nirmatrelvir-ritonavir was 4.6% but included COVID-19-related deaths.[10] Our study showed comparable outpatient rebound rates of 10.5% for molnupiravir and 5.4% for nirmatrelvir-ritonavir. However, we did not set the day of rebound, unlike previous studies,[10,11] because the dynamics of COVID-19 rebound during the Omicron wave are poorly understood.[6] Interestingly, our findings indicated that no rebound occurred before day 7 or after day 14. On the 28th day, all patients experiencing a rebound had fewer symptoms and spontaneously recovered without the need for additional treatment. This may suggest that further antiviral treatments are unnecessary, and careful observation alone may be sufficient to manage the phenomenon of COVID-19 rebound.
This study has several limitations. First, we could not collect respiratory samples to evaluate the SARS-CoV-2 viral load, as most patients did not go through follow-up SARS-CoV-2 PCR tests over the course. The second limitation is the absence of internal and external validations of symptom scoring. However, we adopted the symptom scoring system used in similar settings in prospective cohort studies to overcome this limitation.[9,12] This allowed us to define COVID-19 rebound based on the changes in symptom scores. Third, we were unable to compare the “true” incidence of COVID-19 rebound between the nirmatrelvir-ritonavir and molnupiravir groups in the same conditions, because patients with comorbidities who were taking certain medications that interact with nirmatrelvir-ritonavir were administered molnupiravir instead. Consequently, patients in the molnupiravir group were more likely to have comorbidities, which might have contributed to a higher incidence of COVID-19 rebound in this group.
In conclusion, the incidence of COVID-19 rebound was 7.3% in outpatients taking oral antiviral agents during the Omicron-dominant period. Rebound phenomenon occurred in patients treated with nirmatrelvir-ritonavir or molnupiravir. Patients with high initial symptom scores were associated with a more frequent rebound phenomenon. Early and repeated assessments of symptoms can assist in finding and diagnosing the phenomena of COVID-19 rebound after therapy.
Author contributions
Conceptualization: Euijin Chang.
Data curation: Jaijun Han.
Formal analysis: Jaijun Han.
Funding acquisition: Sung-Han Kim.
Investigation: Jaijun Han.
Methodology: Sung-Han Kim.
Project administration: Euijin Chang.
Supervision: Euijin Chang, Sung-Han Kim.
Writing – original draft: Jaijun Han.
Writing – review & editing: Seongman Bae, Jiwon Jung, Min Jae Kim, Yong Pil Chong, Sang-Oh Lee, Sang-Ho Choi, Yang Soo Kim, Sung-Han Kim.
Supplementary Material
Abbreviations:
- COVID-19
- coronavirus disease 2019
- D
- day
- Dreb =
- day of rebound
- IQR
- interquartile range
- RT-PCR
- reverse transcription-polymerase chain reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
Supplemental Digital Content is available for this article.
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), which is funded by the National Institute of Infectious Diseases, National Institute of Health, Republic of Korea (grant number. HD22C2045).
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Han J, Bae S, Jung J, Kim MJ, Chong YP, Lee S-O, Choi S-H, Kim YS, Chang E, Kim S-H. Clinical characteristics of COVID-19 rebound after nirmatrelvir-ritonavir or molnupiravir therapy: A prospective cohort study. Medicine 2023;102:39(e35094).
Contributor Information
Jaijun Han, Email: jaijun.han@gmail.com.
Seongman Bae, Email: songman.b@gmail.com.
Jiwon Jung, Email: trueblue27@naver.com.
Min Jae Kim, Email: kimsunghanmd@hotmail.com.
Yong Pil Chong, Email: drchong@amc.seoul.kr.
Sang-Oh Lee, Email: soleemd@amc.seoul.kr.
Sang-Ho Choi, Email: sangho@amc.seoul.kr.
Yang Soo Kim, Email: kimsunghanmd@hotmail.com.
Sung-Han Kim, Email: kimsunghanmd@hotmail.com.
References
- [1].Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alshanqeeti S, Bhargava A. COVID-19 rebound after paxlovid treatment: a case series and review of literature. Cureus. 2022;14:e26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang Y, Chen X, Xiao W, et al. Rapid COVID-19 rebound in a severe COVID-19 patient during 20-day course of paxlovid. J Infect. 2022;85:e134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention. Health alert network. Office of readiness and response; 2022. Available at: https://emergency.cdc.gov/han/2022/han00467.asp [access date December 1, 2022].
- [6].Charness ME, Gupta K, Stack G, et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med. 2022;387:1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19; 2020. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [access date January 2, 2023]. [DOI] [PMC free article] [PubMed]
- [8].Kang SW, Park H, Kim JY, et al. Comparison of the clinical and virological characteristics of SARS-CoV-2 Omicron BA.1/BA.2 and omicron BA.5 variants: a prospective cohort study. J Infect. 2023;86:e148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bae S, Kim JY, Lim SY, et al. Dynamics of viral shedding and symptoms in patients with asymptomatic or mild COVID-19. Viruses. 2021;13:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22:1681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deo R, Choudhary MC, Moser C, et al. Symptom and viral rebound in untreated SARS-CoV-2 infection. Ann Intern Med. 2023;176:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park S, Lim SY, Kim JY, et al. Clinical and virological characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.617.2 (Delta) variant: a prospective cohort study. Clin Infect Dis. 2022;75:e27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.