Abstract
Some studies have demonstrated an increased risk of prostate cancer in patients with obstructive sleep apnea (OSA). However, the relationship is unclear and the results are conflicting. This study aims to investigate associations between OSA and prostate cancer using the Korea National Health Insurance Service database.
A total of 152,801 men (≥ 20 years of age) newly diagnosed with OSA between 2007 and 2014 were included. A control group of 764,005 subjects was selected using propensity score matching by age and sex. The mean follow-up time was 4.6 years (range 2.3–6.9). The primary endpoint was newly diagnosed prostate cancer. The prostate cancer hazard ratio (95% confidence interval) was calculated for patients with OSA and compared to the control group.
The incidence of prostate cancer among patients with OSA was significantly higher than that in controls (1.34 [1.23–1.49]). In particular, the incidence of prostate cancer was highest in patients aged 40–65 years (1.51 [1.32–1.72]).
This study provides additional evidence for a link between OSA and prostate cancer.
Keywords: obstructive sleep apnea, prostate cancer
1. Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse during sleep, which induces frequent episodes of nocturnal breathing cessation and oxygen desaturation.[1] It is a common disorder worldwide, affecting over 4% of men and 2% of women, and its prevalence is linked to the obesity epidemic.[2] Untreated OSA can lead to a variety of complications including hypertension, heart attack, stroke, diabetes, depression, insomnia, dementia, and even early death. More recently, evidence supports a potential link between OSA and various cancers.[3]
Prostate cancer is the most common cancer in men.[4] Globally, one in nine men will be diagnosed with prostate cancer. Although prostate cancer has a low mortality rate, the total number of deaths is large because of a high prevalence of this cancer. In the United States, prostate cancer is the 2nd leading cause of cancer death in men, with 33,330 deaths estimated in 2020.[4] In East Asian countries, the incidence of prostate cancer has increased rapidly.[5] In Korea, for example, there were 8.4 cases of prostate cancer per 100,000 population in 1999, and this increased to 25.6 cases in 2014.[5] This represents a nearly three-fold increase in prostate cancer in 15 years.[5] This finding is likely due to a number of factors, including cultural changes incorporating Western dietary patterns and lifestyles, greater availability of testing, and an increase in the elderly population.[5]
Several studies have linked OSA to prostate cancer, while others have found no correlation.[6–8] Fang et al. showed that the risk of prostate cancer in patients with sleep disorders was significantly higher than control patients based on data from two million subjects from the National Health Insurance database in Taiwan.[6] Chung also showed that male patients with OSA had a higher risk of prostate cancer in a 6,200-subject dataset from the Taiwan Longitudinal Health Insurance Database.[7] In contrast, Gozal showed that the adjusted risk of prostate cancer appeared to be lower in patients with OSA based on a cohort of 5.6 million subjects.[8] To confirm the causality between OSA and prostate cancer, additional analysis of long-term, large-scale cohorts are needed. Recently, the Korea National Health Insurance Service (KNHIS) database has become available for research purposes in Korea.
The purpose of this study was to investigate whether the incidence of prostate cancer as increased among patients with OSA by using KNHIS data.
2. Methods
2.1. Data source
The KNHIS is a public medical insurance system which covers about 97% of the Korean population.[9] The KNHIS is managed by the Korean government that insures nearly the entire population of South Korea, providing long term data on more than 50 million people. The KNHIS provides robust data on diagnoses, interventions, prescriptions, and patient demographics. The database utilizes the Korean Classification of Diseases, 6th edition, which is a modified version of the International Classification of Diseases, 10th edition (ICD-10). Researchers can use KNHIS data after approval by the local Institutional Review Board.
2.2. Study population and design
We defined the OSA group as that including men aged ≥ 20 years with newly diagnosed OSA (G47.30) between 2007 and 2014. To select the control group, we used propensity score matching by age in subjects who were not diagnosed with OSA; the number of subjects was five times that of the subjects in the OSA group. The primary endpoint of this study was the incidence of newly diagnosed prostate cancer. Patients diagnosed with any kind of cancer prior to enrollment were excluded. A flowchart shows the enrollment process for this study (Fig. 1).
Figure 1.
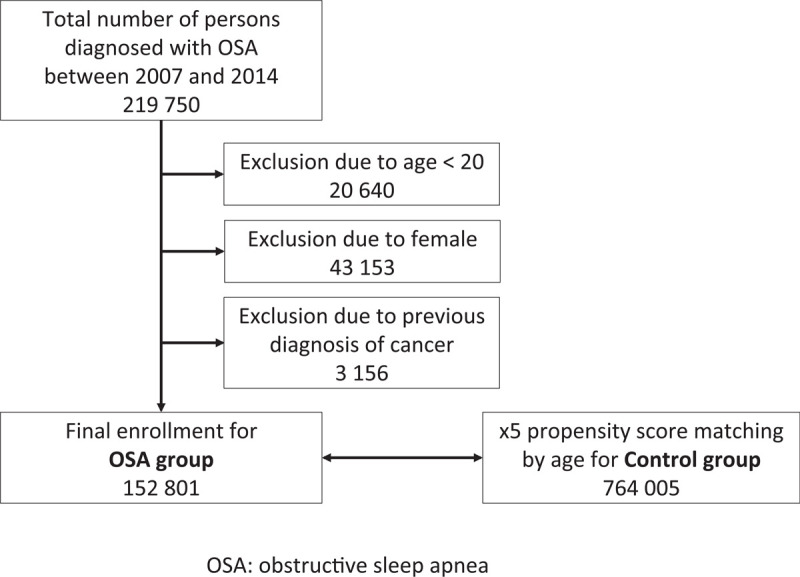
Enrollment flowchart.
2.3. Data collection
We collected the following baseline data from the KNHIS database: age (years) and income level (classified by quintile based on health insurance premium payment). Data on comorbidities, including diabetes, hypertension, dyslipidemia, stroke, chronic obstructive pulmonary disease, and ischemic heart disease, were also collected using insurance claim data (Table 1).
Table 1.
Working definitions derived from insurance claims data.
| Disease | Working definition |
| Obstructive sleep apnea | at least one claim under ICD-10 code G47.3 |
| Prostate cancer | at least one claim under ICD-10 code C61 and registered as a cancer patient in the National Medical Expenses Support Program |
| Diabetes | at least one claim per year for the prescription of anti-diabetic medication under ICD-10 code E11-14 |
| Hypertension | at least one claim per year for the prescription of anti-hypertensive medication under ICD-10 code I10–13 or I15 |
| Dyslipidemia | at least one claim per year for the prescription of anti-dyslipidemic medication under ICD-10 code E78 |
| Stroke | at least one claim under ICD-10 code I63 or I64 |
| COPD | at least one claim under ICD-10 code J41, J42, J43, or J44 |
| IHD | at least one claim under ICD-10 code I20, I21, I22, I23, I24, or I25 |
COPD = chronic obstructive pulmonary disease, ICD = international classification of diseases, IHD = ischemic heart disease.
2.4. Statistical analysis
Data are presented as the mean ± standard deviation (SD) for age and as proportions for the remaining categorical variables. Comparisons between 2 groups were made using Student's t test or the chi-squared test. A Kaplan-Meier plot without covariance correction is presented to analyze the risk of prostate cancer according to the presence or absence of OSA. The incidence of prostate cancer was calculated by dividing the number of events by the person-time product at risk. To determine the hazard ratio of OSA on the relative incidence of prostate cancer, the Cox proportional hazards model was used after stratifying for covariates, including income level and diabetes, hypertension, and dyslipidemia statuses. Two different models were applied. Model 1 was not adjusted by any covariate. Model 2 was adjusted for income level and diabetes, hypertension, and dyslipidemia statuses. In addition, differences in the hazard ratio were also analyzed according to age. The results are presented as the mean and 95% confidence interval (95% CI). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria).
2.5. Ethical approval
The study was exempted from the requirement for informed consent by the Institutional Review Board of Konkuk University Hospital because of the use of publicly available data (KUMC 2020-03-040).
3. Results
A total of 49,570,064 subjects enrolled in the KNHIS in 2007, the first year for which research data are available, and the numbers are comparable for each subsequent year up to 2014. Between 2007 and 2014, 152,801 male patients were newly diagnosed with OSA. A total of 764,005 subjects were selected as controls (Fig. 1). An enrollment flowchart and demographic data are summarized in Figure 1 and Table 2, respectively.
Table 2.
Demographics of patients with OSA and controls.
| OSA | Controls | ||||
| N | % | N | % | P value | |
| Total number | 152 801 | 100.0 | 764 005 | 100.0 | |
| Follow-up duration (yr) | 4.5 ± 2.3 | 4.5 ± 2.3 | 1.000 | ||
| Mean age (years) | 43.8 ± 12.7 | 43.8 ± 12.7 | 1.000 | ||
| Age ≥ 65 years | 9 290 | 6.1 | 46 450 | 6.1 | 1.000 |
| Income in the lowest quintile | 23 647 | 15.5 | 164 421 | 21.5 | < .001 |
| Diabetes | 10 823 | 7.1 | 44 843 | 5.9 | < .001 |
| Hypertension | 36 152 | 23.7 | 104 288 | 13.7 | < .001 |
| Dyslipidemia | 24 354 | 16.0 | 58 834 | 7.7 | < .001 |
| Stroke | 6 469 | 4.2 | 15 330 | 2.1 | < .001 |
| COPD | 22 222 | 14.5 | 66 218 | 8.7 | < .001 |
| IHD | 2 667 | 1.8 | 6 674 | 0.9 | < .001 |
COPD = chronic obstructive pulmonary disease, IHD = ischemic heart disease, OSA = obstructive sleep apnea.
3.1. The incidence of prostate cancer for the OSA group and controls
The Kaplan-Meier plot shows the incidence of prostate cancer without adjustment. Prostate cancer occurred more frequently in the OSA group than in the control group (Fig. 2).
Figure 2.
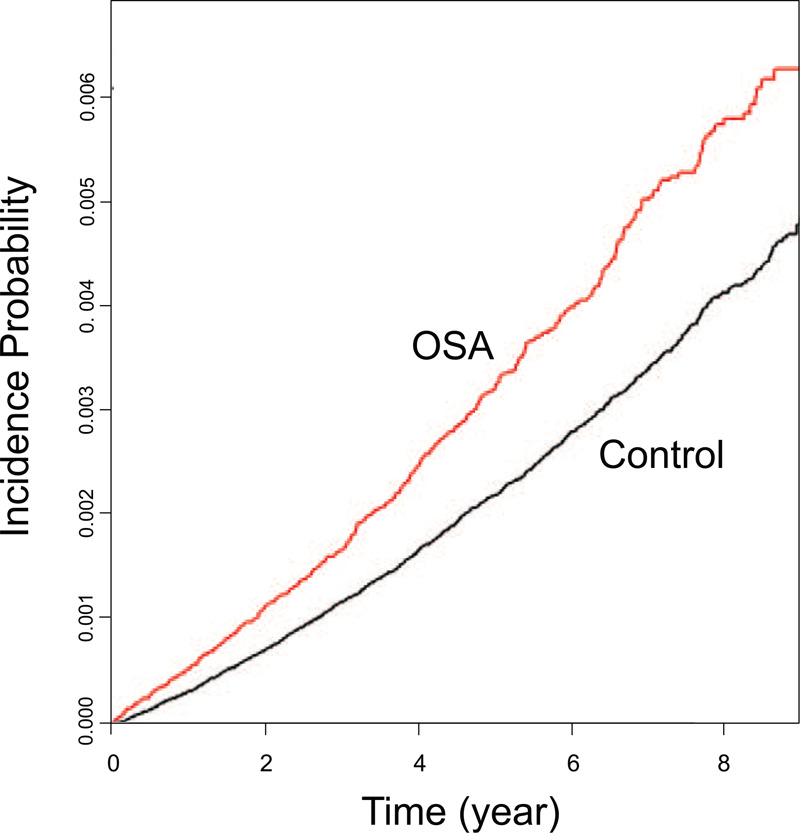
Kaplan-Meier plot of the incidence of prostate cancer among patients with OSA. Prostate cancer occurs more frequently in the OSA group than in the control group. OSA = obstructive sleep apnea.
3.2. The hazard ratio of the OSA group compared with that of the control group regarding prostate cancer incidence
The Cox proportional hazards model showed that the hazard ratio for prostate cancer incidence in the OSA group was significant for both models. The hazard ratio based on Model 1, which was not adjusted, was 1.43 (95% CI 1.29–1.58), while it slightly decreased to 1.34 (95% CI 1.21–1.49) after adjusting for income level and various comorbidities (diabetes, hypertension, and dyslipidemia) in Model 2. Additionally, we have classified the result of Model 2 by 3 age groups. The hazard ratio was highest at 1.51 (95% CI 1.32–1.72) in the age group of 40–65 years, 1.34 (95% CI 0.48–3.24) for those under 40 years of age, and 1.21 (95% CI 1.03–1.42) for those over 65 years of age. These results are summarized in Tables 3 and 4.
Table 3.
OSA hazard ratio for the incidence of prostate cancer.
| N | Event | Rate | Model 1 | Model 2 | |
| Controls | 764 005 | 1 719 | 0.470 | 1 | 1 |
| OSA | 152 801 | 491 | 0.672 | 1.43 (1.29–1.58) | 1.34 (1.21–1.49) |
Model 1: not adjusted; Model 2: adjusted by income level and diabetes, hypertension, and dyslipidemia statuses.
OSA = obstructive sleep apnea.
Hazard ratio was represented with 95% confidence interval (95% CI).
Table 4.
OSA hazard ratio for the incidence of prostate cancer by age group.
| Age (yr) | 20 ≤ age < 40 | 40 ≤ age < 65 | 65 ≤ age |
| Controls | 1 | 1 | 1 |
| OSA | 1.34 (0.48–3.24) | 1.51 (1.32–1.72) | 1.21 (1.03–1.42) |
The result is adjusted by income level and diabetes, hypertension, and dyslipidemia statuses.
OSA = obstructive sleep apnea.
Hazard ratio was represented with 95% confidence interval (95% CI).
4. Discussion
The occurrence of tumor in the prostate has been linked to multiple factors including age, race, diet, genetics, and environment.[10] One of the greatest risk factors for prostate cancer is age. Prostate cancer rarely affects young men; the majority of cases are diagnosed in men over 65 years of age. Moreover, men of African descent are 73% more likely to develop prostate cancer compared with white men, and 2.4 times more likely to die from prostate cancer.[10] Other risk factors for prostate cancer include social and environmental factors—particularly a high-fat, high-carbohydrate diet, genetics, alcohol consumption, and certain occupational exposures.[11] Men who are overweight or obese are at greater risk of ultimately developing an aggressive form of prostate cancer. Recently, OSA has attracted attention as a new risk factor for various cancers. However, there is no clear consensus regarding an association between OSA and prostate cancer.[6–8]
In this study, we demonstrated that prostate cancer occurred more frequently in the OSA group compared with the control group, even after adjustments for income level and diabetes, hypertension, and dyslipidemia statuses. Overall, there was a 1.34-fold increase over the control group, and a 1.51-fold increase in the 40–65-year age group. The greatest strength of this study is the overwhelming number of subjects. Of the 50 million people represented by the dataset, 150,000 men who were diagnosed with OSA during the study period and 760,000 control subjects were analyzed.
In three large population-based studies that looked to identify a link between OSA and cancer, the numbers of patients with OSA were less than 2,200 in Fang's study, 1,200 in Chung's study, and 15,000 in Gozal's study.[6–8] The current study has much greater statistical power compared with the prior studies and accounts for additional important comorbidities. Similar to Chung and Fang's results, our study demonstrated that the incidence of prostate cancer was increased in patients with OSA.[6,7] However, Gozal et al reported that that the incidence of prostate, breast, and colorectal cancer was reduced in patients with OSA, while the risk of melanoma, kidney, and pancreatic cancer were increased.[8] However, the study lacked data on race and ethnicity, weight and BMI, which are all relevant factors to both OSA and cancer.
Another interesting phenomenon in this study is that incidence of prostate cancer, while both higher than control patients, was slightly lower in patients with OSA less than 65 years old compared to those 65 years and older. Shah et al. found that longstanding OSA might have a cardioprotective role during acute myocardial infarction due to ischemic preconditioning.[12] It is possible that elderly patients with OSA might be less susceptible to the damaging effects of hypoxia on cells than younger patients with OSA.[12] This might explain a decrease in the incidence of prostate cancer in the elderly population with OSA. Moreover, prostate cancer is being diagnosed earlier than ever before due the increased utilization of prostate-specific antigen testing.[12] This trend can reflect why the incidence of prostate cancer in patients with OSA was higher than in the older age group due to earlier screening.
The exact mechanism by which OSA contributes to prostate cancer is not yet known; however, several possibilities have been discussed in the literature. First, chronic intermittent hypoxia caused by recurrent apnea and arousal events in OSA is considered to be the strong risk factor. Hypoxic conditions induce the production of hypoxia-inducible factor-1a, which is involved in cancer metastasis and angiogenesis. Hypoxia-inducible factor-1a is also overexpressed in prostate cancer cells, suggesting a link between OSA and prostate cancer.[13,14] In addition, chronic hypoxic conditions are also involved in the early development of prostate cancer, playing an important role in cancer growth and hormone-refractory progression.[15–18] Second, sleep fragmentation, a characteristic finding in patients with OSA, is also suspected to be involved in the development of prostate cancer. Sleep fragmentation induces the dysregulation of circadian rhythms, inadequate sleep duration, and suppression of the melatonin levels. Melatonin plays an important role in regulating other hormones that influence breast and prostate cancers, and studies have identified a link between lower melatonin levels and an increased risk of prostate cancer.[19,20] Third, apnea and subsequent hyperventilation that induce hypo- and hyper-oxygenation repeatedly produces oxygen free radicals. These oxygen radicals cause systemic and local inflammation leading to extensive cytokine production, which can stimulate the development of cancer.[21,22]
The KNHIS data is a valuable tool that facilitated our analysis of a large patient cohort; however, similar to all large registries, it is subject to limitations including the potential for selection, information, and recall bias, as well as coding errors and missing information. A specific weakness of this study is that degree of OSA could not be evaluated from the KNHIS data. Patients with OSA were identified based on the diagnosis code from available claim data, but information on OSA severity is not available. Second, the control group could include patients with unrecognized OSA. However this number should be relatively low as a biennial checkup is recommended for all KNHIS subscribers, and universal healthcare is accessible to all people living in South Korea. Third, no data was available on lifestyle, exercise, smoking, and drinking habits, nutritional status, and body mass index, all of which can be considered to be important confounders of cancer development. Finally, prostate cancer is known to have racial differences, and South Korean is among the world's most ethnically homogeneous countries.
5. Conclusion
The incidence of prostate cancer was significantly higher among patients with OSA than among controls. This study provides additional evidence for a link between OSA and prostate cancer.
Author contributions
Conceptualization: Jae Hoon Cho.
Data curation: Jae Hoon Cho.
Formal analysis: Jae Hoon Cho.
Methodology: Eun Jung Lee, Jeffrey D Suh.
Writing – original draft: Eun Jung Lee, Jeffrey D Suh.
Glossary
Abbreviations: CI = confidence interval, ICD-10 = International Classification of Diseases, 10th edition, KNHIS = Korea National Health Insurance Service, OSA = obstructive sleep apnea, SD = standard deviation.
References
- [1].Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Partinen M. Epidemiology of obstructive sleep apnea syndrome. Curr Opin Pulm Med 1995;1:482–7. [DOI] [PubMed] [Google Scholar]
- [3].Martínez-García MÁ, Campos-Rodriguez F, Barbé F. Cancer and OSA: current evidence from human studies. Chest 2016;150:451–63. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- [5].Jung KW, Won YJ, Oh CM, et al. Cancer statistics in korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat 2017;49:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fang HF, Miao NF, Chen CD, et al. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J Cancer 2015;6:1140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung SD, Hung SH, Lin HC, et al. Obstructive sleep apnea and urological comorbidities in males: a population-based study. Sleep Breath 2016;20:1203–8. [DOI] [PubMed] [Google Scholar]
- [8].Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep 2016;39:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Song SJ, Han K, Choi KS, et al. Trends in diabetic retinopathy and related medical practices among type 2 diabetes patients: Results from the National Insurance Service Survey 2006–2013. J Diabetes Investig 2018;9:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kheirandish P, Chinegwundoh F. Ethnic differences in prostate cancer. Br J Cancer 2011;105:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Discacciati A, Wolk A. Lifestyle and dietary factors in prostate cancer prevention. Recent Results Cancer Res 2014;202:27–37. [DOI] [PubMed] [Google Scholar]
- [12].Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath 2013;17:819–26. [DOI] [PubMed] [Google Scholar]
- [13].Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999;59:5830–5. [PubMed] [Google Scholar]
- [14].Ghafar MA, Anastasiadis AG, Chen MW, et al. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate 2003;54:58–67. [DOI] [PubMed] [Google Scholar]
- [15].Fraga A, Ribeiro R, Principe P, et al. Hypoxia and prostate cancer aggressiveness: a tale with many endings. Clin Genitourinary Cancer 2015;13:295–301. [DOI] [PubMed] [Google Scholar]
- [16].Butterworth KT, McCarthy HO, Devlin A, et al. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- and phenotype. Int J Cancer 2008;123:760–8. [DOI] [PubMed] [Google Scholar]
- [17].Stewart GD, Ross JA, McLaren DB, et al. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int 2010;105:8–13. [DOI] [PubMed] [Google Scholar]
- [18].Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res 2014;74:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sigurdardottir LG, Valdimarsdottir UA, Fall K, et al. Circadian disruption, sleep loss, and prostate cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2012;21:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sigurdardottir LG, Markt SC, Rider JR, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol 2015;67:191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nguyen HL, Zucker S, Zarrabi K, et al. Oxidative stress and prostate cancer progression are elicited by membrane-type 1 matrix metalloproteinase. Mol Cancer Res 2011;9:1305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and IH mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 2012;92:967–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


