Fernando Gonzalez-Ortiz, Michael Turton, Przemysław R. Kac, Denis Smirnov, Enrico Premi, Roberta Ghidoni, Luisa Benussi, Valentina Cantoni, Claudia Saraceno, Jasmine Rivolta, Nicholas J. Ashton, Barbara Borroni, Douglas Galasko, Peter Harrison, Henrik Zetterberg, Kaj Blennow, Thomas K. Karikari. Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain. 2023;146:1152-1165. https://doi.org/10.1093/brain/awac407
The authors apologize for errors in Fig. 3 and Supplementary Table 1, which have now been corrected. The corrections do not affect the results, their interpretation, or the conclusion of the study, and are described below.
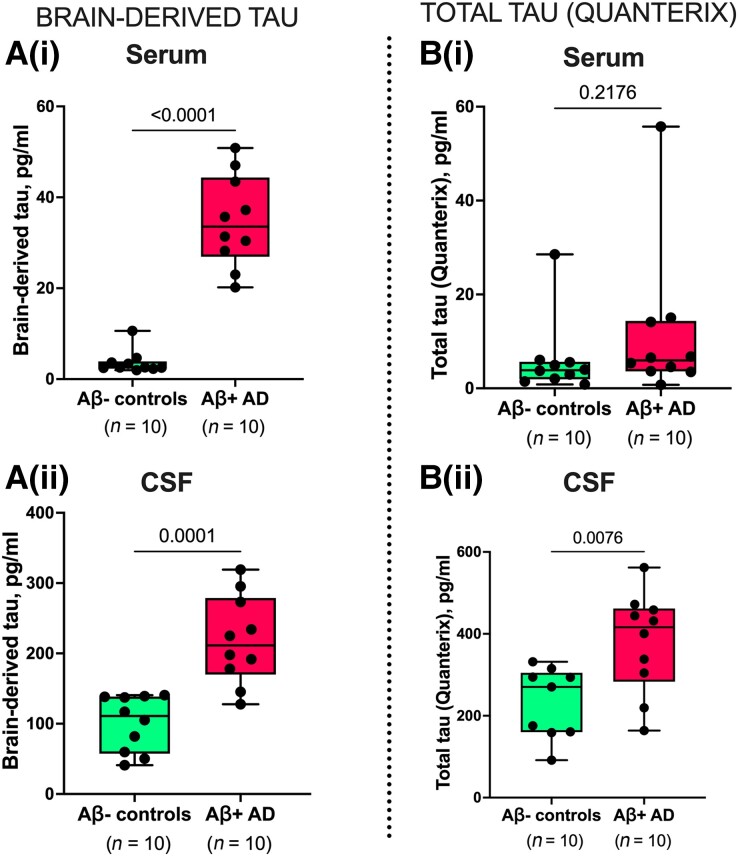
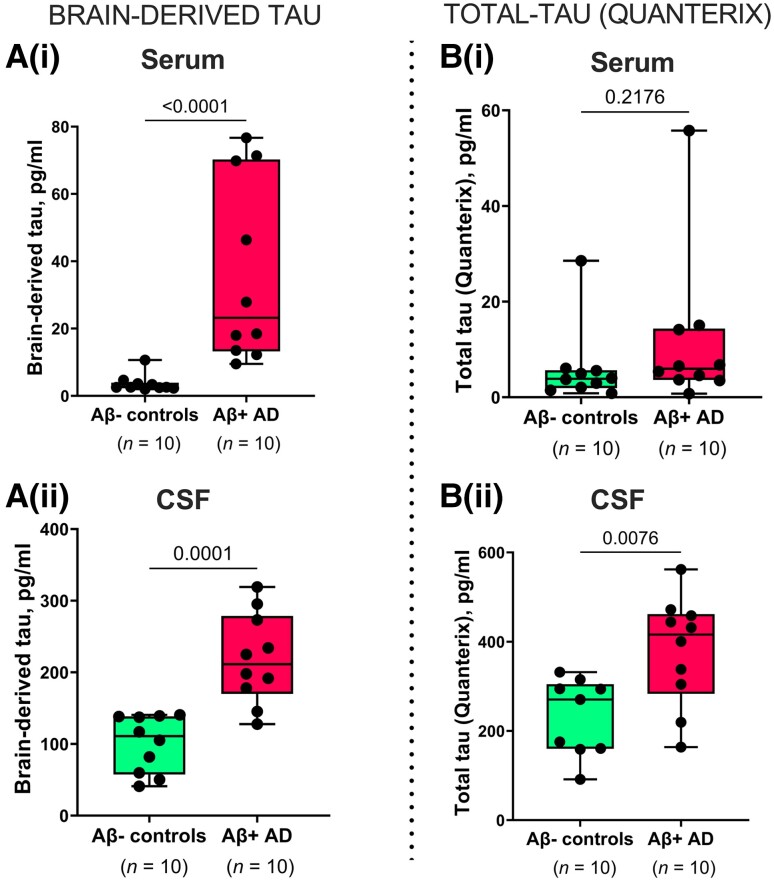
Figure 3.
Concentrations and correlation of BD-tau in paired serum and CSF samples. [A(i and ii)] Concentrations of BD-tau in paired serum and CSF samples showing significant increases in Aβ+ Alzheimer’s disease and Aβ− control individuals classified according to their neurochemical CSF biomarker profiles. The corresponding levels of t-tau (Quanterix) in the same paired serum and CSF samples are shown in B(i) and B(ii), respectively. P-values indicate the results of Mann–Whitney tests. In each box plot, the horizontal bar on top of the coloured area shows the 75% percentile, the middle bar depicts the median and the lower bar shows the 25% percentile. Values that are above the 75% percentile and below the 25% percentile are shown outside the coloured areas.
Further clarification has also been added concerning the association between plasma/serum BD-tau and cortical thickness at the end of the second paragraph of the ‘Results’ subsection ‘BD-tau in blood associates with neurodegeneration markers in Alzheimer’s disease but not in other neurodegenerative diseases’ as follows:
This is in line with our hypothesis because an association of BD-tau with cortical thickness in non-Alzheimer's disease was not expected, given increases in the biomarker levels in Alzheimer's disease but not in non-Alzheimer's disease (Figs 4 and 5). Cortical thickness data were not available to perform a similar analysis for the Alzheimer's disease group in this cohort.
Figure 3 and legend have been changed from:
to:
Figure 3.
Concentrations and correlation of BD-tau in paired serum and CSF samples. [A(i and ii)] Concentrations of BD-tau in paired serum and CSF samples showing significant increases in Aβ+ Alzheimer’s disease and Aβ− control individuals classified according to their neurochemical CSF biomarker profiles. The corresponding levels of t-tau (Quanterix) in the same paired serum and CSF samples are shown in B(i) and B(ii), respectively. For B(ii), one sample in the Aβ− control group returned no measurable signal due to a technical instrument error. Excluding the CSF-serum pair of this sample from the analyses did not change the results. P-values indicate the results of Mann–Whitney tests. In each box plot, the horizontal bar on top of the coloured area shows the 75% percentile, the middle bar depicts the median and the lower bar shows the 25% percentile. Values that are above the 75% percentile and below the 25% percentile are shown outside the coloured areas. Note that there are differences in the absolute concentrations of BD-tau and t-tau in both serum and CSF, which can be explained by the use of different assay designs, analytical technologies, calibrators, and standard curves for each biomarker. This means that the values are a reflection of several factors, including assay sensitivity, and that absolute concentrations are not directly comparable in numerical sense.
In Supplementary Table 1, some data in the bottom row of the ‘Discovery cohort’ column have been updated as follows: in the ‘Control’ subcolumn, the asterisk has been deleted; in the ‘Alzheimer’s disease’ subcolumn, the value has been changed from 16.1 ±5.1 to 11.6 ±16.1.