Abstract
An outbreak due to extended-spectrum β-lactamase-producing Klebsiella pneumoniae (ESBL-KP) was detected from May 1993 to June 1995. A total of 145 patients, particularly patients in intensive care units (ICUs) (107 patients [72%]), were colonized or infected. Infection developed in 92 (63%) patients, and primary bacteremia caused by ESBL-KP was the most frequent infection (40 of 92 patients [43%]). A single clone of ESBL-KP was identified by pulsed-field gel electrophoresis analysis throughout the whole period, and no molecular epidemiological relationship could be found between the epidemic strain and non-ESBL-KP isolates. To determine risk factors for ESBL-KP infection weekly rectal swabs were obtained in three serial incidence surveys (470 patients); the probabilities of carriage of ESBL-KP in the digestive tract were 33% (October and November 1993), 40% (May and June 1994), and 0% (October and November 1995) at 10 days of ICU admission. A logistic regression model identified prior carriage of ESBL-KP in the digestive tract (odds ratio, 3.4; 95% confidence interval 1.1 to 10.4) as an independent variable associated with ESBL-KP infection. A statistically significant correlation was observed between the restricted use of oxyimino-β-lactams (189 defined daily doses [DDD]/1,000 patient-days to 24 DDD/1,000 patient-days) and the trends of ESBL-KP infection (r = 0.7; P = 0.03).
Klebsiella spp. have been prominent among the gram-negative bacilli causing nosocomial infections and an important source of transferable antibiotic resistance (27). During the 1970s, there were frequent epidemics of gentamicin-resistant Klebsiella pneumoniae infections in hospitals (22). In the last several years, following the overuse of the expanded-spectrum cephalosporins, several outbreaks caused by extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae (ESBL-KP) have been reported (1, 4, 6, 11, 16–18, 26, 32, 33, 36).
Several studies suggest that the implementation of rigorous restriction of the use of expanded-spectrum cephalosporins, usually combined with the reinforcement of infection control measures, can decrease the prevalence of ESBL-KP strains (26, 29, 32, 33). However, the successful management of these outbreaks remains controversial.
A recent large outbreak caused by ESBL-KP occurring over a 2-year period in our hospital allowed us to analyze the clinical and molecular epidemiology of ESBL-KP. We sought to establish a correlation between the restricted use of oxyimino-β-lactam consumption during the outbreak caused by ESBL-KP and the eradication of such resistant strains, as well as to define the main risk factors for ESBL-KP infection.
MATERIALS AND METHODS
The study was carried out in the Hospital de Bellvitge, a 1,000-bed teaching hospital for adult patients providing acute medical and surgical care but without wards for pediatrics, obstetrics, and burns. The average yearly admission is 23,000 patients. The hospital has three 12-bed medical-surgical intensive care units (ICUs), with one nurse for every two patients except patients who have undergone organ transplantation, who each have a single nurse. All rooms in ICU are single rooms.
Data collection.
The surveillance program was initiated in May 1993 after the detection of several ESBL-KP isolates in ICUs. A retrospective revision indicated that the first two ESBL-KP strains occurred in March 1993 in one patient in a surgical ward. These ESBL-KP isolates were recovered from bile and a surgical wound.
Patients infected with ESBL-KP isolates were prospectively identified. The double-disk synergy test was performed to detect ESBL-producing strains (20). Daily laboratory surveillance of cultures of clinical samples positive for ESBL-KP was performed by an infectious diseases physician who filled out a form generated by a computer-assisted protocol. Clinical assessment was determined according to the definitions of the Centers for Disease Control and Prevention for nosocomial infections (12). Patients with samples from any body site positive for ESBL-KP but without related signs or symptoms of infection were considered to be colonized. A clinical sample positive for ESBL-KP was considered to be acquired in the ICU if it appeared during a stay in an ICU or was obtained during the first week after discharge from an ICU or if patients had digestive tract colonization during their stay in an ICU. All other clinical samples positive for ESBL-KP were considered not to be acquired in an ICU.
To study the trends in K. pneumoniae infections in the hospital over time, we extended the study period from January 1993 to December 1995. Clinical information on all non-ESBL-KP isolates from clinical samples was also collected retrospectively. For this analysis we included all patients with any clinical sample positive for a non-ESBL-KP or an ESBL-KP isolate during the study period.
We calculated the rates of incidence of ICU-acquired ESBL-KP infection or colonization according to the number of episodes of infection (some patients had more than one infection) or colonization (but not stool sample infection or colonization) per 1,000 daily census.
Incidence surveys.
To determine the risk factors for ESBL-KP infection we did three serial incidence surveys. We carried out an active surveillance program with ICU patients during three different 2-month periods (October and November 1993, May and June 1994, and October and November 1995). Weekly rectal swab samples were obtained to detect digestive tract carriage of ESBL-KP in these patients from the time of admission to an ICU to the time of discharge from an ICU or the time to ESBL-KP infection or colonization. For this purpose, a patient was considered to be infected or colonized with ESBL-KP if any culture of a sample from a clinical site other than stool samples was positive for ESBL-KP.
The data collected from the patients included in the three surveys were demographic characteristics, prior surgery, severity of acute illness on ICU admission by means of the simplified acute physiologic score (19), and severity of underlying disease according to the criteria of McCabe and Jackson (23). We also assessed the numbers of days with devices in place; these devices included intravascular catheters, urinary catheters, and endotracheal tubes. In addition, antibiotic therapy was determined as the number of days of therapy with different groups of antibiotics for each patient until ESBL-KP infection or colonization was detected. The groups of antibiotics analyzed were penicillins (penicillin G, ampicillin, piperacillin, and ticarcillin), cephalothin, cefuroxime, oxyimino-β-lactams (extended-spectrum cephalosporin and aztreonam), amoxicillin-clavulanic acid, piperacillin-tazobactam, aminoglycosides, glycopeptides, fluoroquinolones, and carbapenems.
Interventions. (i) Infection control measures.
Routine preventive measures in all three ICUs included the use of disposable gloves. The use of disposable aprons while caring for ESBL-KP-infected patients was implemented. The patients in non-ICU wards colonized or infected with ESBL-KP were spatially segregated, and the use of disposable gloves and aprons while caring for these patients was also implemented. Health care personnel were informed about the need for appropriate use of gloves, and the need for handwashing was reinforced. These infection control measures were introduced in June 1993, when the initial ESBL-KP isolates were detected.
(ii) Restriction of oxyimino-β-lactam use.
In September 1993 restricted use of oxyimino-β-lactams in ICU patients was introduced. Ceftriaxone or cefotaxime use was restricted to the treatment of community-acquired infections, such as pneumonia and meningitis, and ceftazidime use was restricted to the treatment of infections in neutropenic patients requiring admission to an ICU. Concomitantly, the use of imipenem and piperacillin-tazobactam was instituted for empiric treatment of nosocomial infections in ICUs. Compliance with this antibiotic use policy was monitored by an infectious diseases physician. Consumption was expressed as defined daily doses (DDD), as recommended by the Nordic Council on Medicines (30).
Microbiological studies. (i) Susceptibility studies.
MICs were determined with the MicroScan automated microdilution system (Dade International, West Sacramento, Calif.) and by the diffusion based E-test (AB BIODISK, Solna, Sweden). The antibiotics tested were ampicillin, piperacillin, ticarcillin, amoxicillin-clavulanic acid, piperacillin-tazobactam, ceftazidime, ceftazidime-clavulanic acid, cefotaxime, aztreonam, imipenem, meropenem, cefpirome, ciprofloxacin, gentamicin, tobramycin, and amikacin. The criteria of the National Committee for Clinical Laboratory Standards (28) were used to define susceptibility or resistance to these antimicrobial agents.
(ii) Digestive tract colonization detection.
Rectal swabs were inoculated onto two MacConkey agar plates (Oxoid); one of the plates was supplemented with 4 μg of ceftazidime per ml. The plates were incubated at 37°C for 48 h. K. pneumoniae strains were identified by conventional biochemical tests.
(iii) PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed with 51 ESBL-KP strains isolated from 41 patients during the outbreak period (both blood and fecal isolates from 10 patients were studied). Twenty-two non-ESBL-KP strains, obtained from blood from patients with nosocomially acquired bacteremia, were also studied (10 isolates were obtained during the outbreak and 12 were isolated after June 1995).
Macrorestriction analysis of chromosomal DNA was done by PFGE by published procedures (14) with XbaI (New England Biolabs, Boston, Mass.). PFGE was run in a CHEF-DR III apparatus (Bio-Rad Laboratories, Richmond, Calif.), with pulses ranging from 1 to 30 s at a voltage of 6 V/cm for 23 h at 14°C. The gels were stained with ethidium bromide and photographed.
(iv) β-Lactamase study.
ESBL production by all isolates was detected by the double-disk synergy test (20) and by the ESBL E-test (AB BIODISK).
Twelve strains of K. pneumoniae isolated from blood samples during the outbreak period and exhibiting the common resistance pattern were selected for isoelectric focusing. Bacterial sonicates were purified by ultracentrifugation (20) and were subjected to isoelectric focusing in a PhastSystem apparatus (Pharmacia, Uppsala, Sweden) by using polyacrylamide gels with a pH range of 3 to 9 (PhastGel 3-9; Pharmacia). The gels were stained with nitrocefin (500 μg/ml; Oxoid, Hampsire, England), and pIs were obtained by comparison with those for a set of β-lactamases with known pIs.
Statistical methods.
The relationship between the incidence of ESBL-KP (infection or colonization at clinical sites) in ICUs and oxyimino-β-lactam use was calculated by the use of Spearman correlation coefficients. In order to obtain a larger sample, data obtained over 4-month periods were analyzed.
Risk factors for ESBL-KP infection for all ICU patients from whom weekly rectal swab samples were obtained during the incidence survey were studied by multivariate analysis by logistic regression techniques. The probability of carriage of ESBL-KP in the digestive tract was calculated by using the Kaplan-Meier estimate.
RESULTS
Description of the outbreak.
During the study period (January 1993 to December 1995), a total of 550 clinical isolates of K. pneumoniae were detected in 444 hospitalized patients, with 202 of them (35%) being ESBL-KP isolates (Table 1). There were significant differences in the source of ESBL-KP isolates between the ICUs and the non-ICU wards. In the ICUs isolates were more frequently detected in blood samples (40% in ICUs versus 16% in non-ICU wards) and respiratory tract samples (26% in ICUs versus 3% in non-ICU wards), whereas in the non-ICU wards isolates were more frequently detected in urine samples (23% in ICUs versus 55% in non-ICU wards) and surgical wound samples (18% in ICUs versus 34% in non-ICU wards). Fourteen of 21 (67%) ESBL-KP-infected urine samples from patients in non-ICU wards were from patients with a urinary catheter.
TABLE 1.
Clinical samples infected or colonized with K. pneumoniae during the study period (January 1993 to December 1995)
| Clinical sample | No. (%) of isolates
|
|||
|---|---|---|---|---|
| ICU
|
Non-ICU
|
|||
| ESBL-KP (n = 107)a | Non-ESBL-KP (n = 100) | ESBL-KP (n = 38) | Non-ESBL-KP (n = 199) | |
| Urine | 25 (23) | 34 (34) | 21 (55) | 81 (41) |
| Wound | 19 (18) | 11 (11) | 13 (34) | 47 (24) |
| Respiratory tract | 28 (26) | 42 (42) | 1 (3) | 26 (13) |
| Blood | 43 (40) | 20 (20) | 6 (16) | 27 (13) |
| Catheter | 39 (36) | 24 (24) | 0 | 16 (8) |
| Other | 5 (5) | 6 (6) | 2 (5) | 14 (7) |
| Total | 159 (54) | 137 (46) | 43 (17) | 211 (83) |
n indicates total number of patients.
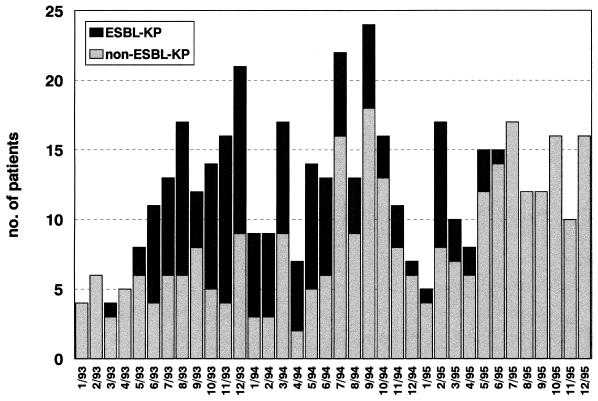
An ESBL-KP outbreak was detected from May 1993 to June 1995. A total of 145 patients were affected, and these included 94 (65%) males and 51 (35%) females, with a mean age of 53.4 ± 19.7 years. Of these, 107 were ICU patients (72%), and the remaining 38 patients (28%) acquired the infection or colonization in a non-ICU setting. The total number of patients colonized or infected with K. pneumoniae over time is presented in Fig. 1.
FIG. 1.
Numbers of patients colonized or infected with K. pneumoniae over the course of the study period.
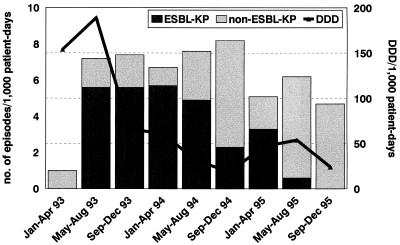
In ICUs the rates of incidence of ESBL-KP strains were the highest during the first year of the outbreak, and the peak incidence of ESBL-KP infection, 5.7 episodes/1,000 patient-days, was reached between January 1994 and April 1994, several months after control measures had been reinforced (Fig. 2). We observed a gradual decrease from May to August 1994 (4.9 episodes/1,000 patient-days) and from May to August 1995 (0.6 episodes/1,000 patient-days).
FIG. 2.
ESBL-KP and non-ESBL-KP incidence rates in ICUs after the implementation of the restricted use of oxyimino-β-lactams in ICUs.
Concomitantly, the rate of incidence of non-ESBL-KP strains at the peak of the outbreak remained close to that observed in the baseline period, increasing at the end of and after the outbreak. The 38 patients with ESBL-KP infection not acquired in an ICU were found to be staying in 17 different hospital wards, with 12 of them being surgical wards. Epidemiological analysis of these 38 patients showed that 16 of them had previously been admitted to ICUs and for another 10 patients there was some evidence of temporal clustering with ESBL-KP-colonized patients; the remaining 12 patients had sporadic cases of infection without evidence of geographical clustering. Of these 38 patients, 23 (60.5%) had undergone urinary tract catheterization and 31 (81.5%) had a surgical wound at the time of isolation of ESBL-KP.
Clinical data.
Among the 145 patients with clinical samples other than stool samples positive for ESBL-KP, 92 (63%) patients were infected and 53 (37%) patients were colonized. The 92 infected patients, 69 in ICUs and 23 in non-ICU wards, had 109 episodes of infection, with primary bacteremia (40 episodes) being the most frequent ESBL-KP infection. Of these 40 episodes, only for 10 episodes could antibiotic treatment be evaluated; the outcomes of the remaining episodes depended both on the antibiotic and on catheter removal. Five of the 10 episodes were treated with imipenem alone, 2 were treated with imipenem and tobramycin, 2 were treated with tobramycin alone, and 1 was treated with piperacillin-tazobactam. Two patients, one treated with imipenem and one treated with tobramycin, developed septic shock and died.
Twenty-three of 28 surgical wound infections were deep surgical wound infections: 18 intra-abdominal infections, 2 cases of osteomyelitis, and 3 cases of meningitis. A total of four episodes of meningitis were observed during the outbreak: three episodes followed neurosurgical procedures and one occurred in a patient with an external cerebrospinal fluid shunt. All 13 episodes of respiratory tract infection were observed in ICU patients, and 5 (38%) were pneumonias. The remaining infections were 25 urinary tract infections and 2 soft-tissue infections. The overall mortality rate as a result of ESBL-KP infections was 38% (35 of 92 patients with ESBL-KP infections).
Risk factors for ESBL-KP infection in ICU patients.
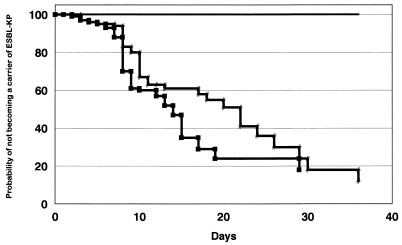
In the incidence surveys, 470 patients were admitted to the ICUs: 141 patients in October and November 1993, 168 patients in May and June 1994, and 161 patients in October and November 1995. There were 35 fecal carriers (24.8%) during the first period, 41 fecal carriers (24.4%) during the second period, and no fecal carriers during the third period, while the probabilities of carriage of ESBL-KP in the digestive tract were 33, 40, and 0%, respectively, at 10 days of ICU admission (Fig. 3).
FIG. 3.
Probability of remaining free of digestive tract colonization with ESBL-KP. Line with slashes, first period; line with squares, second period; solid line, third period.
Among the 470 patients, 31 (6.5%) had ESBL-KP infection or colonization, and all but 4 patients were prior fecal carriers. The mean number of days between carriage of ESBL-KP in the digestive tract and a positive clinical sample was of 24.2 ± 20.1 days (range, 2 to 90 days). In two of the four nonfecal carriers, ESBL-KP isolates were not found in serial stool sample screenings before positive clinical samples were found (one had further negative rectal swabs after the detection ESBL-KP infection). For the remaining two patients only an initial rectal swab was obtained (one patient died as a result of an ESBL-KP infection at 6 days of ICU admission). No differences in the type of infection were found in these noncarrier patients.
A logistic regression model with ESBL-KP infection or colonization as the dependent variable, with adjustment for age, surgery, severity of disease, days of invasive devices, and days of prior antibiotic therapy, identified prior carriage of ESBL-KP in the digestive tract (odds ratio, 3.4; 95% confidence interval, 1.1 to 10.4) as the independent variable associated with ESBL-KP infection or colonization.
Restriction of oxyimino-β-lactam use.
Oxyimino-β-lactams have been used in our hospital since 1985. Among them, ceftazidime is the one that is the most frequently used for the empiric treatment of severe infections in ICU patients. Restricted use of oxyimino-β-lactams was initiated in September 1993 (Fig. 2). Oxyimino-β-lactam use decreased from 189 DDD/1,000 patient-days in the period immediately before the implementation of this restriction (May to August 1993) to 66 DDD/1,000 patient-days after the initiation of the restriction (September to December 1993). Overall, a progressive reduction was observed after restriction of the use of oxyimino-β-lactams was introduced, with these drugs being used for 24 DDD/1,000 patient-days during the last period analyzed. There was a statistically significant correlation between ESBL-KP infection trends in ICU patients and the restricted use of cephalosporins (r = 0.7; P = 0.03).
Concomitantly, an initial increase in imipenem use was observed (from 120 DDD/1,000 patient-days in 1992 to 164 DDD/1,000 patient-days in 1993). However, after the introduction of piperacillin-tazobactam in ICUs in January 1994 (123 DDD/1,000 patient-days in 1994 to 149 DDD/1,000 patient-days in 1995), a further decrease of imipenem consumption was observed (from 158 DDD/1,000 patient-days in 1994 to 132 DDD/1,000 patient-days in 1995). Also, during the outbreak we observed an increase in tobramycin consumption (1992, 30 DDD/1,000 patient-days; 1993, 53 DDD/1,000 patient-days; 1994, 123 DDD/1,000 patient-days; and 1995, 150 DDD/1,000 patient-days) in ICUs.
Microbiology. (i) Antibiotic resistance phenotype.
Throughout the whole outbreak a common multidrug resistance pattern was observed in ESBL-KP strains isolated from 145 patients. The MIC ranges were as follows: gentamicin, 8 to 16 μg/ml; ciprofloxacin, 2 to 8 μg/ml, tobramycin, 1 to 2 μg/ml; and amikacin, 2 μg/ml. Only one ESBL-KP strain isolated in 1994 had a different antibiotype (MICs, 0.5 μg/ml for gentamicin, 1 μg/ml for tobramycin, 2 μg/ml for amikacin; and 0.03 μg/ml for ciprofloxacin). All the isolates had a positive double-disk synergy test result, and the ceftazidime MIC for the isolates decreased when clavulanic acid was added.
Twenty-two nonepidemic K. pneumoniae isolates from patients with nosocomial bacteremia, used as controls, were resistant to ampicillin, piperacillin, and ticarcillin and susceptible to cephalosporins, carbapenems, and aminoglycosides; only one of these strains showed diminished susceptibility to ciprofloxacin (2 μg/ml). None of the nonepidemic strains had a positive double-disk synergy test result.
Table 2 presents the MICs at which 50% and 90% of isolates are inhibited (MIC50 and MIC90, respectively), the MIC range, and the percent susceptibility to cephalosporins, aztreonam, carbapenems, and combinations of a β-lactam antibiotic plus a β-lactamase inhibitor against the blood isolates of ESBL-KP and non-ESBL-KP studied.
TABLE 2.
Activities of β-lactam antibiotics against ESBL-KP and non-ESBL-KP isolates from blood
| Antibiotic | MIC breakpoint (μg/ml) | Non-ESBL-KP (n = 26)
|
ESBL-KP (n = 44)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | % Suscep- tible | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | % Suscep- tible | ||
| Amoxicillin-clavulanic acid | ≤8/4 | 1–4 | 2 | 4 | 100 | 4–16 | 8 | 16 | 68 |
| Piperacillin-tazobactam | ≤16/4 | 2–4 | 2 | 4 | 100 | 4–>256 | 128 | >256 | 45 |
| Ceftazidime | ≤8 | <0.5–1 | 0.5 | 0.5 | 100 | 2–>256 | 128 | >256 | 14a |
| Ceftazidime-clavulanic acid | NDb | <0.12–0.5 | 0.25 | 0.5 | 0.25–4 | 1 | 2 | ||
| Cefotaxime | ≤8 | 0.03–0.25 | 0.06 | 0.25 | 100 | 1–>256 | 8 | 64 | 55a |
| Cefpirome | ND | 0.03–0.25 | 0.06 | 0.25 | 1–>256 | 4 | 16 | a | |
| Aztreonam | ≤8 | 0.01–0.06 | 0.03 | 0.06 | 100 | 1–>256 | 128 | >256 | 39a |
| Imipenem | ≤4 | 0.12–0.25 | 0.25 | 0.25 | 100 | 0.12–1 | 0.12 | 0.25 | 100 |
| Meropenem | ND | 0.03–0.25 | 0.06 | 0.12 | 0.06–2 | 0.06 | 0.06 | ||
K. pneumoniae isolates producing ESBLs may be resistant clinically to all cephalosporins and aztreonam.
ND, not defined.
(ii) PFGE pattern analysis.
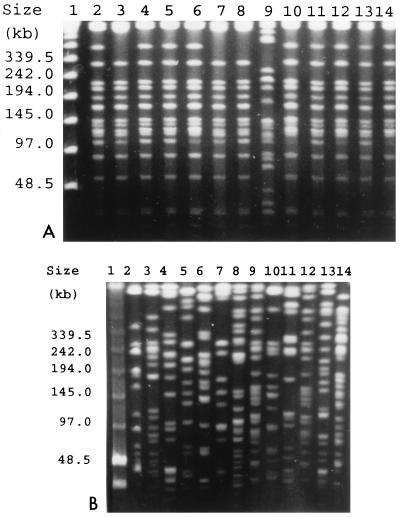
After chromosomal DNA restriction with XbaI, a major PFGE pattern was found in an analysis of the 50 ESBL-KP isolates with the same antibiotypes. Although slight differences in the restriction patterns of some of them were found, they were considered subtypes of the epidemic clone. The PFGE patterns of the isolates from clinical samples and feces from the same patient were identical. The single strain susceptible to gentamicin and ciprofloxacin had a PFGE pattern different from that of the epidemic strain (Fig. 4A, lane 9). The PFGE profiles of isolates selected from nine patients are presented in Fig. 4A.
FIG. 4.
PFGE of total DNA from K. pneumoniae isolates cut with XbaI. (A) ESBL-KP isolates. Lane 1, PFGE marker (New England BioLabs); lanes 2 and 3, isolates from the blood of non-ICU patients; lanes 4 to 6 and 10, isolates from the blood of ICU patients; lanes 7 and 8, 11 and 12, and 13 and 14, isolates from the blood and feces of three patients, respectively; lane 9, blood isolate with a susceptibility pattern different from those of the other isolates. (B) Non-ESBL-KP isolates. Lane 1, PFGE marker (New England BioLabs); lanes 2 to 8, 10, 11, 13, and 14, isolates from the blood of 11 patients, respectively; lanes 9 and 12, non-ESBL-KP strains isolated from the blood of the same patient during two different episodes of bacteremia, respectively.
The 22 non-ESBL-KP strains were multiclonal, and no relationship could be found between ESBL-KP and non-ESBL-KP isolates. Figure 4B shows the PFGE profiles of isolates selected from 12 patients.
(iii) Isoelectric focusing analysis.
All of the ESBL-KP isolates tested expressed β-lactamases with pIs of 7.1 and 7.6. An additional β-lactamase with a pI of 8.2 was detected in two strains. These 12 isolates exhibited the major antibiotype and PFGE pattern. β-Lactamases with pIs of 7.6 and 8.2 could be transferred by conjugation.
DISCUSSION
In recent years ESBL-KP isolates have produced significant outbreaks in hospitals worldwide (1, 4, 6, 11, 16–18, 26, 32, 33, 36). Our data document, according to the results of PFGE analysis of genomic DNA, the identities of different ESBL-KP strains isolated throughout the outbreak; the strains had the same PFGE pattern and belonged to a single clone. Therefore, our outbreak was due to the spread of an epidemic strain of multidrug-resistant K. pneumoniae, in a manner similar to that for other epidemics reported recently (2, 14, 33). No molecular epidemiological relationship could be found between the epidemic ESBL-KP strain and any of the non-ESBL-KP isolates from patients with nosocomial bacteremia acquired at different times before, during, and after the outbreak. We analyzed the possible influence of the ESBL-KP outbreak on decreasing the rates of incidence of endemic non-ESBL-KP infections, since it is well known that exogenous multidrug-resistant bacteria can replace the normal bowel flora when intestinal colonization resistance is impaired (3, 37). However, we did not document a decrease in non-ESBL-KP infections in our hospital during the ESBL-KP outbreak.
ICU patients were mainly involved in most of the previously described outbreaks (1, 4, 6, 11, 16–18, 26, 36) and in our outbreak. ICUs, where large amounts of empiric broad-spectrum antibiotics are consumed and where critically ill patients with low levels of resistance to exogenous colonization are cared for, serve as breeding grounds for epidemic multidrug-resistant bacteria. On the other hand, the role of acquisition in a non-ICU ward was less important. In fact, 42% of patients who acquired infection in a non-ICU ward had recently been discharged from ICUs and so may have been colonized during their stay in an ICU, since rectal colonization in these patients had not specifically been studied. The non-ICU wards where acquisition occurred were mainly surgical wards, and acquisition of ESBL-KP strains occurred among clusters of patients with surgical wounds or urinary catheters, who required many procedures and for whom there was a higher risk of cross transmission.
Among the 109 episodes of ESBL-KP infection, primary bacteremia was the most frequent infection. Thus, in ICU patients ESBL-KP strains showed a particular predisposition toward the production bacteremia in comparison to the predisposition of non-ESBL-KP strains (40 versus 20%). Whether this predisposition depends on specific virulence determinants or epidemiological conditions related to antibiotic resistance remains to be determined. Although most virulence factors seem to be similar in both ESBL-KP and susceptible K. pneumoniae strains, some R plasmids encoding ESBLs have been found to produce a surface protein which facilitates adhesion to intestinal cells and so could promote gut colonization (8, 10).
The role of the intestinal reservoir as an endogenous source of endemic or epidemic gram-negative infections in ICU patients has been referred to by other investigators in their descriptions of K. pneumoniae outbreaks (35), and our data also provide strong evidence for this. The incidence of carriage of ESBL-KP in the digestive tract detected in our patients during the outbreak was higher than that found by others (9), and we analyzed this in a recent report (31). Carriage of ESBL-KP in the digestive tract was often followed by colonization or infection at other body sites (21). Actually, prior rectal colonization was detected in most patients developing ESBL-KP infection and was the major risk factor associated with ESBL-KP infection or colonization. This is supported by the results of our molecular studies, which documented the identities of ESBL-KP-infected fecal and clinical samples. The management of ESBL-KP outbreaks has included the restricted use of oxyimino-β-lactams (5, 15, 24, 26, 29, 32, 33), along with other measures (13), such as reinforcement of the use of barrier measures or selective intestinal decontamination (7), with different degrees of success. While all investigators agree with the fact that the number of ESBL isolates decreased markedly when restrictions on ceftazidime use were put into place, complete eradication of such organisms was achieved only in the smallest outbreaks (29, 32). Prevalence remained at a constant low level after the initial decrease following the restricted use of cephalosporins in two large ESBL-KP epidemics (26, 33).
Our study of 145 patients provides data about the significant role of the rigorous restriction of oxyimino-β-lactam use in the management and successful control of a large nosocomial ESBL-KP outbreak. Since we implemented two different strategies simultaneously (reinforcement of the use of barrier precautions and restricted cephalosporin use), it is not possible to define the relative contribution of each to controlling the outbreak. However, we did not observe any significant reduction in the ESBL-KP incidence rate for the first 6 months after the use of barrier precautions had been reinforced. In contrast, a significant correlation between a progressive reduction of oxyimino-β-lactam consumption and the ESBL-KP incidence rate was established.
Because many recent resistance problems can be traced to excessive use of broad-spectrum antibiotics, antibiotic control policies must be considered mandatory to minimize antibiotic resistance. Practical approaches to antibiotic control include restricting the use of particular agents, specifically, defining indications for use or cycling classes of antibiotics to limit the selective pressure on nosocomial flora (25, 34).
ACKNOWLEDGMENT
This work was supported by National Health Service grant FIS 95/1234 from the Fondo de Investigación Sanitarias. C. Ardanuy was supported by National Health Service grants FIS 96/5176 and FIS 97/5243 from the Fondo de Investigación Sanitarias.
REFERENCES
- 1.Arlet G, Sanson-Le Pors M J, Rouveau M, Fournier G, Marie O, Schlemmer B, Philippon A. Outbreak of nosocomial infections due to Klebsiella pneumoniae producing SHV-4 β-lactamase. Eur J Clin Microbiol Infect Dis. 1990;9:797–803. doi: 10.1007/BF01967377. [DOI] [PubMed] [Google Scholar]
- 2.Arlet G, Rouveau M, Casin I, Buvet P J M, Lagrande P H, Philippon A. Molecular epidemiology of Klebsiella pneumoniae strains that produce SHV-4 β-lactamase and which were isolated in 14 French hospitals. J Clin Microbiol. 1994;32:2553–2558. doi: 10.1128/jcm.32.10.2553-2558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barza M, Giuliano M, Jacobos N V, Gorbach S L. Effect of broad-spectrum parenteral antibiotics on “colonization resistance” of intestinal microflora of humans. Antimicrob Agents Chemother. 1987;31:723–727. doi: 10.1128/aac.31.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauerfeind A, Rosenthal E, Eberlein E, Holley M, Schweighart S. Spread of Klebsiella pneumoniae producing SHV-5 β-lactamase among hospitalized patients. Infection. 1993;21:18–22. doi: 10.1007/BF01739303. [DOI] [PubMed] [Google Scholar]
- 5.Boyce J M. Treatment and control of colonization in the prevention of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:256–261. doi: 10.1086/647289. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson C, Legrand P, Philippon A, Montravers F, Ansquer M, Dural J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987;ii:302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, Meakins J L, Soussy C J, Lemaire F. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med. 1989;110:873–881. doi: 10.7326/0003-4819-110-11-873. [DOI] [PubMed] [Google Scholar]
- 8.Darfeuille-Michaud A, Jallat C, Aubel D, Sirot D, Rich C, Sirot J, Joly B. R-plasmid-encoded adhesive factor in Klebsiella pneumoniae strains responsible for human nosocomial infections. Infect Immun. 1992;60:44–55. doi: 10.1128/iai.60.1.44-55.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Champs C, Sauvant M P, Chanal C, Sirot D, Gazoy N, Malhuret R, Baguet J C, Sirot J. Prospective survey of colonization and infection caused by expended-spectrum β-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J Clin Microbiol. 1989;27:2887–2890. doi: 10.1128/jcm.27.12.2887-2890.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Martino P, Livreli V, Sirot D, Joly B, Darfeuille-Michaud A. A new fimbrial antigen harbored by CAZ-5/SHV-4-producing Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1996;64:2266–2273. doi: 10.1128/iai.64.6.2266-2273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen D, Russell E G, Tymms M, Roper E J, Grayson M L, Turnidge J. Random amplified polymorphic DNA and plasmid analyses used in investigation of an outbreak of multiresistant Klebsiella pneumoniae. J Clin Microbiol. 1995;33:713–717. doi: 10.1128/jcm.33.3.713-717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner J S, Jarvis W R, Emori T G, Horan T C, Hughes J M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 13.Gaynes R P, Weinstein R A, Smith J, Carman M, Kabins S A. Control of aminoglycoside resistance by barrier precautions. Infect Control. 1983;4:221–224. doi: 10.1017/s0195941700058264. [DOI] [PubMed] [Google Scholar]
- 14.Gouby A, Neuwirth C, Bourg G, Bouziges N, Carles-Nurit M J, Despaux E, Ramuz M. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J Clin Microbiol. 1994;32:301–305. doi: 10.1128/jcm.32.2.301-305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbert-Rogres L C F, Heritage J, Gascoyne-Binzi D M, Hawkey P M, Todd N, Lewis I J, Bailey C. Molecular epidemiology of ceftazidime resistant Enterobacteriaceae from patients on a paediatric oncology ward. J Antimicrob Chemother. 1995;36:65–82. doi: 10.1093/jac/36.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby G A, Medeiros A A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactams in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 18.Johnson A P, Weinbren M J, Ayling-Smith B, Du Bois S K, Amyes S G B, George R C. Outbreak of infection in two UK hospitals caused by a strain of Klebsiella pneumoniae resistant to cefotaxime and ceftazidime. J Hosp Infect. 1992;20:97–103. doi: 10.1016/0195-6701(92)90111-x. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall J R, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D, Mercier P, Thomas R, Villers D. A simplified acute physiologic score for ICU patients. Crit Care Med. 1984;12:975–977. doi: 10.1097/00003246-198411000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Legrand P, Fournier G, Buré A, Jarlier V, Nicolas M H, Decre D, Duval J, Philippon A. Detection of extended broad-spectrum beta-lactamases in Enterobacteriaceae in four french hospitals. Eur J Clin Microbiol Infect Dis. 1989;8:527–529. doi: 10.1007/BF01967473. [DOI] [PubMed] [Google Scholar]
- 21.Lucet J C, Chevres S, Decré D, Vanjak D, Macrez A, Bédos J P, Wolff M, Regnier B. Outbreak of multiply resistant Enterobacteriaceae in an intensive care unit: epidemiology and risk factors for acquisition. Clin Infect Dis. 1996;22:430–436. doi: 10.1093/clinids/22.3.430. [DOI] [PubMed] [Google Scholar]
- 22.Martin, C. M., N. S. Ikari, J. Zimmerman, and J. A. Naitz. 1971. A virulent nosocomial Klebsiella with a transferable R factor for gentamicin: emergence and suppression. J. Infect. Dis. 124(Suppl.):S24–S29. [DOI] [PubMed]
- 23.McCabe W R, Jackson G G. Gram-negative bacteremia. II. Clinical, laboratory, and therapeutic observations. Arch Intern Med. 1962;110:856–864. [Google Scholar]
- 24.McGowan J E. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 25.McGowan J E. Do intensive hospital antibiotic control programs prevent the spread of antibiotic resistance? Infect Control Hosp Epidemiol. 1994;15:478–483. doi: 10.1086/646954. [DOI] [PubMed] [Google Scholar]
- 26.Meyer K S, Urban C, Eagan J A, Berger B J, Rahal J J. Nosocomial outbreak of Klebsiella pneumoniae resistant to late-generation cephalosporins. Ann Intern Med. 1993;119:353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Montgomerie J Z. Epidemiology of Klebsiella and hospital-associated infections. Rev Infect Dis. 1979;5:736–753. doi: 10.1093/clinids/1.5.736. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Document M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 29.Naumovski L, Quinn J P, Miyashiro D, Patel M, Bush K, Singer S B, Graves D, Palzkill T, Arvin A M. Outbreak of ceftazidime resistance due to a novel extended-spectrum β-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992;36:1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordic Council on Medicines. Nordic statistics on medicines. Nordic drug index with classification and defined daily doses. Uppsala, Sweden: Nordic Council on Medicines; 1994. [Google Scholar]
- 31.Peña C, Pujol M, Ricart A, Ardanuy C, Ayats J, Liñares J, Garrigosa F, Ariza J, Gudiol F. Risk-factors for fecal carriage of Klebsiella pneumoniae producing extended-spectrum β-lactamases in the intensive care unit. J Hosp Infect. 1997;35:9–16. doi: 10.1016/s0195-6701(97)90163-8. [DOI] [PubMed] [Google Scholar]
- 32.Rice L B, Willey S H, Papanicolaou G A, Medeiros A A, Eliopoulos G M, Moellering R C, Jr, Jacoby G A. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990;34:2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice L B, Eckstein E C, De Vente J, Shlaes D M. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin Infect Dis. 1996;23:118–124. doi: 10.1093/clinids/23.1.118. [DOI] [PubMed] [Google Scholar]
- 34.Sanders W E, Jr, Sanders C C. Cycling of antibiotics: an approach to circumvent resistance in specialized units of the hospital. Clin Microbiol Infect. 1996;1:223–225. doi: 10.1016/s1198-743x(15)60278-6. [DOI] [PubMed] [Google Scholar]
- 35.Selden R, Lee S, Wang W L, Bennett J V, Eickhoff T C. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971;74:657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- 36.Sirot J, Chanal C, Petit A, Sirot D, Labia R, Gerbaud G. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated beta-lactamases markedly active against third-generation cephalosporins: epidemiologic studies. Rev Infect Dis. 1988;10:850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]
- 37.Van der Waaij D, de Vries Berghuis J M, Lekkerkerk van der Wees J E C. Colonization resistance of the digestive tract in conventional and antibiotic treated mice. J Hyg. 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]