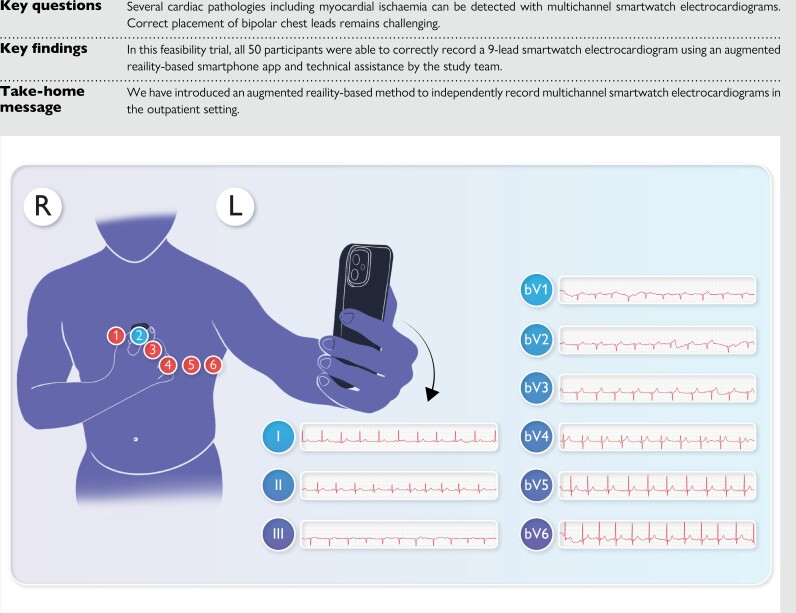
Abstract
Aims
It has been demonstrated that several cardiac pathologies, including myocardial ischaemia, can be detected using smartwatch electrocardiograms (ECGs). Correct placement of bipolar chest leads remains a major challenge in the outpatient population.
Methods and results
In this feasibility trial, we propose an augmented reality–based smartphone app that guides the user to place the smartwatch in predefined positions on the chest using the front camera of a smartphone. A machine-learning model using MobileNet_v2 as the backbone was trained to detect the bipolar lead positions V1–V6 and visually project them onto the user’s chest. Following the smartwatch recordings, a conventional 10 s, 12-lead ECG was recorded for comparison purposes. All 50 patients participating in the study were able to conduct a 9-lead smartwatch ECG using the app and assistance from the study team. Twelve patients were able to record all the limb and chest leads using the app without additional support. Bipolar chest leads recorded with smartwatch ECGs were assigned to standard unipolar Wilson leads by blinded cardiologists based on visual characteristics. In every lead, at least 86% of the ECGs were assigned correctly, indicating the remarkable similarity of the smartwatch to standard ECG recordings.
Conclusion
We have introduced an augmented reality–based method to independently record multichannel smartwatch ECGs in an outpatient setting.
Keywords: Electrocardiogram, Augmented reality, Smartwatch diagnostic, Machine learning, Digital health
Structured Graphical Abstract
Structured Graphical Abstract.
Introduction
Along with the increasing availability of smartwatches worldwide, health-related features of wearables such as photoplethysmography-based heart rate analysis and detection of atrial fibrillation are recognized, and some have already been implemented in the current clinical guidelines.1,2 Data on successful recording of 3- and 12-lead electrocardiograms (ECGs) using smartwatches have been reported.3–5 Furthermore, it has been demonstrated that detection of myocardial ischaemia and other cardiac pathologies using smartwatch ECGs is possible.4–7 Recording a 12-lead ECG in patients at the time of experiencing symptoms is known to be of paramount diagnostic importance. Currently, multichannel ECGs are barely available outside of healthcare settings, which limits remote diagnostic options. To overcome this limitation, novel technologies using prepositioned electrode strips, belts, and 12-lead ECG T-shirts have been proposed.8–10 These methods, however, require purchasing specific equipment and have not been established in standard care. Considering the increasing availability of smartwatches, developing a multichannel ECG protocol for these wearables might enhance diagnostic accuracy for symptomatic patients in an outpatient setting. Correct placement of unipolar leads without additional guidance and instruction, however, remains a major challenge if the user is not instructed by medical staff. At the same time, this is necessary to obtain correct ECG recordings, as the morphology of each lead varies with its location. To date, there is no standardized protocol for self-recorded multichannel smartwatch ECGs. Against this background, in this study, we propose an augmented reality (AR)-based smartphone app that guides the user to place the smartwatch in predefined positions on the chest using the front camera of the smartphone or a touchpad. The goal is to establish a method for self-recorded, instructed smartwatch ECGs and thereby facilitate early detection of cardiac disorders such as myocardial ischaemia in patients who do not have immediate access to full medical care. In this feasibility trial, we focus on the practical issues and limitations of our method as well as assessing the interpretability of the self-instructed ECG recordings when compared with conventional ECGs.
Methods
Study design and participants
This study was an investigator-initiated, single-centre prospective feasibility trial conducted at the University Hospital Basel. It was performed in compliance with the Declaration of Helsinki. Ethics approval was obtained from the local ethics committee (EKNZ BASEC 2020-02470). The trial was registered on clinicaltrials.gov (NCT05425342). Patients 18 years old or older who were able to record a smartwatch ECG and were hospitalized in the University Hospital Basel were eligible for enrolment. Exclusion criteria included allergic reactions, wounds or other local skin alterations that could interfere with the measurements, significant cognitive impairment, and prior knowledge or experience in recording ECGs. Participants were screened on-site and by using the electronic patient records of the university hospital.
The primary endpoint of the trial was the number of independently placed and recorded smartwatch ECG leads by patients. A correctly recorded lead was defined as a complete, 30 s long bipolar electrical signal obtained by the patient with the smartwatch. The secondary endpoints were correct identification of the heart rhythm, heart rate, heart axis, and changes in P waves, PQ interval, QRS complex, ST segments, and T waves in smartwatch ECGs, when compared with the standard 12-lead ECGs, by two independent cardiologists. The percentage of correctly assigned bipolar smartwatch chest leads to unipolar Wilson leads was also determined.
Consent
All participants provided written informed consent.
Test methods
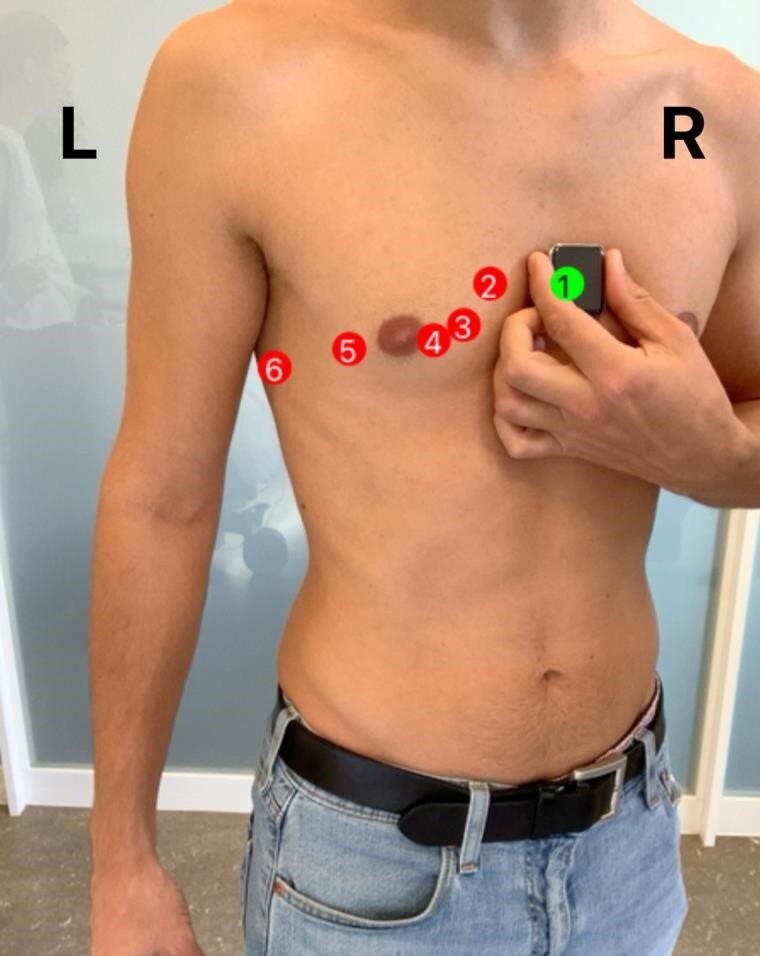
Clinical and demographic data, including medical history, were assessed through personal interviews and electronic patient records. Participants were then instructed on how to record an ECG on the smartwatch (Apple Watch Series 5; Apple Inc., Cupertino, CA, USA), and a general information sheet based on available literature was handed out (see Supplementary material online, Supplements S1 and S2). Einthoven leads were recorded as proposed by Spaccarotella et al.7 For the Wilson-like bipolar chest leads, a recently developed AR app (provided by the Department of Biomedical Engineering, University of Basel) was used. The app uses the front camera of an iPad Pro (Apple Inc.) to visualize the torso and marks the ECG positions V1–V6 (see Figure 1 and Supplementary material online, Video S1) using the model described below.
Figure 1.
Screenshot of the augmented reality mobile app. Using the Apple Core ML framework, the estimated positions of the bipolar chest leads bV1–bV6 are projected live to the anterolateral chest surface. Upon correct placement of the smartwatch, an optical feedback of colour change is provided, as shown at the position bV1.
Patients moved the smartwatch to the suggested position and received a red-green colour feedback as soon as they reached the correct position (see Figure 1 and Supplementary material online, Video S1). In each position, a 30 s ECG was recorded by the participants. In case of technical or positional errors, patients were corrected by the investigator and the assistance was precisely documented. Following the smartwatch recordings, a conventional 10 s, 12-lead ECG (Schiller Cardiovit AT-180m) was recorded by the study team, which was additionally trained for this purpose according to our hospital standards. The correct placement of the Wilson leads was guided by anatomical landmarks such as the intercostal spaces and the clavicle, which reflects our clinical practice. The standard ECG measurements were conducted by a medical professional in supine position, while the smartwatch ECGs were obtained while sitting and by the patients themselves.
Automatic electrocardiogram marker detection
For the automatic detection of the ECG marker V1–V6, we trained a machine-learning model to predict the position of V1–V6 directly from an image. Due to the real-time characteristic of the application, we used the MobileNet_v211 as the backbone. The MobileNet_v2 is a specific neural network architecture based on convolution neural networks12 and an inverted residual structure. It was specifically developed for mobile applications. The model is extended by two branches. The first branch estimates the position of the ECG markers with Tanh(BN(LN(RELU6(BN(LN(RELU6(BN(LN(1280, 256))), 128))), 12))) and the second branch Sigmoid(BN(LN(RELU6(BN(LN(DROP(RELU6(BN(LN(1280, 256))), 0.2),128))), 6))) predicts the visibility of the marker in the given image.
For the training of the model, we used 374 images from 28 female and 61 male subjects. For the evaluation, we used 44 images from 3 female and 7 male subjects. In each image, a medical expert manually labelled the position of the ECG landmarks V1–V6. The study examiner acquired the training and evaluation images with the back camera of a smartphone. For the final application, images will be acquired with the front camera. This makes it necessary to flip the images for the training. Furthermore, the images were cropped and scaled to a final size of 512 × 512 pixels. All colour channels of the images were normalized to a mean of 0.485, 0.485, 0.406 and an SD of 0.225, 0.225, 0.225. No dedicated test set was used as the images used for the training slightly differ from those that will be acquired during the examination. The performance of the final model was evaluated in a real-life scenario.
The complete model, with the pretrained MobileNet_v2 backbone, was trained with the Adam optimizer13 with a learning rate of 0.0001 using PyTorch by the PyTorch Foundation, a project of the Linux Foundation. During training, we used random translation, rotation, scale, scale-crop, changes to brightness, contrast and saturation, and iso-noise as data augmentation. We used the mean square error loss for the landmark position estimation and the binary cross entropy for the prediction of the label visibility. Finally, the trained model was converted to the Apple Core ML format to be used in the mobile application.
Electrocardiogram marker augmentation
The final application for the automatic detection of the ECG landmarks and the user feedback is implemented as a mobile app for Apple iOS and the Apple iPad Pro (Apple Inc.) using the Apple Core ML framework. There are two main parts of the application to support the user in placing the watch at the correct ECG landmark locations. The first part is the detection of the ECG landmarks from the image. Images are continuously acquired with the front camera of the iPad so that users can see themselves on display like a mirror. On each image, the trained model from above is used to predict the locations of the ECG landmarks. In order to prevent large changes in the landmark positions between consecutive images, we use a running average of the predicted locations. Finally, the predicted positions are overlaid onto the current image to show the user the correct location to place the watch. In the second step, we provide an optical feedback to the users if they place the watch at the correct location. To achieve this, the hand of the user is tracked with the hand pose estimation function of the Core ML framework. Using the position of the tip of the index finger and the thumb, we provide feedback to the user by changing the colour of the overlaid landmarks if the user places the watch at the correct position to acquire the ECG.
Analysis
The number of correctly recorded smartwatch ECG leads without assistance was determined and divided by the number of total attempts. The number of assistance interventions and their type (placement vs. technical assistance) was evaluated.
The smartwatch single-lead ECG recordings were compared with those of the conventional ECGs by two blinded independent board-certified cardiologists. In case of an analysis mismatch, a consensus decision was achieved. Sensitivity and specificity of the smartwatch ECG to determine the heart rhythm and detect atrial and ventricular conduction abnormalities were calculated. For this analysis, the conventional ECG interpretations by cardiologists were used as the gold standard. The 95% confidence intervals (CIs) were (95% CI LL, UL), where LL is the lower limit of the CI and UL is the upper limit.
The ratio of correctly identified bipolar chest ECG leads (bV1–bV6) was calculated as the number of successes divided by the sample size. An assignment was defined as correct if the bipolar lead was assigned to the same or a neighbouring unipolar Wilson lead. Results are expressed as means ± SD or in percentage.
Results
Baseline demographic and clinical characteristics of participants
A total of 54 patients were screened, of which 4 were excluded due to prior experience in recording ECGs. Of the 50 patients enrolled, 25 (50%) were males, 34 (68%) had a history of hypertension, 26 (52%) had vascular disease, and 34 (74%) had arrhythmia. The mean age of the participants was 66.2 ± 10.2 years at the time of inclusion. The demographic and clinical characteristics are given in Table 1.
Table 1.
Demographic and clinical characteristics of the participants
| Demographic characteristics | |
| Age mean (years) | 66.2 (10.2) |
| Gender | |
| Male/female | 25/25 (50/50) |
| Clinical characteristics | |
| Congestive heart failure history | 14 (28%) |
| Hypertension | 34 (68%) |
| Stroke/TIA/thromboembolism history | 8 (16%) |
| Vascular disease history | 26 (52%) |
| Diabetes history | 10 (20%) |
| Arrhythmia history | 37 (74%) |
Data are given as means (SD) or n (%).
Primary endpoint: correctly recorded smartwatch electrocardiogram without assistance and number and type of assistance intervention
Seventy-eight per cent of the participants were able to independently record Einthoven Lead I. For Leads II and III, 80 and 68% of patients could successfully carry out the measurement, respectively. The bipolar Wilson-like chest leads bV1–bV6 are presented in Table 2. Altogether, 24% of patients were able to record all limb and chest leads without any assistance from the study team. The rest of the patients needed at least one assistance intervention to conduct the measurements. A total of 42% of patients required assistance interventions related to incorrect lead placement, whereas 48% of them needed assistance due to technical problems such as premature termination of the ECG, pressing the digital crown, or failing to maintain skin contact during the recording. The total number of assistance interventions was 94, corresponding to 1.9 ± 1.7 interventions per patient per complete 9-lead ECG. The maximum number of assistance interventions for one patient was 7.
Table 2.
Number of correctly recorded chest leads (b: bipolar)
| Lead | bV1 | bV2 | bV3 | bV4 | bV5 | bV6 |
| Correctly recorded lead, n (%) | 32 (64) | 43 (86) | 46 (92) | 50 (100) | 42 (84) | 40 (80) |
Secondary endpoint: interpretation of the smartwatch electrocardiogram
Rhythm
Of the 50 participants, 41 (82%) had sinus rhythm in the conventional 12-lead ECG. Atrial fibrillation was detected in 7 cases (14%) in smartwatch ECGs, of which all were confirmed with the gold standard indicating a 100% sensitivity for detecting this arrhythmia (Table 3). Specificity for atrial fibrillation, however, was slightly lower at 93% (95% CI 81, 98). Sensitivity and specificity for other arrhythmias are shown in Table 3.
Table 3.
Heart rhythm as determined by two independent cardiologists
| Pathology | n | n SW | Sens. | 95% CI (LL, UL) | Spec. | 95% CI (LL, UL) |
|---|---|---|---|---|---|---|
| Atrial fibrillation | 7 | 10 | 100 | (65, 100) | 93 | (81, 98) |
| Atrial flutter | 2 | 2 | 50 | (9, 91) | 98 | (89, 100) |
| Junctional rhythm | 0 | 0 | — | (–,–) | 100 | (93, 100) |
| Pacemaker rhythm | 3 | 2 | 33 | (6, 79) | 98 | (89, 100) |
| Supraventricular premature beats |
0 | 3 | — | (–,–) | 94 | (84, 98) |
| Ventricular premature beats | 2 | 8 | 100 | (34, 100) | 88 | (75, 94) |
n, arrhythmias found in the standard ECG; n SW, arrhythmias found in the smartwatch ECG.
Heart rate
The normal heart rate was considered 60–100 b.p.m. Bradycardia was defined as <60 b.p.m., tachycardia as >100 b.p.m. Thirty-nine patients (78%) had a normal heart rate in the 12-lead ECGs. Bradycardia was identified in 5 (10%) of the standard and in none of the smartwatch ECGs. Tachycardia, on the other hand, was found in 6 (12%) cases and could be detected by the smartwatch with a sensitivity of 83% (95% CI 44, 97) and specificity of 80% (95% CI 65, 89).
Heart axis
The heart axis, describing the main direction of electrical heart activity, was assessed using the Cabrera system. The normal heart axis was defined as −30° to +90°. A normal heart axis was found in 40 cases (80%). Left and extreme heart axis were found in 7 (14%) and 3 (6%) cases, respectively. In the smartwatch ECGs, a sensitivity of 57% (95% CI 25, 84) and a specificity of 100% (95% CI 92, 100) for the detection of the left heart axis were calculated. Extreme heart axis was detected with a sensitivity of 67% (95% CI 21, 94) and specificity of 98% (95% CI 89, 100).
P waves and PQ interval
P waves and PQ interval were analysed in the standard and smartwatch ECGs in order to assess atrial and atrioventricular conduction. The cardiologists analysed P waves and PQ interval in arbitrarily chosen leads based on individual morphologies. Physiologic P waves were found in 41 of the cases (82%). P mitrale was diagnosed in two cases, none of which was detected in the smartwatch ECGs. Single cases of atrioventricular blocks I° and III° were identified in both 12-lead and smartwatch ECGs.
QRS complex
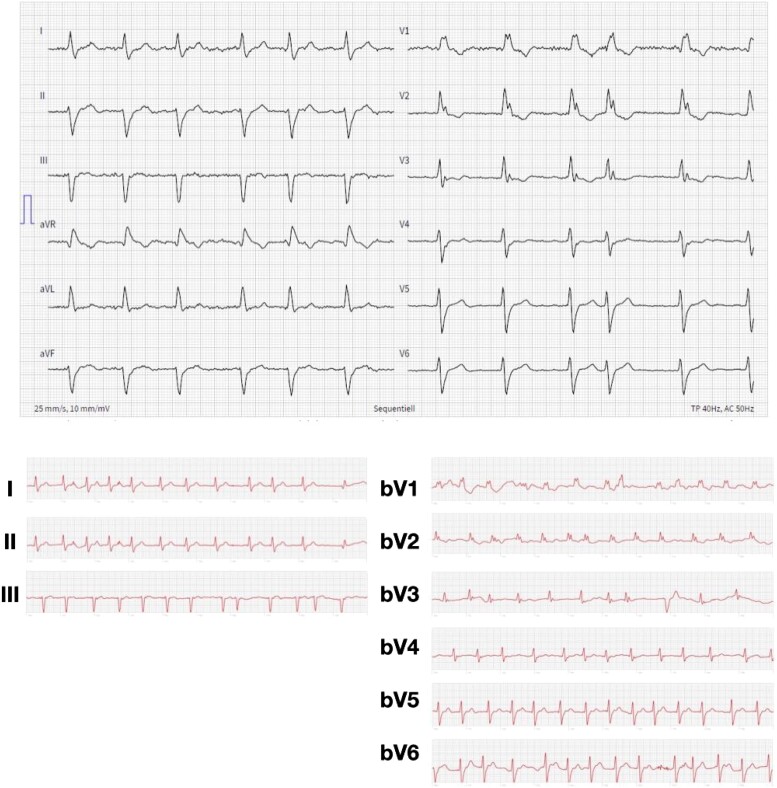
A wide QRS complex was defined as >100 ms and was detected in 5 (10%) cases. Sensitivity of detecting wide QRS complex in the smartwatch ECGs was 60% (95% CI 23, 88) and a specificity of 98% (95% CI 88, 100) was calculated. In some cases, bundle branch blocks, fascicular blocks, and pathologic Q waves were detected (Table 4). An example of a bifascicular block is shown in Figure 2.
Table 4.
QRS complex analysis by two independent cardiologists
| Pathology | n | n SW | Sens. | 95% CI (LL, UL) |
Spec. | 95% CI (LL, UL) |
|---|---|---|---|---|---|---|
| Wide QRS complex (>100 ms) | 5 | 4 | 60 | (23, 88) | 98 | (88, 100) |
| Left bundle branch block | 3 | 2 | 33 | (6, 79) | 98 | (89, 100) |
| Right bundle branch block | 4 | 4 | 75 | (30, 95) | 98 | (89, 100) |
| Left anterior fascicular block | 1 | 1 | 0 | (0, 79) | 98 | (89, 100) |
| Left posterior fascicular block | 0 | 0 | — | (–,–) | 100 | (93, 100) |
| Bifascicular block | 0 | 0 | — | (–,–) | 100 | (93, 100) |
| Pathologic Q waves | 1 | 0 | 0 | (0, 79) | 100 | (93, 100) |
| Poor R-wave progression | 6 | 5 | 0 | (0, 39) | 89 | (76, 95) |
| Signs of left heart hypertrophy (Sokolow–Lyon criteria) | 1 | 3 | 100 | (21, 100) | 96 | (86, 99) |
| Signs of right heart hypertrophy (Sokolow–Lyon criteria) | 1 | 0 | 0 | (0, 79) | 100 | (93, 100) |
n, number of pathologies found in the standard ECG; n SW, number of pathologies found in the smartwatch ECG.
Figure 2.
Example of a 86-year-old female patient with history of hypertension and vascular disease presenting with a bifascicular block (right bundle branch block and a left anterior fascicular block) in the standard 12-lead electrocardiogram (upper panel). Accordingly, resembling QRS morphology and axis deviation were identified in the self-recorded smartwatch electrocardiogram (lower panel). The interval 10–20 s of the smartwatch recordings are presented.
ST segments and T waves
A thorough analysis of ST segments and T waves was performed to assess repolarization abnormalities.
In total, 6 patients (12%) with ST elevation were identified. One of these was detected in the smartwatch ECGs (Table 5), indicating a sensitivity of 17% (95% CI 3, 56) and a specificity of 95% (95% CI 85, 99). ST depression was detected in 13 cases (26%) in the 12-lead ECGs. The sensitivity and specificity for detecting ST depressions in the smartwatch ECGs were 38% (95% CI 18, 64) and 95% (95% CI 82, 99; Table 5). Pathologic T waves were identified in 16 patients (32%) in at least one lead. The sensitivity of detecting this anomaly was 31% (95% CI 14, 56). A specificity of 97% (95% CI 85, 99) for detecting pathologic T waves was noted.
Table 5.
ST segment and T-wave analysis by two independent cardiologists
| Pathology | n | n SW | Sens. | 95% CI (LL, UL) |
Spec. | 95% CI (LL, UL) |
|---|---|---|---|---|---|---|
| ST segment elevation | 6 | 3 | 17 | (3, 56) | 95 | (85, 99) |
| ST segment depression | 13 | 7 | 38 | (18, 64) | 95 | (82, 99) |
| Pathologic T waves | 16 | 6 | 31 | (14, 56) | 97 | (85, 99) |
n, number of pathologies found in the standard ECG; n SW, number of pathologies found in the smartwatch ECG.
Assignment of bipolar smartwatch chest leads to unipolar Wilson leads
Bipolar chest leads recorded with smartwatch ECGs were assigned to standard unipolar Wilson leads by cardiologists based on visual characteristics. In every lead, at least 86% of the ECGs were assigned correctly. bV2 was the most correctly identified lead (92 and 96% by Cardiologists 1 and 2, respectively). The anterior leads bV3 and bV4 had the lowest accuracy of assignment (Table 6). The smartwatch chest leads were most commonly erroneously assigned to the contiguous leads, as shown in Table 6. Two of the 300 chest leads recorded could not be interpreted by Cardiologist 2 due to motion artefacts.
Table 6.
Number of correct bipolar smartwatch chest lead assignments to unipolar Wilson leads and the most commonly erroneously assigned chest leads by two independent cardiologists
| Smartwatch chest lead | Cardiologist 1 | Cardiologist 2 | Most commonly erroneously assigned chest leads |
|---|---|---|---|
| bV1 | 45 (90) | 42 (86) | V2 |
| bV2 | 46 (92) | 47 (96) | V1 |
| bV3 | 41 (82) | 46 (92) | V2, V4 |
| bV4 | 44 (88) | 43 (86) | V3 |
| bV5 | 44 (88) | 48 (96) | V6 |
| bV6 | 38 (76) | 47 (94) | V5 |
The number in the parenthesis is the percentage of correct assignments.
There were no adverse events related to the use of the standard or the smartwatch ECGs.
Discussion
There is emerging evidence that smartwatch ECGs may contribute to the out-of-hospital diagnosis of cardiac disease, particularly arrhythmias, possibly even myocardial ischaemia. However, obtaining a multichannel smartwatch ECG remains challenging. The present feasibility study describes a novel method to facilitate the conduction of a multilead smartwatch ECGs using an AR approach. The goal was to develop a tool for patients to obtain a nine-lead smartwatch ECG.
Despite the advanced age of participants (66.2 ± 10.2 years), we have found that the majority was able to record an interpretable nine-lead ECG using the AR technology and some technical assistance. As expected, the Einthoven leads were less challenging to obtain than the bipolar precordial leads. Our findings generally support the hypothesis that certain cardiac pathologies may be identified with smartwatch ECGs.
The agreement of ST segment changes between smartwatch and standard ECG in patients with acute ST elevation myocardial infarction has previously been reported by Spaccarotella et al.7 Our data show that ST segment changes in asymptomatic patients could not be reliably detected by the smartwatch ECGs. However, considering the feasibility nature of the study, the low number of participants does not allow a conclusive evaluation. Overall, the visual characteristics of the smartwatch leads markedly resemble those of the standard ECG. This was demonstrated by the fact that the vast majority of smartwatch ECG leads could be assigned to the respective or neighbouring standard leads. These findings suggest that future use of the AR app may allow patients to record a self-instructed high-quality smartwatch ECGs in an out-of-hospital setting.
We observed a remarkable discrepancy in the number of participants with bradycardia between the smartwatch ECG and the standard ECG. We assume that the recording conditions account for this difference: while standard 12-lead ECGs were obtained in the supine position requiring no mental or physical effort by the participant, the smartwatch ECGs are recorded in the sitting position, requiring active participation of the patients and are recorded for a period of 30 s per lead as opposed to the standard 10 s ECG. Hence, the difference between the two recordings was reflecting a physiological difference and not a technical problem.
The trial has demonstrated that most participants required some instructions to record a self-instructed ECG with the present version of the AR app. This is why the authors would recommend supplying potential users with a professional video clip that demonstrates the correct use and gives instructions before they use the app. Further improvements to better guide the users during the acquisition of the ECGs include feedback by sound, depicted signs and vibration alerts originating directly from the smartwatch. Also, the implementation of a nine-lead smartwatch ECG report, similar to the one depicted in Figure 2, is under consideration. Furthermore, implementation of automated evaluation of the smartwatch ECGs using the K-means clustering algorithm or two-event related moving-averages or fractional-Fourier-transform algorithms14,15 would further enhance diagnostic accuracy.
There are several limitations to the study. First, the study population consists of hospitalized patients, which limits interpretability for the general population that may be able to record a smartwatch ECG at home. A general limitation of multichannel smartwatch ECGs is the sequential recording of leads, as opposed to the standard 12-lead ECGs, where all leads are obtained simultaneously. In traditional 12-lead surface electrocardiography, the Wilson’s Central Terminal (WCT), an artificially constructed reference point, is defined as the simple average of the limb leads.16 In smartwatch ECG recordings, reconstruction of the WCT and the augmented leads aVF, aVL, and aVR remains challenging due to heart rate and heart rate variability discrepancies between the sequential limb measurements. However, computing leads III, aVF, aVL, and aVR is possible after aligning the R-peaks in I and II (see Supplementary material online, Supplement S3). Implementing this protocol could, after appropriate validation, further enhance diagnostic accuracy and facilitate the recording of a 12-lead smartwatch ECG. The feasibility nature of the study allows no conclusive assessment of the smartwatch ECG interpretation due to the low number of participants and pathologic ECG findings. The AR algorithm was based on a neural network and was trained on the images of the participants who placed their arms straight to their body. This might reduce the quality of the landmark tracking during the application where landmarks can be occluded by the hands of the participants. Extending the training data set with such type of images may improve the quality of the landmark tracking. In the present study, we used an iPad Pro to run the AR app due to technical reasons. A future version of the app may be installed on any mobile device with a front camera. Further training of the AR algorithm will allow us to specify the lead positions and improve the recording quality. Studies in different settings are needed to assess the feasibility of this AR approach in a non-supervised outpatient setting. In this feasibility study, we introduced a novel AR-based method to independently record multichannel smartwatch ECGs. In this era of emerging telemedicine applications, this AR-based technology may play a complementary role in improving out-of-hospital diagnostics of cardiac disease.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Supplementary Material
Contributor Information
Peter Daniel Serfözö, Department of Digitalisation and ICT, Chief Medical Information Officer (CMIO) Office, University Hospital Basel, Hebelstrasse 10, Basel 4031, Switzerland.
Robin Sandkühler, Department of Biomedical Engineering, Center for Medical Image Analysis and Navigation, University of Basel, Gewerbestrasse 14, Allschwil 4123, Switzerland.
Bibiana Blümke, Department of Digitalisation and ICT, Chief Medical Information Officer (CMIO) Office, University Hospital Basel, Hebelstrasse 10, Basel 4031, Switzerland.
Emil Matthisson, Department of Digitalisation and ICT, Chief Medical Information Officer (CMIO) Office, University Hospital Basel, Hebelstrasse 10, Basel 4031, Switzerland.
Jana Meier, Department of Digitalisation and ICT, Chief Medical Information Officer (CMIO) Office, University Hospital Basel, Hebelstrasse 10, Basel 4031, Switzerland.
Jolein Odermatt, Department of Digitalisation and ICT, Chief Medical Information Officer (CMIO) Office, University Hospital Basel, Hebelstrasse 10, Basel 4031, Switzerland.
Patrick Badertscher, Department of Cardiology, University Hospital Basel, Petersgraben 4, Basel 4031, Switzerland.
Christian Sticherling, Department of Cardiology, University Hospital Basel, Petersgraben 4, Basel 4031, Switzerland.
Ivo Strebel, Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, Spitalstrasse 2, Basel 4056, Switzerland.
Philippe C Cattin, Department of Biomedical Engineering, Center for Medical Image Analysis and Navigation, University of Basel, Gewerbestrasse 14, Allschwil 4123, Switzerland.
Jens Eckstein, Department of Digitalisation and ICT, Chief Medical Information Officer (CMIO) Office, University Hospital Basel, Hebelstrasse 10, Basel 4031, Switzerland; Department of Internal Medicine, University Hospital Basel, Petersgraben 4, Basel 4031, Switzerland.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Complementary data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Turakhia MP, Desai M, Hedlin H, Rajmane A, Talati N, Ferris T, et al. . Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am Heart J 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dörr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, et al. . The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol 2019;5:199–208. [DOI] [PubMed] [Google Scholar]
- 3. Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Recording of bipolar multichannel ECGs by a smartwatch: modern ECG diagnostic 100 years after Einthoven. Sensors (Basel) 2019;19:2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: a new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors (Basel) 2019;19:4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobos Gil M. Standard and precordial leads obtained with an Apple Watch. Ann Intern Med 2020;172:436–437. [DOI] [PubMed] [Google Scholar]
- 6. Avila CO. Novel use of Apple Watch 4 to obtain 3-lead electrocardiogram and detect cardiac ischemia. Perm J 2019;23:19-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spaccarotella CAM, Polimeni A, Migliarino S, Principe E, Curcio A, Mongiardo A, et al. . Multichannel electrocardiograms obtained by a smartwatch for the diagnosis of ST-segment changes. JAMA Cardiol 2020;5:1176.– . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang R-K. Resting 12-lead ECG tests performed by patients at home amid the COVID-19 pandemic - results from the first 1000 patients. J Electrocardiol 2022;73:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boehm A, Yu X, Neu W, Leonhardt S, Teichmann D. A novel 12-lead ECG T-shirt with active electrodes. Electronics (Basel) 2016;5:75. [Google Scholar]
- 10. Steijlen AS, Jansen KM, Albayrak A, Verschure DO, Van Wijk DF. A novel 12-lead electrocardiographic system for home use: development and usability testing. JMIR Mhealth Uhealth 2018;6:e10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandler M, Howard A, Zhu M, Zhmoginov A, Chen L. MobileNetV2: inverted residuals and linear bottlenecks. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, 2018.
- 12. Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Advances in Neural Information Processing Systems, 2012.
- 13. Kingma DP, Ba J. Adam: a method for stochastic optimisation. arXiv preprint arXiv:1412.6980v9 [cs.LG]. 2014.
- 14. Balouchestani M, Krishnan S. Advanced K-means clustering algorithm for large ECG data sets based on a collaboration of compressed sensing theory and K-SVD approach. SIViP 2016;10:113–120. [Google Scholar]
- 15. Aziz S, Ahmed S, Alouini MS. ECG-based machine-learning algorithms for heartbeat classification. Sci Rep 2021;11:18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malmivuo J, Plonsey R. Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields. Oxford, USA: Oxford University Press; 1995. 10.1093/acprof:oso/9780195058239.001.0001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Complementary data that support the findings of this study are available from the corresponding author upon reasonable request.