Abstract
The neurotrophic herpes virus cytomegalovirus is a known cause of neuropathology in utero and in immunocompromised populations. Cytomegalovirus is reactivated by stress and inflammation, possibly explaining the emerging evidence linking it to subtle brain changes in the context of more minor disturbances of immune function. Even mild forms of traumatic brain injury, including sport-related concussion, are major physiological stressors that produce neuroinflammation. In theory, concussion could predispose to the reactivation of cytomegalovirus and amplify the effects of physical injury on brain structure. However, to our knowledge this hypothesis remains untested.
This study evaluated the effect of cytomegalovirus serostatus on white and grey matter structure in a prospective study of athletes with concussion and matched contact-sport controls.
Athletes who sustained concussion (n = 88) completed MRI at 1, 8, 15 and 45 days post-injury; matched uninjured athletes (n = 73) completed similar visits. Cytomegalovirus serostatus was determined by measuring serum IgG antibodies (n = 30 concussed athletes and n = 21 controls were seropositive). Inverse probability of treatment weighting was used to adjust for confounding factors between athletes with and without cytomegalovirus. White matter microstructure was assessed using diffusion kurtosis imaging metrics in regions previously shown to be sensitive to concussion. T1-weighted images were used to quantify mean cortical thickness and total surface area. Concussion-related symptoms, psychological distress, and serum concentration of C-reactive protein at 1 day post-injury were included as exploratory outcomes. Planned contrasts compared the effects of cytomegalovirus seropositivity in athletes with concussion and controls, separately.
There was a significant effect of cytomegalovirus on axial and radial kurtosis in athletes with concussion but not controls. Cytomegalovirus positive athletes with concussion showed greater axial (P = 0.007, d = 0.44) and radial (P = 0.010, d = 0.41) kurtosis than cytomegalovirus negative athletes with concussion. Similarly, there was a significant association of cytomegalovirus with cortical thickness in athletes with concussion but not controls. Cytomegalovirus positive athletes with concussion had reduced mean cortical thickness of the right hemisphere (P = 0.009, d = 0.42) compared with cytomegalovirus negative athletes with concussion and showed a similar trend for the left hemisphere (P = 0.036, d = 0.33). There was no significant effect of cytomegalovirus on kurtosis fractional anisotropy, surface area, symptoms and C-reactive protein.
The results raise the possibility that cytomegalovirus infection contributes to structural brain abnormalities in the aftermath of concussion perhaps via an amplification of concussion-associated neuroinflammation. More work is needed to identify the biological pathways underlying this process and to clarify the clinical relevance of this putative viral effect.
Keywords: cytomegalovirus, traumatic brain injury, diffusion kurtosis imaging, inflammation, sport-related concussion
Cytomegalovirus is a common neurotrophic virus that results in lifelong latent infections. Savitz et al. report that cytomegalovirus serostatus is associated with differences in brain structure in athletes with concussion, raising the possibility that latent neurotropic viruses can moderate the effects of traumatic brain injury.
Introduction
Human cytomegalovirus (CMV) is a common neurotrophic herpes virus with an age-adjusted seroprevalence of ∼50% in the USA.1 CMV establishes lifelong latent infections in a variety of cell types, including myeloid lineage cells, vascular pericytes, endothelial cells of the blood–brain barrier, glia, neurons and neuronal precursor cells.2-5 Periods of physiological or psychological stress6-8 and/or inflammation9-11 promote reactivation of the virus via activation of the sympathetic nervous system (SNS)6,12 and AP-1 or NF-κB-induced transcription of the immediate early (IE) CMV promoter,13,14 respectively. This reactivation is usually considered to be benign but may cause serious disease, including neurological sequelae, in individuals who are immunocompromised due to HIV infection, immune suppressive therapy or congenital immunodeficiencies.15,16 Emerging research suggests that reactivation of CMV may also have negative consequences in contexts where the disturbance in immune function is more subtle such as sepsis,17,18 autoimmune disease,19-22 old age23-26 and psychiatric disorders.27-32 With respect to psychiatric disorders, we recently showed that individuals with major depressive disorder (MDD) who were CMV seropositive had reduced grey matter volume, decreased white matter integrity, and altered resting state functional connectivity compared to propensity-matched CMV seronegative participants with MDD.30-32 Similarly, CMV-positive participants with schizophrenia were shown by independent groups to have smaller hippocampi and total cortical surface area compared to CMV-negative participants with schizophrenia.27-29 These data raise the possibility that CMV reactivation has an adverse effect on brain structure in the context of disorders that lead to activation of the SNS and/or the immune system.
Traumatic brain injury (TBI), including sport-related concussion (SRC), is a major physiological stressor that activates the SNS33,34 and induces both neuroinflammatory and systemic inflammatory responses.35-37 Moreover, neurotrauma is a well established cause of systemic immune suppression that increases the risk of subsequent infection.38-40 In theory, these post-injury changes in autonomic and immunological function could predispose to the reactivation of CMV, which could in turn affect brain structure. However, to our knowledge, this hypothesis remains untested in the TBI field. The goal of this work was thus to determine if CMV serostatus moderated the physiological effects of concussion in athletes. Our primary analysis focused on diffusion kurtosis metrics in white matter given that we previously reported abnormalities in these metrics in athletes with SRC in this sample41 and given that alterations in diffusion MRI are one of the most commonly reported sequelae of concussion because of the diffuse injury to white matter that is known to occur.42,43 Cortical thickness and surface area were evaluated in secondary analyses while symptoms and the commonly used, non-specific marker of inflammation, C-reactive protein (CRP), were exploratory outcomes. We hypothesized that CMV seropositivity would be associated with reductions in white matter microstructural integrity, cortical thickness, and surface area in athletes with concussion, but not controls.
Materials and methods
Participants
This study is a secondary analysis of data from a prospective study of concussion in high school and collegiate American football players enrolled between August 2015 and June 2018, described elsewhere.41,44 Parents of minors and adult participants provided written informed consent. Minor participants provided written assent. Study procedures were approved by the Medical College of Wisconsin Institutional Review Board and the US Department of Defense Human Research Protection Office (HRPO).
Exclusion criteria for the parent study included contraindication or injury precluding participation in the study protocol; current narcotic use or psychotic disorder; or conditions known to cause cognitive dysfunction such as moderate-to-severe TBI or epilepsy. American football players participated in pre-season baseline clinical assessments. Athletes sustaining a concussion completed up to four follow-up visits with an MRI session and blood collection, including at 24–48 h (1 day) and 8, 15 and 45 days post-injury. Team physicians or certified athletic trainers identified and diagnosed concussions. The study team screened injuries to ensure they met the study definition of concussion, which was based on the Centers for Disease Control and Prevention HEADS UP initiative:
‘An injury resulting from a forceful bump, blow, or jolt to the head that results in rapid movement of the head and causes a change in the athlete’s behavior, thinking, physical functioning, or the following symptoms: headache, nausea, vomiting, dizziness/balance problems, fatigue, difficulty sleeping, drowsiness, sensitivity to light/noise, blurred vision, memory difficulty, and difficulty concentrating.’
American football players without concussion in the prior 6 months were enrolled as controls and completed similar visits, matched to injured athletes based on level of competition, institution, team, age, estimated intellectual functioning (word reading performance at baseline), race, handedness, concussion history and position. Additional inclusion criteria for the current study included providing consent/assent to allow blood samples to be banked for future analyses and the inclusion in our prior work investigating diffusion kurtosis imaging metrics in this cohort.41
Clinical data
Demographic information, health history and the Wechsler Test of Adult Reading (WTAR; to estimate intellectual functioning) were collected at pre-season baseline. An estimate of socioeconomic status (SES) was calculated using the modified Hollingshead Four Factor Index.45 Concussion-related symptom severity assessed using The Sport Concussion Assessment Tool-Third Edition (SCAT) and symptoms of psychological distress assessed using the Brief Symptom Inventory-18 Global-Severity Index (BSI-GSI) at the 1-day visit were included to characterize the clinical effects of SRC.
C-reactive protein and CMV IgG antibodies
Venous blood was collected in BD Vacutainer® serum tubes, processed following manufacturer’s instructions, and stored at −80°C. Serum concentrations of CRP were measured in duplicate using Meso Scale Discovery V-PLEX assays on a QuickPlex SQ 120 instrument following the manufacturer’s instructions, as previously described.44 One sample had CRP levels above detection limits and was excluded from further analysis. The current study focused on CRP concentrations at the 1-day visit. Finally, a single post-injury visit sample from each participant was used to detect IgG CMV antibodies using a commercially available semi-quantitative enzyme-linked immunosorbent assay (ELISA) (IBL America). A sample was considered CMV seropositive if it had an optical density (OD) value of 20% over the supplied cut-off standard, equivalent to ∼10 international antibody units. All biomarker assays were performed blind to concussion status.
MRI acquisition, processing and metrics
MRI data acquisition has been previously described.41 MRI data were collected on a General Electric MR750 whole body 3 T scanner using a 32-channel head coil. T1-weighted images were collected using a magnetization-prepared rapid gradient-echo sequence with 1 mm isotropic voxels. Diffusion weighted images covering the whole brain were obtained using a single-shot spin-echo echo-planar sequence with 3 mm isotropic voxels and b-values of 1000 and 2000s/mm2 with 30 directions for each b-value.
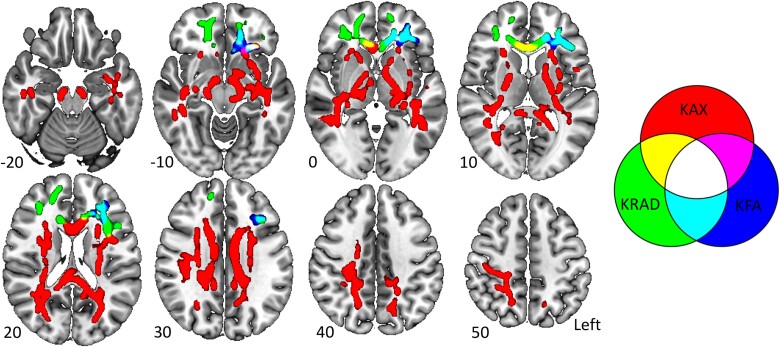
Processing of diffusion data included susceptibility distortion correction, motion correction and eddy current correction using FSL version 6.0.0.46,47 Kurtosis metrics were calculated via the DKE software48,49 with the robust option to detect and remove outliers during model fitting.50 In our prior work, the tract-based spatial statistics program51 was used to identify differences in diffusion kurtosis metrics between athletes with and without concussion at each post-injury visit.41 We previously reported increased axial kurtosis in the corpus callosum, genu and splenium in the SRC group relative to controls at the 1 day time point, which spread to the corticospinal tracts, superior longitudinal fasciculus and inferior longitudinal fasciculus, bilaterally, by 8 days post-injury.41 In contrast, radial kurtosis was significantly reduced in the SRC group relative to controls. The changes in radial kurtosis were also more delayed, becoming evident at Day 15 in the major white matter tracts of the frontal cortex.41 Finally, the pattern of changes in kurtosis fractional anisotropy resembled that of radial kurtosis emerging at Day 15 and persisting to Day 45 predominantly in the frontal cortex. Here, we aimed to evaluate the moderating effects of CMV on white matter microstructural integrity following SRC. We therefore selected three different regions of interest (ROIs; one for each metric) based on the largest cluster of significant voxels at the visit with the largest difference between groups in our previous work.41 Specifically, the axial kurtosis ROI was obtained from the 8 day comparison while the radial kurtosis and kurtosis fractional anisotropy ROIs were obtained from the 15 day comparisons. The average axial kurtosis, radial kurtosis and kurtosis fractional anisotropy from within these ROIs were then obtained in all athletes at each post-injury visit for subsequent analysis. ROIs are illustrated in Fig. 1.
Figure 1.
Diffusion kurtosis imaging regions of interest. Regions of interest (ROIs) from the skeletonized data have been inflated using tbss_fill for visualization. The Axial kurtosis (KAX) ROI, the kurtosis-fractional anisotropy (KFA) ROI, the radial kurtosis (KRAD) ROI, and areas of overlap between ROIs are displayed. Z-coordinates are displayed for each slice. Images are displayed using radiological convention. The figure was made using MRIcroGL.
Cortical reconstruction and volume segmentation of T1-weighted images was conducted using the longitudinal processing stream in FreeSurfer version 5.3.52-54 Mean cortical thickness was estimated for each hemisphere. Total cortical surface area for each hemisphere was calculated as the sum of individual surface areas from cortical regions defined by the Desikan-Killiany atlas for that hemisphere.55,56 The test-retest reliability and segmentation accuracy of FreeSurfer have been previously established,57-59 and the same processing pipeline and quality assessment procedures were applied to all participants. All MRI processing was conducted blind to CMV status. Although analyses were not technically blind to injury group, as it could be deduced from subject labels, the same processing pipeline and quality control procedures were applied to all participants and no manual edits were made to generated outputs to avoid potential bias. Of the 558 total MRI datasets included in this study, 19 failed quality assessment procedures (e.g. due to excessive head motion on the T1-weighted image) and were excluded from FreeSurfer analyses.
Statistical analysis
Statistical analyses were performed using SPSS version 27 unless otherwise specified. Participant matching in the parent study was focused on matching athletes with concussion to controls; athletes were not matched upon enrolment based on CMV serostatus. Therefore, inverse probability of treatment weighting (IPTW) was used to adjust for confounding factors between each unique athlete with and without CMV following recommended guidelines, as in our prior work in other clinical populations.31,32,60 The propensity score was defined as the likelihood of being CMV-positive (CMV+) based on multivariate logistic regression with the following variables in R: body mass index (BMI), age, socioeconomic status (SES), ethnicity, race, WTAR standard score, and prior number of concussions. Prior to IPTW, mean imputation of one WTAR standard score was conducted for a single participant that did not complete WTAR testing. Generalized linear models (GLM) compared demographic variables between SRC and controls for descriptive purposes. Similarly, GLM were used to test for group differences in CMV+ and CMV-negative (CMV−) before and after IPTW to assess covariate balance.
A priori analyses focused on the effect of CMV within each group (i.e. concussed and controls) for outcome measures of interest. For primary (kurtosis metrics) and secondary outcomes (left and right hemisphere thickness and area), planned contrasts were specified within the confines of linear mixed-effect models. These models included the effects of visit (modelled as a repeated factor across participants), injury group (SRC or control), CMV status (positive or negative), all two-way interactions, and the three-way interaction while accounting for estimated propensity weights. Estimated intracranial volume was included as a covariate for surface area analyses. A limited number of participants did not complete MRI sessions at all visits (Supplementary material); mixed models can include these participants with the missing at random assumption. Analysis of exploratory markers (CRP and symptoms) was limited to the 1-day visit using GLM to compare CMV+ versus CMV− within SRC and controls, separately, accounting for propensity weights. Because symptoms were often not endorsed or were endorsed at low levels in the control group, SCAT symptom severity and BSI-GSI scores were binarized (absent/present) and a GLM was fitted using a binomial distribution with logit link to compare controls with and without CMV. For SCAT and BSI-GSI (T-scores) in patients with SRC, as well as CRP across each group, a GLM with a normal distribution was used. CRP levels were log transformed to normalize their distribution prior to analysis. Bonferroni correction was used to account for multiple comparisons within each level of analyses (primary analyses: P < 0.017 for three tests; secondary analyses: P < 0.0125 for four tests; exploratory analyses: P < 0.017 for three tests). All results are reported from the two-tailed tests.
Data availability
Data from the parent project (e.g. MRI, clinical, demographic data) are publicly available from the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR; https://fitbir.nih.gov/study_profile/279). CRP and CMV data and relevant analysis protocols are available from the corresponding author upon reasonable request with the execution of necessary data-use agreements.
Results
A total of 1174 athletes were enrolled at baseline for the parent study; 106 sustained SRC and completed follow-up visits while 91 matched controls completed similar visits. As previously described, 10 athletes with SRC and nine control athletes were removed due to failing imaging quality control procedures (e.g. excessive head motion) resulting in diffusion kurtosis imaging data in 96 SRC and 82 controls.41 From this subset of participants, 88 American football players with SRC and 73 controls met the current study criteria (i.e. enrolled in biosample banking) and were included in analyses. Of the 88 unique athletes with SRC, 30 (34%) were seropositive for CMV, while 21 of the 73 (29%) controls were seropositive for CMV, relatively consistent with the CMV prevalence expected in a sample with mean age of ∼19 years and comparable demographics.61 Demographic characteristics of athletes with SRC and controls based on CMV status are summarized in Table 1. After propensity weighting, there were no significant group differences in demographic/background factors between athletes with and without CMV.
Table 1.
Demographic data and impact of propensity weighting
| SRC: CMV+ | SRC: CMV− | CC: CMV+ | CC: CMV− | SRC versus CC | CMV+ versus CMV− (pre-IPTW) | CMV+ versus CMV− (post-IPTW) | |
|---|---|---|---|---|---|---|---|
| n | 30 | 58 | 21 | 52 | – | – | – |
| Age | 19.58 (1.82) | 18.89 (1.79) | 19.82 (1.38) | 19.33 (1.94) | t(159) = −1.22, P = 0.22 | F(1159) = 3.63, P = 0.058 | F(1159) = 0.05, P = 0.83 |
| Race (n White) | 17 | 48 | 15 | 41 | χ2 = 0.17, P = 0.68a | χ2 = 5.97, P = 0.015a | χ2 = 0.25, P = 0.62a |
| Ethnicity | |||||||
| n Hispanic or Latino | 4 | 2 | 3 | 1 | OR = 1.26, P = 0.73b | OR = 6.04, P = 0.013b | OR = 0.80, P = 0.58b |
| n Unknown or not reported | 2 | 4 | 3 | 2 | OR = 1.01, P = 0.99b | OR = 2.16, P = 0.23b | OR = 1.18, P = 0.72b |
| n Not Hispanic or Latino | 24 | 52 | 15 | 49 | – | – | – |
| WTAR SS | 91.88 (16.27) | 102.97 (12.08) | 95.90 (9.91) | 100.71 (13.19) | t(159) = −0.07, P = 0.95 | F(1159) = 14.27, P < 0.001 | F(1159) = 0.03, P = 0.87 |
| SES | 42.62 (12.35) | 42.56 (12.32) | 40.52 (12.59) | 42.15 (12.25) | t(159) = 0.46, P = 0.65 | F(1159) = 0.09, P = 0.77 | F(1159) = 0.02, P = 0.89 |
| Prior Con | 0.57 (0.68) | 1.22 (1.46) | 0.57 (1.21) | 0.71 (0.96) | χ2 = 5.00, P = 0.03c | χ2 = 6.82, P = 0.009c | χ2 = 1.52, P = 0.22c |
| BMI | 28.93 (5.47) | 29.14 (6.04) | 28.81 (5.63) | 28.70 (5.05) | t(159) = 0.38, P = 0.70 | F(1159) = 0.00, P = 0.96 | F(1159) = 0.53, P = 0.47 |
Mean (standard deviation) shown in each cell unless indicated. P < 0.05 results are bolded. BMI = body mass index; CC = contact control; CMV = cytomegalovirus; IPTW = inverse probability treatment weighting; OR = odds ratio; Prior Con = prior concussion; SES = socioeconomic status; SRC = sport-related concussion; WTAR SS = Wechsler Test of Adult Reading standard score.
Binomial distribution.
Multinomial distribution with ‘Not Hispanic or Latino’ as reference.
Poisson distribution.
Primary outcomes: effect of CMV on white matter microstructure
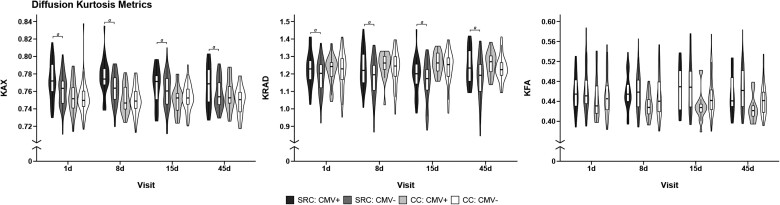
Estimated marginal means for all a priori contrasts are provided in the Supplementary Table 1. Descriptive statistics for kurtosis metrics for each group across each visit are illustrated in Fig. 2 and provided in Supplementary Table 2. There was a significant effect of CMV on axial kurtosis in athletes with SRC [t(155) = 2.72, P = 0.007, mean difference (standard error) (MD) = 0.01 (0.004), 95% confidence interval (CI) (0.003, 0.017), Cohen’s d = 0.44], but not in controls [t(152) = −0.57, P = 0.57, MD = −0.002 (0.004), 95% CI (−0.01, 0.005), d = 0.09]. Similarly, there was a significant effect of CMV on radial kurtosis in athletes with SRC [t(165) = 2.61, P = 0.010, MD = 0.043 (0.016), 95% CI (0.01, 0.075), d = 0.41], but not controls [t(162) = 0.48, P = 0.63, MD = 0.008 (0.018), 95% CI (−0.026, 0.043), d = 0.08]. For both axial and radial kurtosis, CMV+ athletes with SRC had elevated kurtosis relative to CMV− athletes with SRC. CMV status did not affect kurtosis fractional anisotropy levels in either athletes with SRC [t(162) = −0.48, P = 0.63, MD = −0.003 (0.007), 95% CI (−0.017, 0.01), d = 0.08] or controls [t(159) = −1.21, P = 0.23, MD = −0.009 (0.008), 95% CI (−0.024, 0.006), d = 0.19].
Figure 2.
Association of cytomegalovirus and diffusion kurtosis metrics. Trimmed violin plots with box plots of axial kurtosis (KAX), radial kurtosis (KRAD) and kurtosis fractional anisotropy (KFA) in athletes with sport-related concussion (SRC) and contact controls (CC) with (CMV+) and without cytomegalovirus (CMV−). Box plots show first quartile, median and third quartile; whiskers extend to the largest or smallest observation up to 1.5× the interquartile range. 1d = 1 day, 8d = 8 day, 15d = 15 day, 45d = 45 day visit.
a Effects of CMV that survived multiple comparison correction.
Secondary outcomes: effect of CMV on grey matter structure
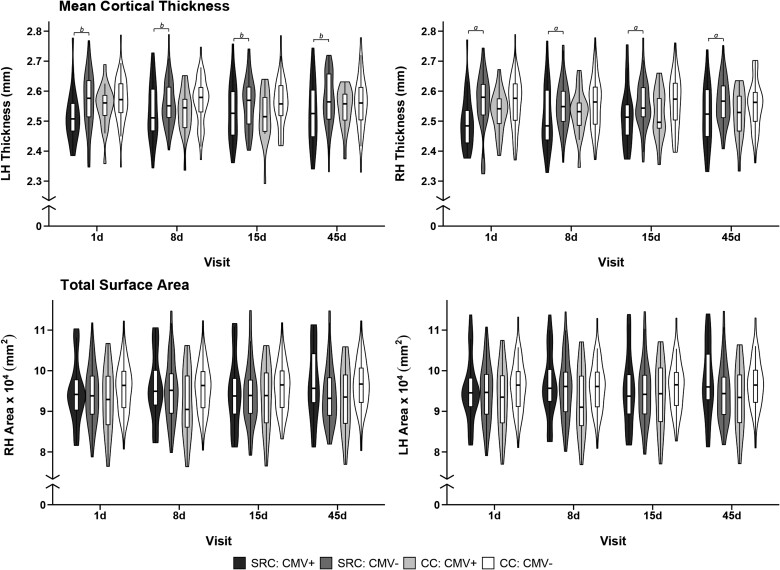
Descriptive statistics for structural metrics for each group across each visit are illustrated in Fig. 3 and provided in Supplementary Table 3. There was a significant effect of CMV status on right hemisphere mean cortical thickness in athletes with SRC [t(159) = −2.66, P = 0.009, MD = −0.044 (0.017) mm, 95% CI (−0.078, −0.011), d = 0.42], but not in controls [t(157) = −0.92, P = 0.36, MD = −0.017 (0.018) mm, 95% CI (−0.053, 0.019), d = 0.15]. A similar effect of CMV in athletes with SRC was observed on left hemisphere mean cortical thickness though it did not survive strict multiple comparison correction [t(161) = −2.12, P = 0.036, MD = −0.033 (0.016) mm, 95% CI (−0.064, −0.002), d = 0.33]. As in the right hemisphere, there was no association of CMV with left hemisphere thickness in controls [t(158) = −0.84, P = 0.40, MD = −0.014 (0.017) mm, 95% CI (−0.048, 0.019), d = 0.13].
Figure 3.
Association of cytomegalovirus with cortical thickness and surface area. Trimmed violin plots with box plots of mean cortical thickness and total surface area in left hemisphere (LH) and right hemisphere (RH) in athletes with sport-related concussion (SRC) and contact controls (CC) with (CMV+) and without cytomegalovirus (CMV−). Box plots show first quartile, median and third quartile; whiskers extend to the largest or smallest observation up to 1.5× the interquartile range. 1d = 1 day, 8d = 8 day, 15d = 15 day, 45d = 45 day visit.
a Effects of CMV that survived multiple comparison correction.
b Effects that did not survive multiple comparison correction.
For total surface area, there was no significant CMV effect for either athletes with SRC or controls for the left hemisphere [t(155) = 1.49, P = 0.14, MD = 1189.63 (800.22) mm2, 95% CI (−391.09, 2770.35), d = 0.24; t(155) = 1.00, P = 0.32, MD = 883.07 (883.28) mm2, 95% CI (−861.75, 2627.90), d = 0.16] or right hemisphere [t(155) = 1.66, P = 0.10, MD = 1325.77 (797.80) mm2, 95% CI (−250.18, 2901.71), d = 0.27; t(155) = 1.30, P = 0.20, MD = 1144.44 (880.58) mm2, 95% CI (−595.04, 2883.93), d = 0.21].
Exploratory analysis: effect of CMV on symptoms and CRP
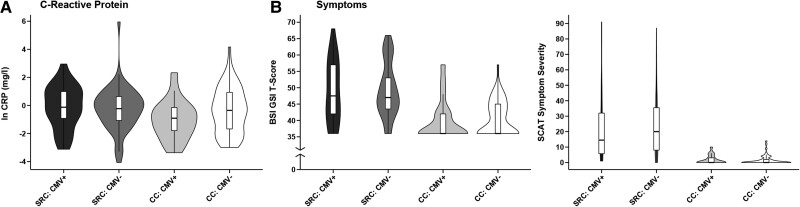
Descriptive statistics for symptom measures and CRP for each group at the 1-day visit are illustrated in Fig. 4 and provided in Supplementary Table 4. The effect of CMV seropositivity on SCAT symptom severity scores was not significant in athletes with SRC [χ2 = 0.014, P = 0.91, MD = 0.50 (4.21), 95% CI (−7.75, 8.74), d = 0.03] or controls [χ2 = 0.001, P = 0.98, MD = −0.01 (0.35), 95% CI (−0.70, 0.68), d = 0.01]. Similarly, the effect of CMV status on BSI-GSI scores was not significant in SRC [χ2 = 0.001, P = 0.97, MD = 0.07 (1.93), 95% CI (−3.72, 3.86), d = 0.01] or controls [χ2 = 0.17, P = 0.68, MD = −0.14 (0.35), 95% CI (−0.82, 0.54), d = 0.10]. Finally, there was no significant effect of CMV on log-transformed serum CRP levels (mg/l) in SRC [χ2 = 2.119, P = 0.15, MD = 0.44 (0.30), 95% CI (−0.15, 1.02), d = 0.32] or controls [χ2 = 3.525, P = 0.06, MD = −0.69 (0.37), 95% CI (−1.42, 0.03), d = 0.47].
Figure 4.
Association of cytomegalovirus with C-reactive protein and symptoms. Trimmed violin plots with box plots for log-transformed C-reactive protein (ln CRP, A), as well as (B) Brief Symptom Inventory Global Severity Index (BSI-GSI), and symptom severity score from the Sport Concussion Assessment Tool (SCAT) at the 1-day visit in athletes with sport-related concussion (SRC) and contact controls (CC) with (CMV+) and without cytomegalovirus (CMV−). Box plots show first quartile, median and third quartile; whiskers extend to the largest or smallest observation up to 1.5× the interquartile range.
Sensitivity analyses
Sensitivity analyses were conducted for all analyses to determine whether results differed when propensity weighting was not applied. As reported in the Supplementary material, the exclusion of propensity weights on our models did not alter results.
Additional exploratory analyses were conducted to ensure that present results were not due to other underlying differences between CMV+ and CMV− athletes within each group (SRC or controls). Few athletes self-reported health conditions upon enrolment and the proportion of CMV+ and CMV− athletes with self-reported health conditions did not differ in athletes with SRC or in controls (P-values > 0.05). Moreover, inclusion of the presence of any self-reported health condition as a covariate had no impact on our results. These analyses are presented in the Supplementary material.
Discussion
This study revealed that CMV seropositive athletes with SRC had elevated axial and radial kurtosis in white matter, but not kurtosis fractional anisotropy compared with CMV seronegative athletes with SRC. The CMV+ and CMV− groups were propensity weighted based on seven different potentially confounding variables. The CMV+ participants with SRC also showed reductions in both left and right cortical thickness but not surface area compared to CMV− participants with SRC although only the right hemisphere thickness remained statistically significant after Bonferroni correction. In contrast, there were no significant effects of CMV serostatus on kurtosis metrics or cortical thickness in the control sample.
Participants with a SRC who tested positive for CMV showed increased axial and radial kurtosis compared to seronegative participants with an SRC. Unlike diffusion tensor imaging (DTI), which assumes a Gaussian distribution of diffused water molecules, diffusion kurtosis imaging assumes a non-Gaussian distribution of diffusion, which theoretically better reflects the underlying biology of complex tissues with high structural heterogeneity such as the brain.62,63 It has therefore been postulated that diffusion kurtosis imaging may be more sensitive than conventional DTI measures to the complex microstructural changes that occur following concussion. In theory, the higher the kurtosis, the more the diffusion pattern deviates from a Gaussian distribution, indicating a more restricted and complex diffusion environment.48,49 Thus, increased axial kurtosis is indicative of impeded diffusion along the longitudinal axis of the white matter fibre tracts while increased radial diffusion indicates impeded diffusion in the transverse orientation. On a biological level these changes are thought to reflect increased intra-axonal debris or cellularity.41,64,65 Neuroinflammation is one possible explanation as damaged axons would attract activated microglia, leading to elevated tissue structural complexity and hence increased kurtosis values.66 In fact, experimental TBI studies in preclinical models have provided some empirical support for a positive association between axial or radial kurtosis and the density of microglial staining,67,68 while microgliosis was associated with an increase of radial kurtosis during the acute inflammatory demyelination phase in a cuprizone model of multiple sclerosis.69
Interestingly, while the effects of CMV on axial kurtosis were in the same direction as those of SRC (both elevated), radial kurtosis was decreased across all athletes with SRC relative to controls whereas CMV status in SRC was associated with elevated radial kurtosis. This finding appears counterintuitive. However, both increased and decreased radial kurtosis can be indicative of pathology because the effects of SRC on white matter microstructure are complex and include any combination of neuroinflammation, oedema, axonal beading, or neurodegeneration, among many possibilities.64,70 The putative effects of CMV on white matter microstructure are then superimposed upon those of SRC. Thus, one conceivable explanation is that CMV and SRC both exert negative effects on brain structural integrity, but the different biological processes engendered by CMV and SRC have opposing effects on the radial kurtosis metric. For instance, the decreased radial kurtosis observed due to SRC may be reflective of a degenerative process whereas CMV seropositivity results in a parallel increase in radial kurtosis due to the cellular complexity associated with inflammation. Ultimately, in vivo diffusion imaging does not have the resolution to definitively clarify the underlying pathology at this time.
We previously reported replicable associations between exposure to CMV and widespread reductions in grey matter volume in the context of MDD.31,32 Cortical volume is determined by two components: surface area and thickness, which are genetically and ontogenetically independent of each other.71,72 Andreou et al.27 recently reported an association between CMV seropositivity and reductions in total cortical surface area but not thickness in participants with schizophrenia spectrum disorders. We therefore selected cortical surface area and thickness as secondary outcomes in this study. We found that CMV seropositive athletes with SRC showed reductions in both left and right hemisphere cortical thickness (though only right hemisphere survived strict multiple comparison correction) but not surface area compared to CMV negative athletes with SRC.
The cortex consists of columns of neurons that run perpendicular to the cortical surface. While surface area reflects the number of cortical columns, the amount of neuropil and number of cells within each cortical column determines the cortical thickness.73,74 Thus, a difference in surface area may be more likely to reflect a disruption in neurodevelopmental processes while changes in cortical thickness may be more reflective of dynamic shifts in the environment such as an inflammatory process. Indeed, increased concentrations of circulating inflammatory mediators have been widely linked with reductions in cortical thickness in several different disorders,75-81 while reductions in cortical thickness are commonly reported in neuroinflammatory diseases such as multiple sclerosis82-85 as well as viral infections such as HIV.86-88 Nevertheless, as with the kurtosis data, we are unable to definitively attribute the observed cross-sectional differences in cortical thickness to specific pathology. Moreover, it is potentially noteworthy that the CMV+ controls had qualitatively thinner cortex than the CMV− athletes (Fig. 3). It is conceivable that the significant reductions in cortical thickness observed in the CMV+ athletes with SRC could have resulted from the combined effects of both the current episode and previous reactivation events.
As in the case of white matter microstructural integrity, there was no significant effect of CMV serostatus on cortical thickness or surface area in the control group. This finding suggests that the association between CMV infection and structural brain abnormalities in the SRC group is unlikely to have resulted solely from the premorbid, neurodevelopmental effects of the virus, but is rather linked to the current concussive episode in some manner. Although we cannot rule out pre-existing cortical thickness differences between concussed athletes with and without CMV, we raise the possibility that concussion-induced inflammation and/or SNS activation leads to the reactivation of latent CMV infection, which in turn exerts biological effects over and above the initial concussive insult. These putative biological effects could include the direct pathological effects of CMV on infected brain cells or the indirect influence of an amplification of concussion-associated inflammatory processes. While we have no direct evidence for this hypothesis, co-infection with CMV is a well known risk factor for inflammation-related morbidity in the context of people living with HIV,89,90 doubles the risk of hospitalization due to SARS-CoV-2 infection,91 and has been implicated in the aetiology of inflammation-linked diseases such as type 2 diabetes92,93 and cardiovascular disease.94,95 Although CMV serostatus did not have a significant influence on plasma CRP concentrations post-concussion, we did observe non-significantly higher CRP concentrations in the CMV+ SRC group 1 day post-concussion (d = 0.32). It is possible that a larger sample size or the measurement of CRP at additional time points would have yielded statistically significant effects. On the other hand, it is also possible that specific markers of viral infection such as CXCL10,96 or other components of the interferon pathway, would be more sensitive than CRP to CMV’s impact on the immune system. Indeed, in our previous work on the effects of CMV in MDD there were also no differences between CMV+ and CMV− groups with respect to CRP concentration.30,31
The immediate clinical significance of the current findings are unclear given the lack of significant CMV effects on acute concussion symptoms. Additional work is needed to investigate other clinical outcomes and with longer periods of follow-up. However, previous evidence suggests that the greater observed differences in structural MRI in athletes with SRC and CMV are meaningful. First, preliminary evidence suggests that persistent diffusion abnormalities in white matter that occur after symptom resolution following SRC increase the risk for subsequent concussion,97 consistent with the preclinical literature demonstrating a window of cerebral vulnerability following an initial injury.98 Therefore, the current results suggest that CMV seropositivity may impact the time course of physiological recovery following SRC, which has important implications for decisions about when athletes can safely return to play following concussion.99 Second, there is evidence that a history of concussion or mild TBI is associated with long-term effects on brain structure, including thinner cortex and other measures of grey matter structure.100-103 In older adults, observable changes in brain structure are commonly observed before the manifestation of clinical symptoms, such as in the context of neurodegenerative disease.104,105 CMV seropositivity is one potential moderating factor that should be considered in future work characterizing the chronic and cumulative effect of neurotrauma, which is consistent with the growing support for a viral role in a variety of neurodegenerative diseases that are also associated with changes in brain structure.106
Several limitations deserve mention. First, the analyses focused on CMV serostatus rather than a quantifiable marker of CMV reactivation. This is because CMV viraemia is rare even in immunosuppressed populations and there is no ‘gold standard’ marker of reactivation. Anti-CMV antibody titre is often employed as a surrogate measure of reactivation, but this approach has significant limitations. For instance, a hypothetical post-concussion increase in IgG antibody titre would take time to occur, and IgG antibodies have a half-life of up to a month suggesting that it would be a lagging indicator of reactivation. We were also unable to use this approach because CMV serostatus was determined from samples collected at different time points (i.e. Day 1 to Day 45) in different participants due to limited availability of serum samples. Second, it is conceivable that other herpes viruses could also affect brain structure in the context of concussion and this possibility should be evaluated in future studies. Nevertheless, CMV is known to have the most pronounced long-term effects on the immune system and has been strongly linked with neuropathology in other contexts.15,107-109 Third, caution is suggested in the interpretation of the kurtosis diffusion imaging results as the biological correlates of changes in these metrics have not been fully resolved. Fourth, the current study was limited to adolescent and young-adult American football players, and thus whether the observed results generalize to other populations of TBI patients is uncertain (e.g. female athletes, non-sport TBI, more severe TBI). Fifth, this study focused on the potential effects of CMV in American football players with acute concussion. Growing literature supports potential cumulative effects of multiple concussions as well as effects other repetitive head impacts that occur through routine contact sport participation.110-112 Because repeated head injury and chronic TBI have also been associated with neuroinflammatory processes,113,114 future work is needed to characterize any potential moderation of CMV seropositivity on the effects of repeated head injury and exposure. Finally, although unlikely based on the propensity weighting approach and sensitivity analysis results, we cannot rule out the possibility that some unmeasured variable (e.g. psychosocial factors) may differ between patients with and without CMV and partially explain some of the observed changes. Additional studies are needed to more thoroughly investigate the role of such factors.
In summary, the current study raises the possibility that the presence of a CMV infection negatively impacts white matter microstructure and cortical thickness after SRC. Observed effect sizes were small-to-moderate. For example, the effect size of CMV seropositivity on kurtosis metrics was smaller than the effect size of concussion across all athletes in this study (e.g. d’s = 0.41–0.44 versus d’s = 0.60–0.70). In contrast, the effect sizes of CMV on cortical thickness (d’s = 0.33–0.42) are in line with recent reports on thickness differences after mild TBI in paediatric patients relative to controls,115,116 though they exceed effects from multiple null findings of cortical thickness reported in collegiate athletes with concussion.117,118 These data suggest that the presence of latent neurotropic viruses may be one facet of the proposed bio-psycho-socio-ecological model of TBI that potentially impacts individual’s response to injury and subsequent physiological recovery.119 Further research is necessary to follow-up on these initial findings to determine whether the observed physiological effects of CMV in athletes with concussion eventually resolve or accumulate over multiple injuries. Furthermore, additional work is also needed to determine the acute or long-term clinical implications of these findings (if any) for TBI patients. If CMV specifically amplifies the neuropathological effects of TBI then this may open up a novel avenue of treatment given the existence of approved anti-CMV medications120-122 and the ongoing efforts to develop CMV vaccines.122,123
Supplementary Material
Acknowledgements
The authors thank Ashlee Taylor and Brenda Davis from the University of Oklahoma Integrative Immunology Center for handling of blood biospecimens; Ashley LaRoche and Alexa Wild from the Department of Neurosurgery at the Medical College of Wisconsin for study coordination; Carisa Bergner from the Comprehensive Injury Center at the Medical College of Wisconsin for statistical consultation; Dan Huber and Lezlie España from the Department of Neurosurgery at the Medical College of Wisconsin for database management and data processing. This work was completed in part with computational resources and technical support provided by the Research Computing Center at the Medical College of Wisconsin.
Contributor Information
Jonathan Savitz, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Oxley College of Health Sciences, The University of Tulsa, Tulsa, OK 74119, USA.
Bryna D Goeckner, Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Bart N Ford, Department of Pharmacology and Physiology, Oklahoma State University Center for Health Sciences, Tulsa, OK 74107, USA.
T Kent Teague, Department of Psychiatry, The University of Oklahoma School of Community Medicine, Tulsa, OK 74135, USA; Department of Surgery, The University of Oklahoma School of Community Medicine, Tulsa, OK 74135, USA; Department of Pharmaceutical Sciences, University of Oklahoma College of Pharmacy, Tulsa, OK 74135, USA.
Haixia Zheng, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Jaroslaw Harezlak, Department of Epidemiology and Biostatistics, School of Public Health-Bloomington, Indiana University, Bloomington, IN 47405, USA.
Rebekah Mannix, Division of Emergency Medicine, Boston Children’s Hospital, Boston, MA 02115, USA; Department of Pediatrics, Harvard Medical School, Boston, MA 02115, USA.
L Tugan Muftuler, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Benjamin L Brett, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Department of Neurology, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Michael A McCrea, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Department of Neurology, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Timothy B Meier, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Department of Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Department of Cell Biology, Neurobiology and Anatomy, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Funding
This work was supported by the Defense Health Program under the US Department of Defense Broad Agency Announcement for Extramural Medical Research through Award No. W81XWH-14-1-0561. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the US Department of Defense. Support for this work was also provided by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R21NS099789. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.S. acknowledges support from the William K. Warren Foundation, the National Institute of Mental Health (R01MH123652), and the National Institute of General Medical Sciences (P20GM121312). The REDCap electronic database and the Adult Translational Research Unit used for this project were supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436.
Competing interests
T.B.M. receives compensation as a member of the Clinical and Scientific Advisory Board for Quadrant Biosciences Inc. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Petersen MR, Patel EU, Abraham AG, Quinn TC, Tobian AAR. Changes in cytomegalovirus seroprevalence among U.S. Children aged 1-5 years: The national health and nutrition examination surveys. Clin Infect Dis. 2021;72:e408–e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsutsui Y, Kosugi I, Kawasaki H. Neuropathogenesis in cytomegalovirus infection: Indication of the mechanisms using mouse models. Rev Med Virol. 2005;15:327–345. [DOI] [PubMed] [Google Scholar]
- 3. Poland SD, Costello P, Dekaban GA, Rice GP. Cytomegalovirus in the brain: In vitro infection of human brain-derived cells. J Infect Dis. 1990;162:1252–1262. [DOI] [PubMed] [Google Scholar]
- 4. Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation. 2012;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fish KN, Soderberg-Naucler C, Mills LK, Stenglein S, Nelson JA. Human cytomegalovirus persistently infects aortic endothelial cells. J Virol. 1998;72:5661–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prosch S, Wendt CE, Reinke P, et al. A novel link between stress and human cytomegalovirus (HCMV) infection: Sympathetic hyperactivity stimulates HCMV activation. Virology. 2000;272:357–365. [DOI] [PubMed] [Google Scholar]
- 7. Paterson DL, Staplefeldt WH, Wagener MM, Gayowski T, Marino IR, Singh N. Intraoperative hypothermia is an independent risk factor for early cytomegalovirus infection in liver transplant recipients. Transplantation. 1999;67:1151–1155. [DOI] [PubMed] [Google Scholar]
- 8. Rector JL, Dowd JB, Loerbroks A, et al. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun. 2014;38:133–141. [DOI] [PubMed] [Google Scholar]
- 9. Docke WD, Prosch S, Fietze E, et al. Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343:268–269. [DOI] [PubMed] [Google Scholar]
- 10. Hargett D, Shenk TE. Experimental human cytomegalovirus latency in CD14 + monocytes. Proc Natl Acad Sci U S A. 2010;107:20039–20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soderberg-Naucler C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest. 1997;100:3154–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wieduwild E, Girard-Madoux MJ, Quatrini L, et al. β2-adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J Exp Med. 2020;217(4):e20190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein J, Volk HD, Liebenthal C, Kruger DH, Prosch S. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. J Gen Virol. 1993;74(Pt 11):2333–2338. [DOI] [PubMed] [Google Scholar]
- 14. Liu XF, Jie C, Zhang Z, et al. Transplant-induced reactivation of murine cytomegalovirus immediate early gene expression is associated with recruitment of NF-kappaB and AP-1 to the major immediate early promoter. J Gen Virol. 2016;97:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol. 2021;19:759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croen KD. Latency of the human herpesviruses. Annu Rev Med. 1991;42:61–67. [DOI] [PubMed] [Google Scholar]
- 17. Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. Jama. 2008;300:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imlay H, Limaye AP. Current understanding of cytomegalovirus reactivation in critical illness. J Infect Dis. 2020;221(Suppl 1):S94–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halenius A, Hengel H. Human cytomegalovirus and autoimmune disease. Biomed Res Int. 2014;2014:472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanheusden M, Broux B, Welten SPM, et al. Cytomegalovirus infection exacerbates autoimmune mediated neuroinflammation. Sci Rep. 2017;7:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maksimowicz-McKinnon K, Zhou J, Hudy J, Hegab S, McKinnon JE. Subclinical CMV viremia is associated with increased nosocomial infections and prolonged hospitalization in patients with systemic autoimmune diseases. J Clin Virol. 2021;140:104849. [DOI] [PubMed] [Google Scholar]
- 22. Gugliesi F, Pasquero S, Griffante G, et al. Human cytomegalovirus and autoimmune diseases: Where are we? Viruses. 2021;13:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: The Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2001;121(1–3):187–201. [DOI] [PubMed] [Google Scholar]
- 24. Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: The Swedish NONA immune study. Exp Gerontol. 2002;37(2–3):445–453. [DOI] [PubMed] [Google Scholar]
- 25. Savva GM, Pachnio A, Kaul B, et al. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12:381–387. [DOI] [PubMed] [Google Scholar]
- 26. Strandberg TE, Pitkala KH, Tilvis RS. Cytomegalovirus antibody level and mortality among community-dwelling older adults with stable cardiovascular disease. Jama. 2009;301:380–382. [DOI] [PubMed] [Google Scholar]
- 27. Andreou D, Jorgensen KN, Nerland S, et al. Cytomegalovirus infection associated with smaller total cortical surface area in schizophrenia Spectrum disorders. Schizophr Bull. 2022;48:1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Houenou J, d’Albis MA, Daban C, et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:142–148. [DOI] [PubMed] [Google Scholar]
- 29. Andreou D, Jorgensen KN, Nerland S, et al. Cytomegalovirus infection associated with smaller dentate gyrus in men with severe mental illness. Brain Behav Immun. 2021;96:54–62. [DOI] [PubMed] [Google Scholar]
- 30. Zheng H, Bergamino M, Ford BN, et al. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng H, Ford BN, Bergamino M, et al. A hidden menace? Cytomegalovirus infection is associated with reduced cortical grey matter volume in major depressive disorder. Mol Psychiatry. 2021;26:4234–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng H, Ford BN, Kuplicki R, et al. Association between cytomegalovirus infection, reduced grey matter volume, and resting-state functional hypoconnectivity in major depressive disorder: A replication and extension. Transl Psychiatry. 2021;11:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Purkayastha S, Stokes M, Bell KR. Autonomic nervous system dysfunction in mild traumatic brain injury: A review of related pathophysiology and symptoms. Brain Inj. 2019;33:1129–1136. [DOI] [PubMed] [Google Scholar]
- 34. La Fountaine MF. An anatomical and physiological basis for the cardiovascular autonomic nervous system consequences of sport-related brain injury. Int J Psychophysiol. 2018;132(Pt A):155–166. [DOI] [PubMed] [Google Scholar]
- 35. Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci. 2009;14:3795–3813. [DOI] [PubMed] [Google Scholar]
- 36. Steenerson K, Starling AJ. Pathophysiology of sports-related concussion. Neurol Clin. 2017;35:403–408. [DOI] [PubMed] [Google Scholar]
- 37. Meier TB, Savitz J. The kynurenine pathway in traumatic brain injury: Implications for psychiatric outcomes. Biol Psychiatry. 2022;91:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sribnick EA, Popovich PG, Hall MW. Central nervous system injury-induced immune suppression. Neurosurg Focus. 2022;52:E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hazeldine J, Lord JM, Belli A. Traumatic brain injury and peripheral immune suppression: Primer and prospectus. Front Neurol. 2015;6:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma R, Shultz SR, Robinson MJ, et al. Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain Behav Immun. 2019;79:63–74. [DOI] [PubMed] [Google Scholar]
- 41. Muftuler LT, Meier TB, Keith M, Budde MD, Huber DL, McCrea MA. Serial diffusion kurtosis magnetic resonance imaging study during acute, subacute, and recovery periods after sport-related concussion. J Neurotrauma. 2020;37:2081–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benjamini D, Iacono D, Komlosh ME, Perl DP, Brody DL, Basser PJ. Diffuse axonal injury has a characteristic multidimensional MRI signature in the human brain. Brain. 2021;144:800–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCrea M, Meier T, Huber D, et al. Role of advanced neuroimaging, fluid biomarkers and genetic testing in the assessment of sport-related concussion: A systematic review. Br J Sports Med. 2017;51:919–929. [DOI] [PubMed] [Google Scholar]
- 44. Meier TB, Huber DL, Bohorquez-Montoya L, et al. A prospective study of acute blood-based biomarkers for sport-related concussion. Ann Neurol. 2020;87:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hollingshead A. Four factor index of social status. Yale Journal of Sociology. 2011;8:21–52. [Google Scholar]
- 46. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage. 2003;20:870–888. [DOI] [PubMed] [Google Scholar]
- 47. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jensen JH, Helpern JA. MRI Quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–1440. [DOI] [PubMed] [Google Scholar]
- 50. Tax CM, Otte WM, Viergever MA, Dijkhuizen RM, Leemans A. REKINDLE: Robust extraction of kurtosis INDices with linear estimation. Magn Reson Med. 2015;73:794–808. [DOI] [PubMed] [Google Scholar]
- 51. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 52. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 54. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 56. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 57. Jovicich J, Marizzoni M, Sala-Llonch R, et al. Brain morphometry reproducibility in multi-center 3 T MRI studies: A comparison of cross-sectional and longitudinal segmentations. Neuroimage. 2013;83:472–484. [DOI] [PubMed] [Google Scholar]
- 58. McCarthy CS, Ramprashad A, Thompson C, Botti JA, Coman IL, Kates WR. A comparison of FreeSurfer-generated data with and without manual intervention. Front Neurosci. 2015;9:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo C, Ferreira D, Fink K, Westman E, Granberg T. Repeatability and reproducibility of FreeSurfer, FSL-SIENAX and SPM brain volumetric measurements and the effect of lesion filling in multiple sclerosis. Eur Radiol. 2019;29:1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–1151. [DOI] [PubMed] [Google Scholar]
- 62. Steven AJ, Zhuo J, Melhem ER. Diffusion kurtosis imaging: An emerging technique for evaluating the microstructural environment of the brain. AJR Am J Roentgenol. 2014;202:W26–W33. [DOI] [PubMed] [Google Scholar]
- 63. Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-Gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006;19:236–247. [DOI] [PubMed] [Google Scholar]
- 64. Budde MD, Skinner NP. Diffusion MRI in acute nervous system injury. J Magn Reson. 2018;292:137–148. [DOI] [PubMed] [Google Scholar]
- 65. Yang L, Cheng Y, Sun Y, et al. Combined application of quantitative susceptibility mapping and diffusion kurtosis imaging techniques to investigate the effect of iron deposition on microstructural changes in the brain in Parkinson's disease. Front Aging Neurosci. 2022;14:792778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Braeckman K, Descamps B, Pieters L, Vral A, Caeyenberghs K, Vanhove C. Dynamic changes in hippocampal diffusion and kurtosis metrics following experimental mTBI correlate with glial reactivity. Neuroimage Clin. 2019;21:101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu F, Shukla DK, Armstrong RC, et al. Repetitive model of mild traumatic brain injury produces cortical abnormalities detectable by magnetic resonance diffusion imaging, histopathology, and behavior. J Neurotrauma. 2017;34:1364–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang ML, Yu MM, Yang DX, Liu YL, Wei XE, Li WB. Longitudinal microstructural changes in traumatic brain injury in rats: A diffusional kurtosis imaging, histology, and behavior study. AJNR Am J Neuroradiol. 2018;39:1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guglielmetti C, Veraart J, Roelant E, et al. Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. Neuroimage. 2016;125:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jha SC, Xia K, Schmitt JE, et al. Genetic influences on neonatal cortical thickness and surface area. Hum Brain Mapp. 2018;39:4998–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. [DOI] [PubMed] [Google Scholar]
- 74. Stockmeier CA, Mahajan GJ, Konick LC, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meier TB, Drevets WC, Wurfel BE, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun. 2016;53:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. North HF, Bruggemann J, Cropley V, et al. Increased peripheral inflammation in schizophrenia is associated with worse cognitive performance and related cortical thickness reductions. Eur Arch Psychiatry Clin Neurosci. 2021;271:595–607. [DOI] [PubMed] [Google Scholar]
- 77. Kose M, Pariante CM, Dazzan P, Mondelli V. The role of peripheral inflammation in clinical outcome and brain imaging abnormalities in psychosis: A systematic review. Front Psychiatry. 2021;12:612471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Qin Y, Wu J, Chen T, et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131:e147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Green C, Shen X, Stevenson AJ, et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav Immun. 2021;92:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Velzen LS, Schmaal L, Milaneschi Y, et al. Immunometabolic dysregulation is associated with reduced cortical thickness of the anterior cingulate cortex. Brain Behav Immun. 2017;60:361–368. [DOI] [PubMed] [Google Scholar]
- 81. Jacomb I, Stanton C, Vasudevan R, et al. C-Reactive protein: Higher during acute psychotic episodes and related to cortical thickness in schizophrenia and healthy controls. Front Immunol. 2018;9:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fujimori J, Fujihara K, Wattjes M, Nakashima I. Patterns of cortical grey matter thickness reduction in multiple sclerosis. Brain Behav. 2021;11:e02050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cruz-Gomez AJ, Forero L, Lozano-Soto E, et al. Cortical thickness and Serum NfL explain cognitive dysfunction in newly diagnosed patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Narayana PA, Govindarajan KA, Goel P, et al. Regional cortical thickness in relapsing remitting multiple sclerosis: A multi-center study. Neuroimage Clin. 2013;2:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Steenwijk MD, Geurts JJ, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain. 2016;139(Pt 1):115–126. [DOI] [PubMed] [Google Scholar]
- 86. Casagrande CC, Lew BJ, Taylor BK, et al. Impact of HIV-infection on human somatosensory processing, spontaneous cortical activity, and cortical thickness: A multimodal neuroimaging approach. Hum Brain Mapp. 2021;42:2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ruiz-Saez B, Garcia MM, de Aragon AM, et al. Effects of perinatal HIV-infection on the cortical thickness and subcortical grey matter volumes in young adulthood. Medicine (Baltimore). 2021;100:e25403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sanford R, Ances BM, Meyerhoff DJ, et al. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis. 2018;67:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis. 2015;211:178–186. [DOI] [PubMed] [Google Scholar]
- 90. Schnittman SR, Hunt PW. Clinical consequences of asymptomatic cytomegalovirus in treated human immunodeficency virus infection. Curr Opin HIV AIDS. 2021;16:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Alanio C, Verma A, Mathew D, et al. Cytomegalovirus latent infection is associated with an increased risk of COVID-19-related hospitalization. J Infect Dis. 2022;226:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yoo SG, Han KD, Lee KH, La Y, Kwon DE, Han SH. Impact of cytomegalovirus disease on new-onset type 2 diabetes Mellitus: Population-based matched case-control cohort study. Diabetes Metab J. 2019;43:815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Woelfle T, Linkohr B, Waterboer T, et al. Health impact of seven herpesviruses on (pre)diabetes incidence and HbA1c: Results from the KORA cohort. Diabetologia. 2022;65:1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang H, Peng G, Bai J, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): A meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6:e005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gianella S, Moser C, Vitomirov A, et al. Presence of asymptomatic cytomegalovirus and epstein–barr virus DNA in blood of persons with HIV starting antiretroviral therapy is associated with non-AIDS clinical events. AIDS. 2020;34:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brett BL, Wu YC, Mustafi SM, et al. The association between persistent white-matter abnormalities and repeat injury after sport-related concussion. Front Neurol. 2020;10:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prins ML, Alexander D, Giza CC, Hovda DA. Repeated mild traumatic brain injury: Mechanisms of cerebral vulnerability. J Neurotrauma. 2013;30:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med. 2017;51:935–940. [DOI] [PubMed] [Google Scholar]
- 100. Meier TB, Bellgowan PSF, Bergamino M, Ling JM, Mayer AR. Thinner Cortex in collegiate football players with, but not without, a self-reported history of concussion. J Neurotrauma. 2016;33:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Major B, Symons GF, Sinclair B, et al. White and grey matter abnormalities in Australian footballers with a history of sports-related concussion: An MRI study. Cereb Cortex. 2021;31:5331–5338. [DOI] [PubMed] [Google Scholar]
- 102. Goswami R, Dufort P, Tartaglia MC, et al. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Structure and Function. 2016;221:1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Misquitta K, Dadar M, Tarazi A, et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. NeuroImage: Clinical. 2018;19:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Verfaillie SCJ, Slot RE, Tijms BM, et al. Thinner cortex in patients with subjective cognitive decline is associated with steeper decline of memory. Neurobiol Aging. 2018;61:238–244. [DOI] [PubMed] [Google Scholar]
- 105. Mak E, Gabel S, Mirette H, et al. Structural neuroimaging in preclinical dementia: From microstructural deficits and grey matter atrophy to macroscale connectomic changes. Ageing Res Rev. 2017;35:250–264. [DOI] [PubMed] [Google Scholar]
- 106. Levine KS, Leonard HL, Blauwendraat C, et al. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron. 2023;111:1086–1093.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Picarda G, Benedict CA. Cytomegalovirus: Shape-shifting the immune system. J Immunol. 2018;200:3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zheng H, Savitz J. Effect of cytomegalovirus infection on the central nervous system: Implications for psychiatric disorders. Curr Top Behav Neurosci. 2023;61:215–241. [DOI] [PubMed] [Google Scholar]
- 110. Manley G, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med. 2017;51:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stephen SJ, Hasman L, Goldenberg M, et al. Short-Term neurologic manifestations of repetitive head impacts among athletes: A scoping review. J Head Trauma Rehabil. 2022;37:318–325. [DOI] [PubMed] [Google Scholar]
- 112. Nowinski CJ, Bureau SC, Buckland ME, et al. Applying the bradford hill criteria for causation to repetitive head impacts and chronic traumatic encephalopathy. Front Neurol. 2022;13:938163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McKee AC. The neuropathology of chronic traumatic encephalopathy: The Status of the literature. Semin Neurol. 2020;40:359–369. [DOI] [PubMed] [Google Scholar]
- 114. Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ware AL, Goodrich-Hunsaker NJ, Lebel C, et al. Post-Acute cortical thickness in children with mild traumatic brain injury versus orthopedic injury. J Neurotrauma. 2020;37:1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mayer AR, Meier TB, Dodd AB, et al. Prospective study of gray matter atrophy following pediatric mild traumatic brain injury. Neurology. 2023;100:e516–e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. España LY, Lee RM, Ling JM, Jeromin A, Mayer AR, Meier TB. Serial assessment of grey matter abnormalities after sport-related concussion. J Neurotrauma. 2017;34:3143–3152. [DOI] [PubMed] [Google Scholar]
- 118. Bobholz SA, Brett BL, España LY, et al. Prospective study of the association between sport-related concussion and brain morphometry (3T-MRI) in collegiate athletes: Study from the NCAA-DoD CARE consortium. Br J Sports Med. 2021;55:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. National Academies of Sciences, Engineering, and Medicine ; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy; Committee on Accelerating Progress in Traumatic Brain Injury Research and Care; Matney C, Bowman K, Berwick D, eds. Traumatic brain injury: A roadmap for accelerating progress. Washington, DC: The National Academies Press; 2022. [PubMed] [Google Scholar]
- 120. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–2444. [DOI] [PubMed] [Google Scholar]
- 121. Coen DM, Schaffer PA. Antiherpesvirus drugs: A promising spectrum of new drugs and drug targets. Nat Rev Drug Discov. 2003;2:278–288. [DOI] [PubMed] [Google Scholar]
- 122. Griffiths P. New vaccines and antiviral drugs for cytomegalovirus. J Clin Virol. 2019;116:58–61. [DOI] [PubMed] [Google Scholar]
- 123. Scarpini S, Morigi F, Betti L, Dondi A, Biagi C, Lanari M. Development of a vaccine against human cytomegalovirus: Advances, barriers, and implications for the clinical practice. Vaccines (Basel). 2021;9:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the parent project (e.g. MRI, clinical, demographic data) are publicly available from the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR; https://fitbir.nih.gov/study_profile/279). CRP and CMV data and relevant analysis protocols are available from the corresponding author upon reasonable request with the execution of necessary data-use agreements.