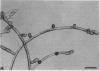
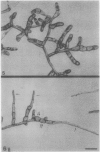
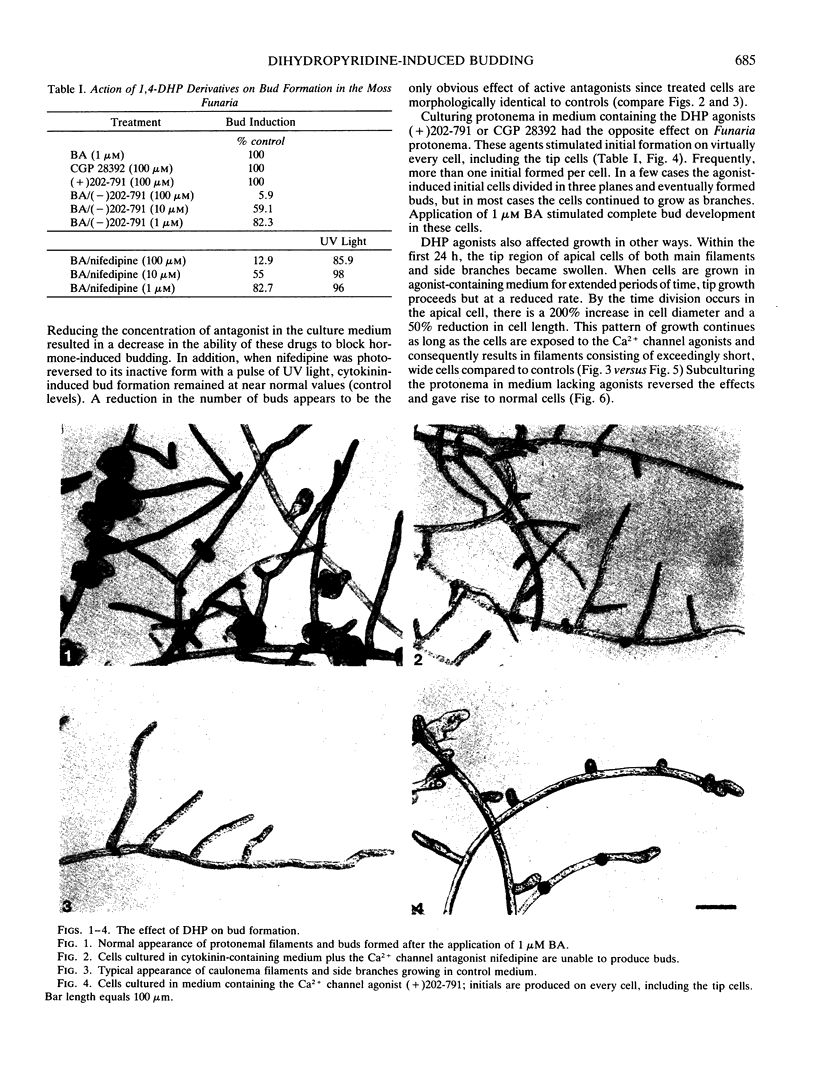
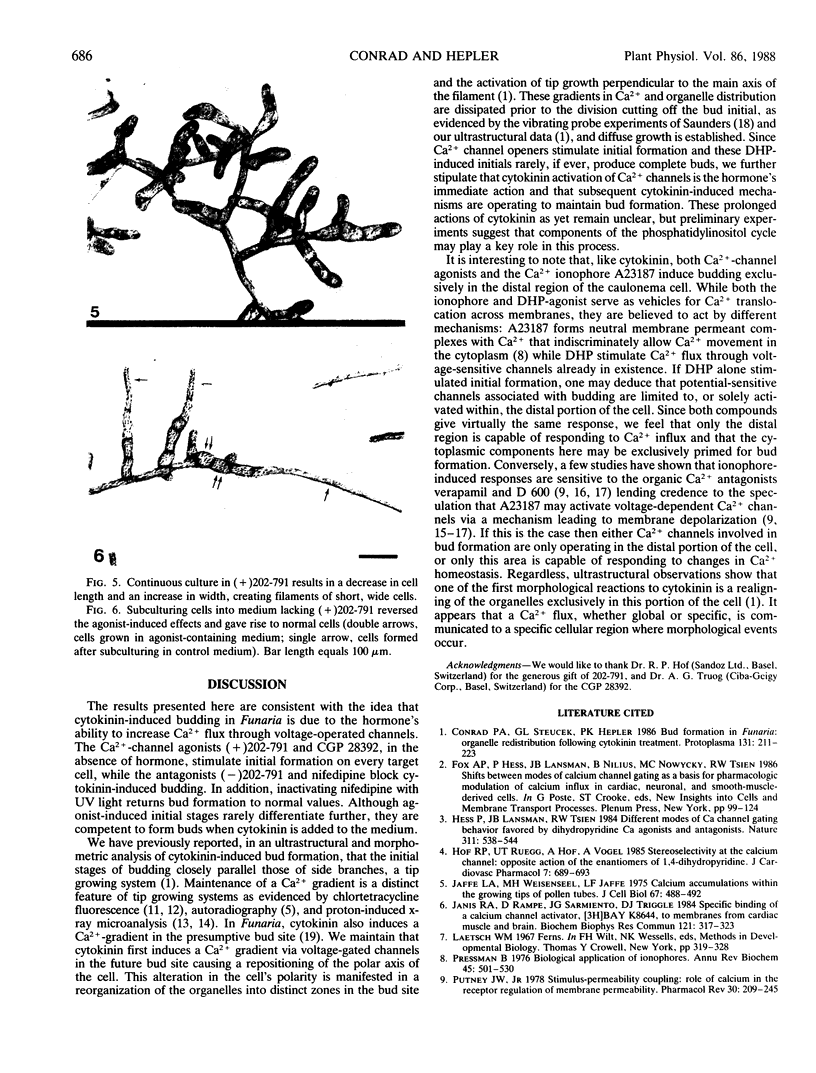
Abstract
The plant hormone cytokinin stimulates target caulonemata of Funaria to form buds that develop into the leafy gametophyte. Previous reports have shown that increases in intracellular Ca2+ occur during hormone-activated budding concomitant with an alteration in the polarity of the organelles in the bud site. In order to ascertain the involvement of voltage-dependent Ca2+ channels in this phenomenon, we have employed dihydropyridines (DHP), compounds noted for their ability to alter Ca2+ flux through potential-sensitive channels. Addition of the DHP agonists (+)202-791 and CGP 28392 (100 micromolar) induces bud initials on every target cell including the tip cell. Application of the DHP antagonist (−)202-791, in the presence of cytokinin (1 micromolar benzyladenine), inhibits budding 96%. Similarly, nifedipine blocks cytokinin-induced budding 87% and its effect on budding can be inactivated with a pulse of ultraviolet light. These results are consistent with the idea that cytokinin induces the budding response by increasing Ca2+ entry through voltage-operated channels. We suggest that cytokinin activation of Ca2+ channels is the first action of the hormone and that subsequent cytokinin-induced mechanisms are operating to maintain budding, since DHP-induced initials rarely develop into complete buds.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hof R. P., Rüegg U. T., Hof A., Vogel A. Stereoselectivity at the calcium channel: opposite action of the enantiomers of a 1,4-dihydropyridine. J Cardiovasc Pharmacol. 1985 Jul-Aug;7(4):689–693. doi: 10.1097/00005344-198507000-00012. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Weisenseel M. H., Jaffe L. F. Calcium accumulations within the growing tips of pollen tubes. J Cell Biol. 1975 Nov;67(2PT1):488–492. doi: 10.1083/jcb.67.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis R. A., Rampe D., Sarmiento J. G., Triggle D. J. Specific binding of a calcium channel activator, [3H]BAY k 8644, to membranes from cardiac muscle and brain. Biochem Biophys Res Commun. 1984 May 31;121(1):317–323. doi: 10.1016/0006-291x(84)90725-3. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978 Jun;30(2):209–245. [PubMed] [Google Scholar]
- Rampe D., Janis R. A., Triggle D. J. BAY K 8644, a 1,4-dihydropyridine Ca2+ channel activator: dissociation of binding and functional effects in brain synaptosomes. J Neurochem. 1984 Dec;43(6):1688–1692. doi: 10.1111/j.1471-4159.1984.tb06096.x. [DOI] [PubMed] [Google Scholar]
- Rosenberger L. B., Triggle D. J. The mechanism of action of ionophore A 23187 on guinea pig intestinal smooth muscle. Can J Physiol Pharmacol. 1979 Apr;57(4):348–358. doi: 10.1139/y79-053. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Saunders M. J., Hepler P. K. Calcium ionophore a23187 stimulates cytokinin-like mitosis in funaria. Science. 1982 Sep 3;217(4563):943–945. doi: 10.1126/science.217.4563.943. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Su C. M., Swamy V. C., Triggle D. J. Calcium channel activation in vascular smooth muscle by BAY K 8644. Can J Physiol Pharmacol. 1984 Nov;62(11):1401–1410. doi: 10.1139/y84-233. [DOI] [PubMed] [Google Scholar]