Abstract
Increasing evidence shows that disease spreading in amyotrophic lateral sclerosis (ALS) follows a preferential pattern with more frequent involvement of contiguous regions from the site of symptom onset. The aim of our study was to assess if: (i) the burden of upper (UMN) and lower motor neuron (LMN) involvement influences directionality of disease spreading; (ii) specific patterns of disease progression are associated with motor and neuropsychological features of different ALS subtypes (classic, bulbar, primary lateral sclerosis, UMN-predominant, progressive muscular atrophy, flail arm, flail leg); and (iii) specific clinical features may help identify ALS subtypes, which remain localized to the site of onset for a prolonged time (regionally entrenching ALS).
A single-centre, retrospective cohort of 913 Italian ALS patients was evaluated to assess correlations between directionality of the disease process after symptom onset and motor/neuropsychological phenotype. All patients underwent an extensive evaluation including the following clinical scales: Penn Upper Motor Neuron Score (PUMNS), MRC Scale for Muscle Strength and the Edinburgh Cognitive and Behavioural ALS Screen (ECAS).
The most frequent initial spreading pattern was that towards adjacent horizontal regions (77.3%), which occurred preferentially in patients with lower MRC scores (P = 0.038), while vertical diffusion (21.1%) was associated with higher PUMNS (P < 0.001) and with reduced survival (P < 0.001). Non-contiguous disease spreading was associated with more severe UMN impairment (P = 0.003), while contiguous disease pattern with lower MRC scores. Furthermore, non-contiguous disease spreading was associated with more severe cognitive impairment in both executive and visuospatial ECAS domains. Individuals with regionally entrenching ALS were more frequently female (45.6% versus 36.9%; P = 0.028) and had higher frequencies of symmetric disease onset (40.3% versus 19.7%; P < 0.001) and bulbar phenotype (38.5% versus 16.4%; P < 0.001).
Our study suggests that motor phenotypes characterized by a predominant UMN involvement are associated with a vertical pattern of disease progression reflecting ipsilateral spreading within the motor cortex, while those with predominant LMN involvement display more frequently a horizontal spreading from one side of the spinal cord to the other. These observations raise the hypothesis that one of the mechanisms underlying disease spreading in ALS pathology is represented by diffusion of toxic factors in the neuron microenvironment. Finally, it is possible that in our cohort, regionally entrenching ALS forms are mainly observed in patients with atypical bulbar phenotypes, characterized by a slowly progressive course and relatively benign prognosis.
Keywords: amyotrophic lateral sclerosis (ALS), motor neuron disease (MND), disease progression, site of onset, motor phenotype, somatotopic organization of motor system
Maranzano et al. present evidence suggesting that disease progression in ALS follows the somatotopic organization of the motor system, reflecting the different neuroanatomical networks of upper and lower motor neurons. Diffusion of toxic factors through the neuron microenvironment may contribute to the spread of pathology.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by the progressive loss of upper (UMNs) and lower motor neurons (LMNs) causing paralysis of voluntary muscles.1 It is now accepted that ALS should be considered as a multisystem disease, in which the pathological process is not only limited to the motor system, but also extends to brain areas related to cognition and behaviour, belonging to the same genetic, clinical and neuropathological spectrum of frontotemporal dementia.2,3
A striking aspect of ALS is its heterogeneity in terms of site of disease onset, burden of UMN and LMN involvement and pattern of disease progression, which has led to the identification of different motor phenotypes,4,5 as also reported by a classic description by Gowers:
‘From the part first affected, the disease spreads to other parts of the same limb. Before it has attained a considerable degree in one limb, it usually shows itself in the corresponding limb on the other side (homologous part)’.6
This statement is further supported by detailed autopsy studies of ALS patients confirming that the loss of both UMNs and LMNs is most marked at the site of onset and diminishes in a gradient moving away from that region.7 Conversely, other studies have shown that disease progression may occasionally skip directly to non-contiguous regions of the CNS rather than following the ‘single seed and simple propagation’ hypothesis, suggesting the possibility of multifocal hits of ALS pathology.8 With regard to theories on pathology spreading, it has been debated whether anterograde ‘dying-forward’ trans-neuronal degeneration originating in the primary motor cortex9 or retrograde ‘dying-back’ degeneration starting in the LMNs10,11 represents the main mechanism underlying disease progression in ALS. Indeed, one of the most discussed hypotheses related to disease progression postulates that TAR DNA-binding protein-43 (TDP-43) aggregates propagate via axonal transport towards topographically distant regions that are connected by the corticospinal tract. This biological model would easily explain the co-occurrent involvement of agranular motor cortex and ventral horns of the spinal cord in ALS.12 However, other studies suggest the alternative hypothesis of independent pathogenic processes for neurodegeneration of UMNs and LMNs. Indeed, neuropathological examination of CNS tissues of ALS patients did not find a direct association between the entity of neuron loss in the primary motor cortex and in the spinal cord.13,14 Moreover, it seems that UMN and LMN impairment follows distinct regional spreading patterns reflecting differences in the somatotopic anatomy of the two motor neuron subpopulations.5 Finally, on rare occasions, the disease process may progress very slowly or may even remain localized to a specific region of the neuroaxis for a long time before generalization.15,16 Considering all these unsolved issues in ALS pathology, a thorough investigation of clinical features of disease progression and their relationship to site of disease onset, as well as burden of UMN and LMN dysfunction is crucial to better understand the pathophysiological mechanisms underlying ALS phenotypic heterogeneity. Therefore, the aim of this study was to investigate: (i) if site of disease onset and burden of UMN and LMN involvement may influence pattern of disease progression; (ii) if different patterns of disease spreading are associated with specific motor/neuropsychological profiles; and (iii) if specific clinical features may help identify, at an early stage, those patients in whom the disease process remains limited to a specific region for a prolonged time.
Materials and methods
Patients
An in-patient cohort of 913 Italian patients (561 males and 352 females) diagnosed with ALS and other motor neuron diseases [primary lateral sclerosis (PLS) and progressive muscular atrophy (PMA)] according to the revised El Escorial criteria17 was recruited at IRCCS Istituto Auxologico Italiano, Milan, between 2002 and 2021. The following demographic and clinical data were collected: sex; age at onset; family history of ALS; motor phenotype [classic, bulbar, respiratory, flail arm, flail leg, UMN-predominant (UMN-p), PLS, PMA]18; revised ALS Functional Rating Scale (ALSFRS-R) scores at evaluation and disease progression rate (ΔFS), calculated according to the following formula: (48 – ALSFRS-R score) / number of months from symptom onset to evaluation19,20; eye movement abnormalities (saccadic and pursuit movement impairment, upgaze palsy, oculomotor apraxia and ophthalmoplegia); disease duration and survival. We received approval for this study from the Ethics Committee of IRCCS Istituto Auxologico Italiano (18 May 2021). Written informed consent for using anonymized clinical data for research purposes was obtained at the time of evaluation from all patients included in the analysis. This study conforms with the Declaration of Helsinki on human research.
Site of disease onset and pattern of regional disease progression
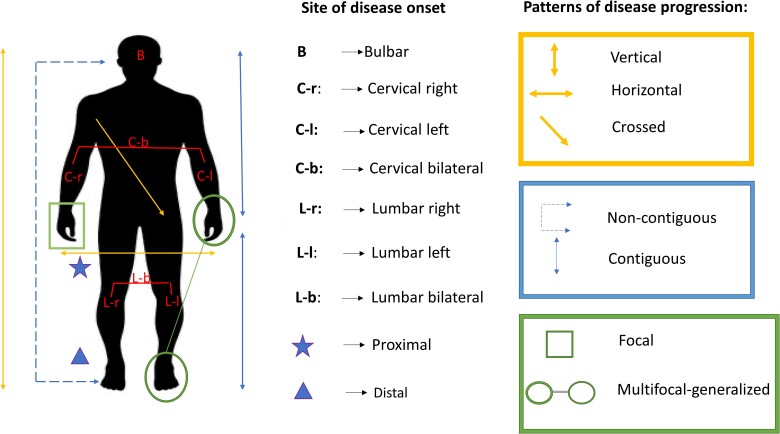
Data concerning site of disease onset and spreading were collected from patient history. Site of onset was defined as the region where motor symptoms first appeared (bulbar, cervical, thoracic or lumbosacral). For limb onset, the following characteristics were also evaluated: side of onset (left versus right), symmetry/asymmetry and involvement of distal versus proximal muscles. Whenever symptoms were reported to simultaneously affect two or more different segments, or the patient was not able to clearly specify the first affected site because symptom appearance was almost concomitant in more than one region, disease onset was considered to be multifocal-generalized. Based on the direction of the first step from site of onset towards the subsequent affected body region, the pattern of disease progression could be defined according to a triple classification: (i) horizontal/vertical/crossed; (ii) contiguous/non-contiguous; and (iii) focal/multifocal-generalized. The methodology followed to classify ALS patients according to different patterns of disease spreading is reported below and illustrated in Supplementary Table 1.
Horizontal/vertical/crossed
Directionality of disease spreading was considered horizontal when the disease spread within the same region from the site of onset to the contralateral corresponding limb, vertical when it progressed from the site of onset to the rostrally or caudally located ipsilateral region, and crossed when it spread to the contralateral rostral or caudal region. Patients with bulbar onset were not considered in this analysis because it was not possible to establish the laterality of disease onset and therefore the directionality of the first step in disease spread. Patients in whom the disease process moved to multiple regions at the same time were equally excluded from the analysis because it was not possible to establish the directionality of disease progression.
Contiguous/non-contiguous
Disease spreading was considered non-contiguous when signs and symptoms moved from the site of onset to a distant, non-adjacent region (i.e. lumbar to bulbar or bulbar to lumbar) and contiguous when they moved to a neighbouring region (i.e. bulbar to cervical, cervical to bulbar, cervical to lumbar or lumbar to cervical).
Focal/multifocal-generalized
Progression was considered focal when signs and symptoms spread to a single region after the site of onset, and multifocal-generalized when they moved simultaneously to two or more different regions (e.g. from bulbar to cervical and lumbar segments simultaneously).
Patients with thoracic onset or involvement of thoracic segments as the first step of disease progression were excluded from all the analyses for the following reasons: (i) low sensitivity of clinical signs and symptoms of motor neuron—especially UMN—involvement in this region; or (ii) impossibility of clinically establishing whether respiratory symptoms were related to cervical or thoracic spinal involvement given the different innervation of respiratory muscles.
Characteristics of disease onset and pattern of disease progression are summarized in Fig. 1. Patients in whom disease spread was still limited at the site of onset when first clinical evaluation was performed, with neither horizontal nor vertical or crossed disease progression, were assigned to the group of regionally entrenching ALS (re-ALS), while those in whom the disease process had already spread to other regions were assigned to the group of disseminating ALS (d-ALS). Considering that time of first clinical assessment was not uniform across the cohort, we corrected each analysis comparing re-ALS with d-ALS groups for the time interval between symptom onset and first evaluation in our centre.
Figure 1.
Summary of pattern of disease progression. Classification of site of disease onset and patterns of disease progression.
Motor and neuropsychological assessment
The burden of UMN and LMN signs was assessed in all patients using different scoring systems. UMN regional involvement was measured with the Penn Upper Motor Neuron Score (PUMNS), a semiquantitative scale ranging from 0 to 32 (0–4 for the bulbar segment, 0–7 for each limb), with higher scores corresponding to greater disease burden.21 LMN signs were assessed using a modified version of the Lower Motor Neuron Score (LMNS), as previously described.22,23 Spinal LMN involvement was also measured using the MRC Muscle Scale, assessing the strength of three muscle groups for each limb (shoulder abductors, elbow flexors, wrist dorsiflexors, hip flexors, knee extensors and ankle dorsiflexors; total score 0–60). The Edinburgh Cognitive and Behavioural ALS Screen (ECAS; Italian version) was used to perform an extensive evaluation of both cognitive and behavioural profile of the study population.24 As for the cognitive domains, language, verbal fluency and executive functions subtests were used to assess the ALS-specific impairment, while memory and visuospatial subtests served to assess ALS non-specific deficits. Behavioural impairment was evaluated using the score (range 0–10) of the ECAS Carer Interview as well as the number of behavioural symptoms registered therein, namely disinhibition, apathy/inertia, loss of sympathy/empathy, perseverative/stereotyped/compulsive/ritualistic behaviours and hyperorality/altered food preferences. Furthermore, the distribution of different patterns of disease progression was compared amongst different cognitive phenotypes according to the Strong revised criteria, i.e. ALScn (cognitively normal), ALSbi (behaviourally impaired), ALSci (cognitively impaired), ALScbi (cognitively and behaviourally impaired), respectively.25 Behavioural symptoms were further investigated using a dedicated scale, namely the Frontal Behavioural Inventory (FBI),26 which consists of two subscales (FBI-A and FBI-B), exploring negative and positive/disinhibited behaviours, respectively.
Statistical analysis
Statistical analysis was conducted with IBM Statistical Package for Social Science (SPSS) version 27. Survival analysis was performed with Kaplan–Meier curves and the log-rank test was used to compare survival across groups. Chi-squared and post hoc chi-squared tests were used to compare ordinal/nominal variables to each other or to compare the distribution of these variables with a hypothetical model predicting random distribution. The Mann-Whitney and Kruskal–Wallis one-way ANOVA were used as non-parametric methods to compare two or more independent groups, respectively. When appropriate, post hoc analysis was conducted to perform comparisons between subgroups. P-values <0.05 were considered statistically significant. Linear or binary logistic regression was used for modelling the relationship between scalar or binomial response and one or more explanatory variables (predictors). When exploring the phenotypical differences between re-ALS and d-ALS individuals, the variable ‘time to first evaluation’, indicating the time interval between symptom onset and first clinical assessment at our centre, was used as a covariate.
Data availability
The data supporting the findings of this study have been published on Zenodo (doi:10.5281/zenodo.7050276) and are available upon request.
Results
Demographic cohort data
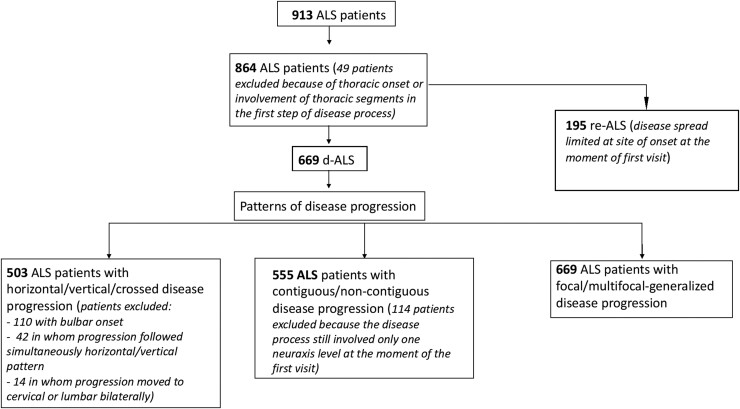
In this study, we analysed a cohort of 913 ALS patients. After the exclusion of 49 individuals with thoracic disease onset or involvement of the thoracic segment in the first step of the disease process, we evaluated the clinical records of 864 ALS patients (male: 528; female: 336). Family history (n = 860) was positive for ALS in 89 (10.3%) patients. The mean (± standard deviation, SD) age at onset was 59.3 (±12.6) years and the median survival was 54.9 (48.3–61.4) months. Site of disease onset was bulbar in 185 (21.5%), spinal in 671 (77.6%) and multifocal-generalized in eight (0.9%) patients. The cohort was divided into 669 (77.4%) d-ALS and 195 (22.6%) re-ALS. Figure 2 describes the number of patients for whom it was possible to define specific patterns of disease progression. Tables 1 and 2 report the main clinical features that characterize our patient cohort overall and in relation to pattern of disease progression.
Figure 2.
Flow chart of patients analysed for each pattern of disease progression. Flow chart describing number of patients for whom it was possible to define specific patterns of disease progression. ALS = amyotrophic lateral sclerosis; re-ALS = regionally entrenching ALS; d-ALS = disseminating ALS.
Table 1.
Association of progression patterns with demographic features of the ALS cohort
| Total cohort n = 913 |
Pattern 1, n = 495 | Pattern 2, n = 555 | Pattern 3, n = 669 | Pattern 4, n = 864 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizontal n = 389 |
Vertical n = 106 |
P | Contiguous n= 500 |
Non-contiguous
n = 55 |
P | Focal n = 595 |
Generalized n = 74 |
P | d-ALS n = 669 |
re-ALS n = 195 |
P | ||
| Sex | |||||||||||||
| Male | 561 (61.4%) | 248 (78.5%) | 68 (21.5%) | 0.940 | 175 (87.1%) | 26 (12.9%) | 0.722 | 379(89.6%) | 44 (10.4%) | 0.476 | 247 (73.5%) | 89 (26.5%) | 0.028 |
| Female | 352 (38.6%) | 141 (78.8%) | 38 (21.2%) | 325 (91.8%) | 29 (8.2%) | 216 (87.8%) | 30 (12.2%) | 422 (79.9%) | 106 (20.1%) | ||||
| Age at onset | 60.9 (59.3–62.2) |
60.0 (58.5–62.3) |
59.2 (57.0–64.1) |
0.518 | 59.2 (58.1–61.0) |
64.7 (61.2–68.3) |
0.003 | 59.7 (58.5–61.2) |
62.0 (57.3–64.1) |
0.675 | 59.9 (58.8–61.9) |
61.1 (58.2–63.5) |
0.446 |
| Family history | |||||||||||||
| FALS | 90 (9.9%) | 36 (69.2%) | 16 (30.8%) | 0.297 | 54 (93.1%) | 4 (6.9%) | 0.412 | 60 (85.7%) | 10 (14.3%) | 0.372 | 70 (78.7%) | 19 (21.3%) | 0.733 |
| SALS | 819 (90.1%) | 350 (79.5%) | 90 (20.5%) | 444 (89.7%) | 51 (10.3%) | 532 (89.3%) | 64 (10.7%) | 596 (77.3%) | 175 (22.7%) | ||||
| Site of onset | |||||||||||||
| Bulbar | 210 (23.0%) | – | – | n.a. | 82 (74.5%) | 28 (25.5%) | <0.001 | 100 (86.2%) | 16 (13.8%) | 0.312 | 110 (59.5%) | 75 (40.5%) | <0.001 |
| Spinal | 703 (77.0%) | 389 (78.6%) | 106 (21.4%) | 418 (93.9%) | 27 (6.1%) | 492 (89.5%) | 58 (10.5%) | 559 (82.3%) | 120 (17.7%) | ||||
| Proximal | 166 (24.2%) | 106 (89.1%) | 13 (10.9%) | <0.001 | 99 (91.7%) | 9 (8.3%) | 0.219 | 118 (88.3%) | 9 (11.7%) | 0.136 | 126 (79.2%) | 33 (20.8%) | 0.249 |
| Distal | 521 (75.8%) | 281 (75.7%) | 90 (24.3%) | 315 (94.9%) | 17 (5.1%) | 376 (92.9%) | 50 (7.1%) | 427 (83.2%) | 86 (16.8%) | ||||
| Symmetrical | 168 (24.3%) | 110 (100%) | 0 (0%) | <0.001 | 98 (90.7%) | 10 (9.3%) | 0.085 | 392 (87.5%) | 56 (12.5%) | 0.003 | 110 (66.9%) | 48 (30.4%) | <0.001 |
| Asymmetrical | 523 (75.7%) | 279 (72.7%) | 85 (27.3%) | 319 (95.2%) | 16 (4.8%) | 106 (97.2%) | 3 (2.8%) | 447 (86.3%) | 71 (13.7%) | ||||
| Survival | 53.2 (46.9–69.4) |
63.6 (53.0–74.1) |
37.5 (30.3–44.6) |
<0.001 | 51.5 (44.3–58.6) |
53.9 (25.0–82.7) |
0.908 | 55.4 (48.7–62.0) |
42.4 (35.1–49.6) |
0.264 | 54.7 (47.5–61.5) |
51.7 (31.6–71.7) |
0.855 |
Chi-squared and chi-squared post hoc analysis (with Bonferroni correction) testing differences in distribution of patterns of disease progression across different demographic features. Significant P-values are in bold.
d-ALS = disseminating ALS; FALS = familial amyotrophic lateral sclerosis; n = number of patients; n.a. = not applicable; re-ALS = regionally entrenching ALS; SALS = sporadic amyotrophic lateral sclerosis.
Table 2.
Association of progression patterns with motor and neuropsychological phenotypes of the ALS cohort
| Total cohort n = 913 |
Pattern 1, n = 495 | Pattern 2, n = 555 | Pattern 3, n = 669 | Pattern 4, n = 864 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizontal n = 389 |
Vertical n = 106 |
P | Contiguous n = 500 |
Non-contiguous n = 55 |
P | Focal n = 595 |
Generalized n = 74 |
P | d-ALS n = 669 |
re-ALS n = 195 |
P | ||
| Motor phenotype | |||||||||||||
| Bulbar | 185 (20.3%) | – | – | – | 76 (76.8%) | 23 (23.2%) | <0.001** | 86 (86.0%) | 14 (14.0%) | 0.322 | 100 (59.9%) | 67 (40.1%) | <0.001** |
| Classic | 492 (53.9%) | 276 (77.3%) | 81 (22.7%) | 0.207 | 320 (95.2%) | 16 (4.8%) | <0.001** | 361 (88.3%) | 48 (11.7%) | 0.48 | 409 (83.8%) | 79 (16.2%) | <0.001** |
| Flail arm | 36 (3.9%) | 24 (100%) | 0 (0%) | 0.009** | 14 (100%) | 0 (0%) | 0.194 | 25 (100%) | 0 (0%) | 0.072 | 25 (73.5%) | 9 (26.5%) | 0.549 |
| Flail leg | 20 (2.2%) | 14 (93.3%) | 1 (6.7%) | 0.159 | 6 (85.7%) | 1 (14.3%) | 0.689 | 15 (100%) | 0 (0%) | 0.165 | 15 (75.0%) | 5 (25.0%) | 0.764 |
| UMN-p | 68 (7.4%) | 27 (65.8%) | 14 (34.2%) | 0.041* | 34 (82.9%) | 7 (17.1%) | 0.009* | 45 (91.8%) | 4 (8.2%) | 0.133 | 50 (76.9%) | 15 (23.1%) | 0.424 |
| PLS | 53 (5.8%) | 17 (63.0%) | 10 (37.0%) | 0.003** | 27 (77.1%) | 8 (22.9%) | 0.11 | 31 (81.6%) | 7 (18.4%) | 0.131 | 38 (73.9%) | 14 (26.1%) | 0.317 |
| PMA | 41 (4.5%) | 31 (100%) | 0 (0%) | 0.038* | 23 (100%) | 0 (0%) | 0.11 | 32 (97.0%) | 1 (3.0%) | 0.489 | 32 (84.2%) | 6 (15.8%) | 0.92 |
| Respiratory | 18 (2.0%) | – | – | – | – | – | – | – | – | – | – | – | – |
| Cognitive phenotype | |||||||||||||
| ALScn | 101 (38.8%) | 47 (74.6%) | 16 (25.4%) | 0.617 | 53 (94.6%) | 3 (5.4%) | 0.472 | 70 (94.6%) | 4 (5.4%) | 0.208 | 74 (74.7%) | 25 (25.3%) | 0.689 |
| ALSci | 73 (28.1%) | 35 (79.5%) | 9 (20.5%) | 0.701 | 40 (88.9%) | 5 (11.1%) | 0.424 | 28 (82.4%) | 4 (17.6%) | 0.904 | 53 (76.8%) | 12 (23.2%) | 0.644 |
| ALSbi | 51 (19.6%) | 22 (84.6%) | 4 (15.4%) | 0.324 | 26 (96.3%) | 1 (3.7%) | 0.046* | 49 (92.5%) | 6 (7.5%) | 0.038* | 34 (73.9%) | 16 (26.1%) | 0.841 |
| ALScbi | 35 (13.5%) | 15 (68.2%) | 7 (31.8%) | 0.317 | 20 (90.9%) | 2 (11.1%) | 0.246 | 23 (92.0%) | 2 (8.0%) | 0.794 | 26 (81.3%) | 6 (18.8%) | 0.484 |
Chi-squared and chi-squared post hoc analysis (with Bonferroni correction) testing differences in distribution of patterns of disease progression across different motor and neuropsychological ALS phenotypes. Significant P-values are in bold. ALSbi = ALS with behavioural impairment; ALScbi = ALS with cognitive and behavioural impairment; ALSci = ALS with cognitive impairment; ALScn = cognitively normal ALS; d-ALS = disseminating ALS; n = number of patients; PLS = primary lateral sclerosis; PMA = progressive muscular atrophy; re-ALS = regionally entrenching ALS; UMN-p = upper motor neuron predominant.
*Significant P-values only before Bonferroni correction.
**Significant P-values also after Bonferroni correction.
Features of disease progression based on site of onset
Directionality of disease spread based on site of disease onset is graphically illustrated in Supplementary Fig. 1. Interactive Supplementary Fig. 2 displays all successive steps of disease progression from site of onset for group of patients presenting the same pattern of disease spreading. Disease progression in patients with bulbar onset involved preferentially cervical rather than lumbar segments (P < 0.001), with no preference of side. In a large group of bulbar-onset patients (42.7%), the disease process was still limited to the site of disease onset at the time of first clinical assessment. Asymmetrical cervical and lumbar spinal onset was more frequently associated with a horizontal rather than vertical pattern of disease progression. Moreover, cervical onset was more frequently followed by lumbar rather than bulbar involvement (43.8% versus 10.8%; P < 0.001) while lumbar spinal onset was more often followed by involvement of the cervical segment rather than the bulbar one. Patients with symmetrical spinal onset showed more frequently a bilateral (disease spreading to both sides of another spinal segment, e.g. from cervical bilateral to lumbar bilateral) rather than unilateral disease progression from site of onset (37.5% versus 15.0%; P < 0.001). Proximal limb onset was more frequently associated with symmetrical disease onset when compared to distal limb onset (46.8% versus 16.1%; P < 0.001). Conversely, distal limb onset was more frequently associated with an asymmetric one (83.8% versus 54.4%; P < 0.001).
Clinical phenotype differences in patients with horizontal versus vertical spreading pattern
As for patients with spinal onset, it was possible to clearly assess the directionality of disease progression from site of onset for 503 patients, with horizontal pattern of disease spread (389 individuals, 77.3%) being more frequently observed compared to vertical one (106 individuals, 21.1%). This difference is highly significant when compared to a hypothetical random distribution (χ2 = 88.0, P < 0.001). Considering that only eight (1.6%) patients showed a crossed pattern of disease spreading, no analysis was performed for this specific group. No significant differences were observed in terms of directionality of disease progression between cervical versus lumbar and right versus left spinal onset. Moreover, no differences were appreciated in terms of sex, age of disease onset and ALS family history between patients with a horizontal pattern of disease progression and those with a vertical one. As for the motor phenotype, horizontal disease progression was more frequently associated with PMA and flail arm phenotypes when compared to vertical disease progression, whereas vertical disease spread was more frequently observed in patients with UMN-p and PLS phenotypes (Tables 1 and 2).
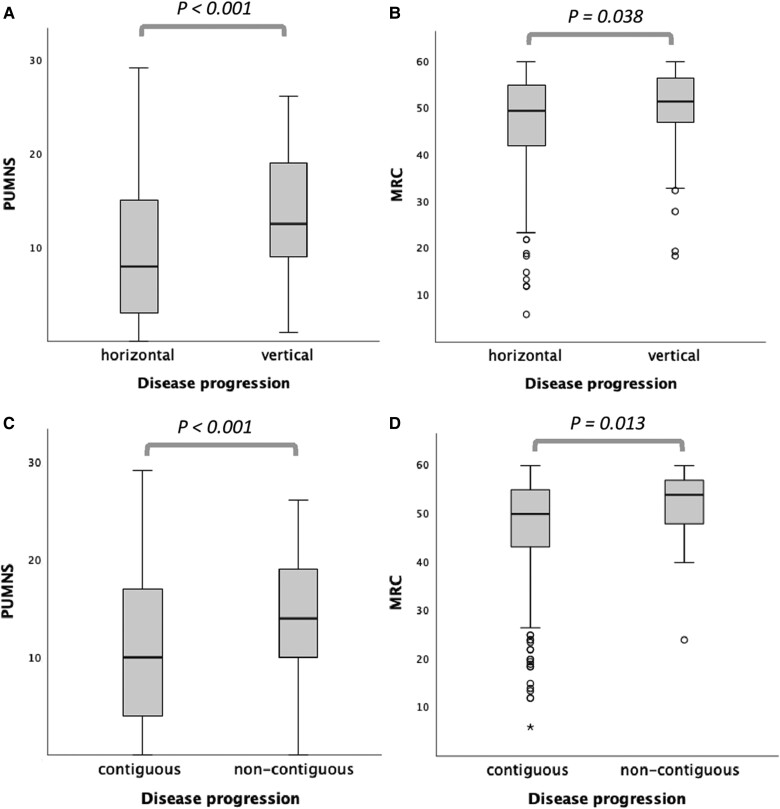
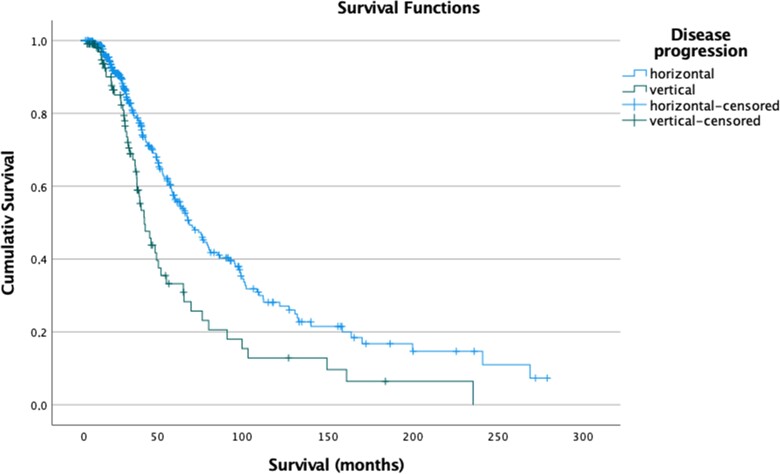
Higher PUMNS values, indicating more extensive UMN involvement, were observed in patients with vertical disease spread when compared to individuals with horizontal progression (median values: 12.5 versus 8.0; P < 0.001) (Fig. 3A). On the contrary, lower scores at MRC, indicating more severe impairment of LMNs, were more frequently found in patients with horizontal pattern of disease progression compared to those with vertical disease progression (median values: 49.5 versus 51.5; P = 0.038) (Fig. 3B). Spinal onset involving proximal limb muscles was more likely to be observed in patients with horizontal compared to vertical spreading (frequency: 27.4% versus 12.6%; P < 0.002), while involvement of distal limb muscles was associated with vertical rather than horizontal progression (frequency: 87.2% versus 72.6%; P < 0.002). Furthermore, patients with vertical disease spreading had reduced survival compared to those with horizontal progression (median values: 37.5 versus 63.6 months; log-rank test, P < 0.001) (Fig. 4). The neuropsychological profile, assessed using both ECAS and FBI, was available for 166 patients. No differences were observed between the two groups both for cognitive and for behavioural domains.
Figure 3.
Kruskal-Wallis analysis to compare motor features among different patterns of disease progression. Distribution of upper motor neuron involvement using the Penn Upper Motor Neuron Score (PUMNS) and lower motor neuron involvement using the Medical Research Council Muscle Scale (MRC) in patients with vertical versus horizontal (A and B) and contiguous versus non-contiguous (C and D) pattern of disease progression from site of onset. Kruskal-Wallis test for independent samples. For each group, the bold horizontal line shows the median, the grey box includes the middle 50% of the data and whiskers show the minimum and maximum values. Empty circles represent outliers (above Q3 + 1.5 IQR and below Q1 − 1.5 IQR, respectively).
Figure 4.
Survival analysis in patients with horizontal/vertical pattern of disease progression. Kaplan-Meyer curves of survival probabilities: patients with horizontal disease progression (light blue line) had significantly prolonged survival when compared to patients with vertical spreading (green line) (log-rank: χ2 = 11.083; P < 0.001).
Clinical phenotype differences in patients with contiguous versus non-contiguous spreading pattern
Contiguous/non-contiguous pattern of disease spread could be determined for 555 patients. Among these, 55 (9.9%) individuals showed a non-contiguous pattern of progression with signs and symptoms spreading directly from bulbar to lumbar segments (28 patients, 51.0%) and from lumbar to bulbar segments in the remaining 27 cases (49.0%). Patients with non-contiguous disease progression were significantly older than those with contiguous spread at the time of symptom onset (64.7 versus 59.2 years; P = 0.003). No differences were observed in terms of sex and ALS family history. Regarding motor phenotype, non-contiguous disease spreading was more frequently observed in bulbar and UMN-p phenotypes, while contiguous disease progression was the predominant pattern in classic ALS (Tables 1 and 2). The non-contiguous pattern was significantly associated with more severe UMN impairment, as evidenced by higher PUMNS values, when compared to the contiguous one (median values: 14.0 versus 10.0; P < 0.001) (Fig. 3C). Conversely, patients with contiguous disease progression showed more extensive LMN involvement, as evidenced by significantly lower scores at MRC (median values: 50.0 versus 54.0; P = 0.013) (Fig. 3D) and higher scores at LMNS when compared to individuals with non-contiguous progression (5.0 versus 4.0, P = 0.037). No differences were observed in terms of survival. However, patients with non-contiguous disease spreading had significantly lower scores at ALSFRS-R (median values: 35.0 versus 38.5; P = 0.038). Neuropsychological assessment with ECAS was available for 149 patients (138 with contiguous and 11 with non-contiguous progression). Non-contiguous disease spreading was associated with more severe cognitive impairment when compared to contiguous spreading, as indicated by significantly lower scores in the following ECAS domains/scores: executive (median values: 30.0 versus 35.0; P = 0.048), visuospatial (median values: 11.0 versus 12.0; P = 0.024), ALS non-specific (median values: 24.0 versus 28.0; P = 0.041), and total (median values: 92.0 versus 104.0; P = 0.047) (Supplementary Fig. 3). Concerning the behavioural domains explored by ECAS and FBI, no differences were observed.
Clinical phenotype differences in patients with focal versus multifocal-generalized spreading pattern
Among the 669 patients analysed, 595 (88.9%) presented with a focal pattern of disease spreading, while 74 (11.1%) with a multifocal-generalized pattern. No differences were observed in terms of age at disease onset, sex, family history, motor phenotype, burden of UMN and LMN involvement, and cognitive and behavioural profile between the two groups. Conversely, patients with multifocal-generalized disease spreading presented more frequently with upgaze palsy when compared to patients with focal pattern (frequencies: 9.7% versus 3.0%; P = 0.005).
Clinical phenotype differences in patients with disseminating versus regionally entrenching ALS
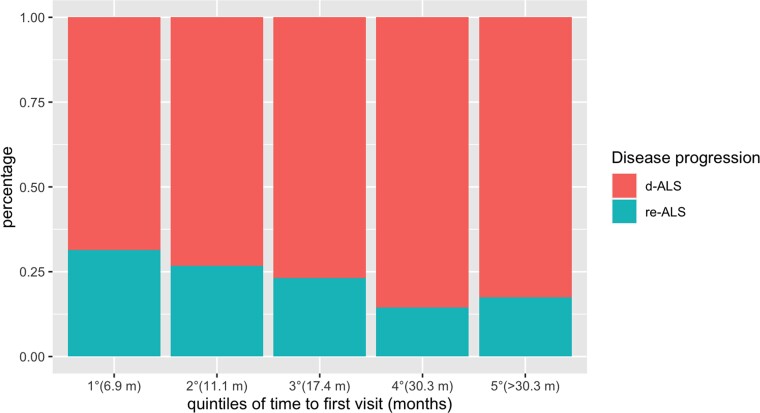
Considering that clinical data were retrospectively collected from patients’ first clinical assessment in our centre, we compared clinical features between d-ALS and re-ALS individuals after adjusting for time to first evaluation, expressed as number of months from symptom onset, to reduce heterogeneity bias (median value 24.3 versus 20.5; P < 0.001). Figure 5 illustrates per cent distribution of both d-ALS and re-ALS individuals along five consecutive quintiles of time to first visit.
Figure 5.
Distribution of d-ALS and re-ALS individuals among successive quintiles of time to first visit. Per cent distribution of d-ALS and re-ALS individuals among successive quintiles of time to first visit. The quintile distribution was as follows: 1° from 1.1 to 6.9 months; 2° from 6.9 to 11.1 months; 3° from 11.1 to 17.4 months; 4° from 17.4 to 30.3 months; 5° more than 30.3 months. ALS = amyotrophic lateral sclerosis; d-ALS = disseminating ALS; re-ALS = regionally entrenching ALS.
Regionally entrenching ALS individuals were more frequently female (45.6% versus 36.9%; P = 0.028) and had higher frequencies of symmetric disease onset (40.3% versus 19.7%; P < 0.001) and bulbar phenotype (38.5% versus 16.4%; P < 0.001). Bulbar UMN and LMN signs were equally distributed between re-ALS and d-ALS individuals. No statistically significant differences were observed between the two groups pertaining to the other clinical and neuropsychological variables.
Discussion
The main findings from our study reveal that patterns of disease progression are related to somatotopic organization of the motor system, with UMN impairment driving mainly a vertical and non-contiguous pattern of disease spreading and LMN dysfunction a horizontal and contiguous one. Horizontal disease spreading was also more frequently associated with proximal spinal onset, while vertical progression with a distal spinal onset. Moreover, patients with proximal spinal onset also showed more frequently a symmetric spinal onset followed by a bilateral pattern of disease progression. Finally, vertical disease spreading was associated with reduced survival when compared to horizontal spreading.
The relationship between patterns of disease progression and extent of UMN and LMN loss has already been described in the literature, raising the hypothesis that UMN and LMN deficits propagate following different trajectories because of their differing somatotopic anatomy.5 According to this, given that the anatomical distance between cortical columns pertaining to different body segments within the primary motor cortex of a single brain hemisphere is shorter compared to the one separating corresponding cortical columns between the two hemispheres, it would be relatively easy for a cortical degenerative process involving UMN cell bodies to follow a vertical spreading process as opposed to a horizontal one. It must be recognized, however, that other mechanisms of anatomical disease progression have been hypothesized in ALS, including a dying-back axonopathy, as suggested by the neuroradiological evidence of maximal reduction of fractional anisotropy in the distal intracranial segment of the corticospinal tracts.27,28 On the other hand, a horizontal spreading pattern is expected to be most likely observed at the spinal cord level, where the anatomical distance between LMN groups innervating corresponding muscles of opposite sides of the body is significantly shorter compared to intersegmental distances. Nevertheless, a horizontal spreading modality has also been described at the UMN level via transcallosal axonal pathways.29 The association between UMN impairment and non-contiguous pattern of disease spreading is more difficult to explain. In the context of the limited anatomical extent of the motor cortex, one could hypothesize a role for putative local toxic factors, which might not only diffuse through the interstitial fluid to contiguous cells but also be more distantly conveyed by the CSF circulation.
Importantly, the different influence of UMN and LMN involvement on disease spreading has been investigated by a recent study based on a large cohort of ALS patients recruited in five centres across Europe.30 In this multicentric, prospective study, the authors explored disease spreading in relation to regional onset of UMN and LMN signs, supporting the hypothesis of a regional progression of LMN degeneration, mostly by contiguity while UMN pathology accelerates rostro-caudal LMN loss. Although these results suggest an independent pathway of spreading for UMN and LMN signs, our findings indicate a horizontal disease progression within the same spinal segment in patients with predominant LMN degeneration as opposed to a vertical progression in individuals with predominant UMN involvement. The topographic organization of the motor cortex and the spinal cord might be responsible for this difference in directionality of disease progression, reflecting somatotopic features of the upper and the recently proposed lower motor homunculus.31 Moreover, while the abovementioned study relied on qualitative assessment of clinical signs, our work used semiquantitative scales to quantify the burden of UMN and LMN involvement.
Remarkably, we also observed that vertical disease progression was associated with spinal disease onset involving distal parts of limbs, while horizontal spread was more frequently observed in proximal limb onset. To further explain this association, it should be noted that a subtle impairment of fine fractioned hand control often precedes the clinical appearance of weakness and atrophy,32 and that the motor cortex plays a disproportionate role in determining dexterity of distal limb movements.33 Considering this point, it is likely that vertical disease progression in distal limb onset is driven once again by a greater impairment of UMNs, which are more involved in the control of fine hand movements than in gross motor activity of proximal limb muscles. This difference could be also reflected in somatotopic and functional organization of motor neurons in the spinal cord. Indeed, motor neurons innervating distal limb muscles are located more laterally in the anterior horns and receive a greater number of afferences from motor cortex compared to those innervating axial and proximal muscles, which are located more medially.34,35 Additionally, it is worth mentioning that medially descending pathways (anterior corticospinal, vestibulospinal and tectospinal tracts) exert bilateral control on LMNs innervating axial and proximal limb muscles through synapses with commissural interneurons whose axons decussate in the spinal cord.36 This somatotopic difference with the lateral corticospinal tract, which follows instead a unilateral pattern of innervation, could explain why, in our cohort, proximal spinal onset tends to be more frequently symmetrical when compared to distal one, as well as more frequently followed by a bilateral pattern of disease progression.
Finally, variable spreading patterns across ALS phenotypes also reflect different involvement of UMNs and LMNs, with UMN-p and PLS on one hand mostly showing a vertical disease progression pattern, while flail arm and PMA phenotypes—on the other hand—a horizontal progression. Vertical disease progression was associated with reduced survival, while patients with non-contiguous disease spreading had lower scores on ALSFRS-R and more severe cognitive impairment in both ALS-specific and non-specific domains. These results may indicate that a major involvement of the motor cortex, resulting more frequently in a vertical and non-contiguous pattern of disease progression, comes with a diffuse involvement of the CNS, leading to a higher degree of disability and cognitive impairment and, therefore, to an increased risk of death.37,38 A similar consideration could be made for patients with multifocal-generalized pattern of progression in whom a more widespread disease type seems to be associated with involvement of extra-motor areas, as indicated by higher occurrence of eye movement dysfunction.
As for the observed association between vertical disease progression and reduced survival, it must be noticed that such patients have, by definition, an earlier involvement of multiple body regions compared to those with a horizontal pattern. This more widespread disease process may in turn lead to a worse prognosis.39
Finally, we studied clinical features of ALS individuals in whom the disease process was still limited to site of onset when the first clinical evaluation was performed (re-ALS). The interval between symptom onset and time to first assessment was used as a covariate to mitigate the fact that time to first visit was not uniform across our cohort. Our results show that these patients are often females with bulbar disease onset. In agreement with existing literature,40 it is likely that some of our re-ALS cases might represent those rare forms of isolated bulbar ALS that, unlike classic bulbar phenotypes portending a reduced survival, are instead associated with a long disease course, limited to bulbar segment, with a relatively benign prognosis. Indeed, in this specific phenotype, the disease process remains localized to the bulbar region or spreads to spinal segments only after several years. It must be noticed, however, that contrary to what has previously been reported by other authors, no predominance of UMN signs was found in the bulbar region among re-ALS individuals studied in our cohort.41
Our study has some limitations. First, site of disease onset and pattern of disease progression were collected from patient history, which does not allow the identification of subtle deficits or clinically silent disease progression. Indeed, this may have biased our search towards LMN involvement, because initial UMN dysfunction might result in less prominent symptoms and therefore be reported to a lesser extent by patients. Futhermore, as already explained in the ‘Materials and methods’ section, we were forced to remove from our analysis patients with thoracic onset or those with involvement of the thoracic segment as the first step of disease spreading, partially limiting the generalizability of our models of disease progression. Likewise, patients with bulbar onset were excluded from the evaluation of directionality (horizontal/vertical/crossed) of disease progression limiting our findings to spinal onset ALS individuals for this specific analysis. Last, the availability of neuropsychological data only for a subset of ALS patients and the use of a screening tool such as the ECAS, rather than a full testing battery, limits the generalizability of the observed associations between disease spreading patterns and cognitive-behavioral phenotype. As such, more comprehensive neuropsychological batteries shall be employed in future studies investigating this topic.
Conversely, our work is one of the largest studies analysing disease progression in ALS and providing a comprehensive description of clinical features in relation to pattern of disease spreading.
Conclusion
Our study suggests that the burden of UMN and LMN involvement plays a crucial role in determining directionality of disease spreading in ALS pathology and indicates that disease progression follows different anatomical patterns reflecting motor system organization of the CNS. Second, we demonstrated that different patterns of disease spreading are associated with different clinical ALS phenotypes, highlighting the importance of a detailed observation of the first steps of disease progression in order to predict evolution of ALS symptoms. Finally, we described the main clinical features of a group of ALS patients in which the disease process remains localized to the site of disease onset or at most progresses very slowly (re-ALS). Further longitudinal studies, possibly exploiting neurophysiological, neuroradiological and/or neurochemical biomarkers of UMN and LMN involvement, are required to confirm our findings and to further explore the relationship between disease progression and clinical phenotypes.
Supplementary Material
Acknowledgements
The authors are thankful to patients, patients’ families, and healthcare professionals involved in patient care, and thank Mrs Patrizia Nelli for administrative support. The authors also thank Dr Alessandro Gaeta for his help in creating the interactive pie chart. The authors acknowledge the ERN Euro-NMD for support.
Contributor Information
Alessio Maranzano, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Federico Verde, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy; Department of Pathophysiology and Transplantation, ‘Dino Ferrari’ Center, Università degli Studi di Milano, Milan, 20122, Italy.
Eleonora Colombo, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Barbara Poletti, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Alberto Doretti, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Ruggero Bonetti, Neurology Residency Program, Università degli Studi di Milano, Milan, 20122, Italy.
Delia Gagliardi, Department of Pathophysiology and Transplantation, ‘Dino Ferrari’ Center, Università degli Studi di Milano, Milan, 20122, Italy; Neurology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, 20122, Italy.
Megi Meneri, Department of Pathophysiology and Transplantation, ‘Dino Ferrari’ Center, Università degli Studi di Milano, Milan, 20122, Italy; Neurology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, 20122, Italy.
Luca Maderna, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Stefano Messina, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Stefania Corti, Department of Pathophysiology and Transplantation, ‘Dino Ferrari’ Center, Università degli Studi di Milano, Milan, 20122, Italy; Neurology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, 20122, Italy.
Claudia Morelli, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy.
Vincenzo Silani, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy; Department of Pathophysiology and Transplantation, ‘Dino Ferrari’ Center, Università degli Studi di Milano, Milan, 20122, Italy.
Nicola Ticozzi, Department of Neurology and Laboratory of Neuroscience, IRCCS Istituto Auxologico Italiano, Milan, 20149, Italy; Department of Pathophysiology and Transplantation, ‘Dino Ferrari’ Center, Università degli Studi di Milano, Milan, 20122, Italy.
Funding
This work was financially supported by the Ministero della Salute (Grants RF-2013-02355764 and GR-2016-02364373), Fondazione Italiana di Ricerca per la SLA (Grant Azygos 2.0) and Università degli Studi di Milano (SEED 2019-GenderALS).
Competing interests
A.M., F.V., E.C., B.P., A.D., R.B., D.G. M.M., L.M., S.M., S.C. and C.M. report no disclosure. V.S. received compensation for consulting services and/or speaking activities from AveXis, Cytokinetics, Italfarmaco, LiquidWeb Srl and Novartis Pharma AG. He receives or has received research support from the Italian Ministry of Health, AriSLA, and E-Rare Joint Translational Call. He is on the Editorial Board of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, European Neurology, American Journal of Neurodegenerative Disease and Frontiers in Neurology. N.T. received compensation for consulting services from Amylyx Pharmaceutical and Zambon Biotech SA. He received research funding from the Italian Ministry of Health and AriSLA. He is associate editor of Frontiers in Aging Neuroscience.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. [DOI] [PubMed] [Google Scholar]
- 2. Burrell JR, Halliday GM, Kril JJ, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388:919–931. [DOI] [PubMed] [Google Scholar]
- 3. Verde F, Tredici KD, Braak H, Ludolph A. The multisystem degeneration amyotrophic lateral sclerosis—Neuropathological staging and clinical translation. Arch Ital Biol. 2017;155:118–130. [DOI] [PubMed] [Google Scholar]
- 4. Grad LI, Rouleau GA, Ravits J, Cashman NR. Clinical Spectrum of amyotrophic lateral sclerosis (ALS). Cold Spring Harb Perspect Med. 2017;7:a024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravits JM, La Spada AR. ALS Motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gowers WR. A manual of diseases of the nervous system. 1st ed. J. & A. Churchill; 1886-1888. [Google Scholar]
- 7. Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: Rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68:1576–1582. [DOI] [PubMed] [Google Scholar]
- 8. Sekiguchi T, Kanouchi T, Shibuya K, et al. Spreading of amyotrophic lateral sclerosis lesions–multifocal hits and local propagation? J Neurol Neurosurg Psychiatry. 2014;85:85–91. [DOI] [PubMed] [Google Scholar]
- 9. Pamphlett R, Kril J, Hng TM. Motor neuron disease: A primary disorder of corticomotoneurons? Muscle Nerve. 1995;18:314–318. [DOI] [PubMed] [Google Scholar]
- 10. Chou SM, Norris FH. Issues & opinions: Amyotrophic lateral sclerosis: Lower motor neuron disease spreading to upper motor neurons. Muscle Nerve. 1993;16:864–869. [DOI] [PubMed] [Google Scholar]
- 11. Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Exp Neurol. 2004;185:232–240. [DOI] [PubMed] [Google Scholar]
- 12. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis: ALS stages. Ann Neurol. 2013;74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Attarian S, Vedel JP, Pouget J, Schmied A. Progression of cortical and spinal dysfunctions over time in amyotrophic lateral sclerosis: Progressive dysfunction in ALS. Muscle Nerve. 2008;37:364–375. [DOI] [PubMed] [Google Scholar]
- 14. Kiernan JA, Hudson AJ. Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain. 1991;114:843–853. [DOI] [PubMed] [Google Scholar]
- 15. Grohme K, Maravic MV, Gasser T, Borasio GD. A case of amyotrophic lateral sclerosis with a very slow progression over 44 years. Neuromuscul Disord. 2001;11:414–416. [DOI] [PubMed] [Google Scholar]
- 16. Zhang H, Chen L, Tian J, Fan D. Differentiating slowly progressive subtype of lower limb onset ALS from typical ALS Depends on the time of disease progression and phenotype. Front Neurol. 2022;13:872500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brooks BR, Miller RG, Swash M, Munsat TL. El escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 18. Chio A, Calvo A, Moglia C, Mazzini L, Mora G; PARALS study group . Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. J Neurol Neurosurg Psychiatry. 2011;82:740–746. [DOI] [PubMed] [Google Scholar]
- 19. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21. [DOI] [PubMed] [Google Scholar]
- 20. Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66:265–267. [DOI] [PubMed] [Google Scholar]
- 21. Quinn C, Edmundson C, Dahodwala N, Elman L. Reliable and efficient scale to assess upper motor neuron disease burden in amyotrophic lateral sclerosis. Muscle Nerve. 2020;61:508–511. [DOI] [PubMed] [Google Scholar]
- 22. Devine MS, Ballard E, O’Rourke P, Kiernan MC, Mccombe PA, Henderson RD. Targeted assessment of lower motor neuron burden is associated with survival in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(3–4):184–190. [DOI] [PubMed] [Google Scholar]
- 23. Maranzano A, Poletti B, Solca F, et al. Upper motor neuron dysfunction is associated with the presence of behavioural impairment in patients with amyotrophic lateral sclerosis. Euro J Neurol. 2022;29:1402–1409. [DOI] [PubMed] [Google Scholar]
- 24. Poletti B, Solca F, Carelli L, et al. The validation of the Italian Edinburgh cognitive and behavioural ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(7–8):489–498. [DOI] [PubMed] [Google Scholar]
- 25. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis—Frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3–4):153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alberici A, Geroldi C, Cotelli M, et al. The frontal behavioural inventory (Italian version) differentiates frontotemporal lobar degeneration variants from Alzheimer’s disease. Neurol Sci. 2007;28:80–86. [DOI] [PubMed] [Google Scholar]
- 27. Iwata NK, Kwan JY, Danielian LE, et al. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain. 2011;134:2642–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Floeter MK, Mills R. Progression in primary lateral sclerosis: A prospective analysis. Amyotroph Lateral Scler. 2009;10(5–6):339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flynn L, Stephen M, Floeter MK. Disease spread through contiguity and axonal tracts in primary lateral sclerosis: Short reports. Muscle Nerve. 2014;49:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gromicho M, Figueiral M, Uysal H, et al. Spreading in ALS: The relative impact of upper and lower motor neuron involvement. Ann Clin Transl Neurol. 2020;7:1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravits J, Stack J. The lower motor neuron homunculus. Brain. 2022;145:3727–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eisen A, Turner MR, Lemon R. Tools and talk: An evolutionary perspective on the functional deficits associated with amyotrophic lateral sclerosis: Issues & opinions: Evolutionary aspects of ALS. Muscle Nerve. 2014;49:469–477. [DOI] [PubMed] [Google Scholar]
- 33. Eisen A, Braak H, Del Tredici K, Lemon R, Ludolph AC, Kiernan MC. Cortical influences drive amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88:917–924. [DOI] [PubMed] [Google Scholar]
- 34. Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–440. [DOI] [PubMed] [Google Scholar]
- 35. Catani M. A little man of some importance. Brain. 2017;140:3055–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. [DOI] [PubMed] [Google Scholar]
- 37. Rizzo G, Marliani A, Battaglia S, et al. Diagnostic and prognostic value of conventional brain MRI in the clinical work-up of patients with amyotrophic lateral sclerosis. JCM. 2020;9:2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beeldman E, Raaphorst J, Klein Twennaar M, de Visser M, Schmand BA, de Haan RJ. The cognitive profile of ALS: A systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry. 2016;87:611–619. [DOI] [PubMed] [Google Scholar]
- 39. Westeneng HJ, Debray TPA, Visser AE, et al. Prognosis for patients with amyotrophic lateral sclerosis: Development and validation of a personalised prediction model. Lancet Neurol. 2018;17:423–433. [DOI] [PubMed] [Google Scholar]
- 40. Zhang HG, Chen L, Tang L, Zhang N, Fan DS. Clinical features of isolated bulbar palsy of amyotrophic lateral sclerosis in Chinese population. Chin Med J. 2017;130:1768–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burrell JR, Vucic S, Kiernan MC. Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:283–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study have been published on Zenodo (doi:10.5281/zenodo.7050276) and are available upon request.