Abstract
Aims
Deep neural network artificial intelligence (DNN-AI)–based Heart Age estimations have been presented and used to show that the difference between an electrocardiogram (ECG)-estimated Heart Age and chronological age is associated with prognosis. An accurate ECG Heart Age, without DNNs, has been developed using explainable advanced ECG (A-ECG) methods. We aimed to evaluate the prognostic value of the explainable A-ECG Heart Age and compare its performance to a DNN-AI Heart Age.
Methods and results
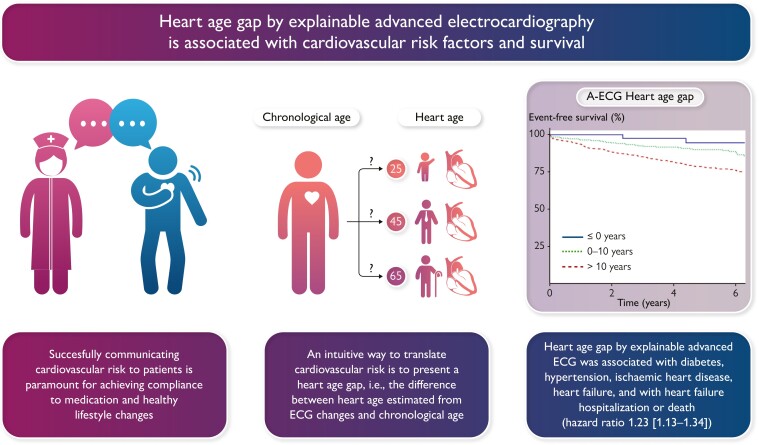
Both A-ECG and DNN-AI Heart Age were applied to patients who had undergone clinical cardiovascular magnetic resonance imaging. The association between A-ECG or DNN-AI Heart Age Gap and cardiovascular risk factors was evaluated using logistic regression. The association between Heart Age Gaps and death or heart failure (HF) hospitalization was evaluated using Cox regression adjusted for clinical covariates/comorbidities. Among patients [n = 731, 103 (14.1%) deaths, 52 (7.1%) HF hospitalizations, median (interquartile range) follow-up 5.7 (4.7–6.7) years], A-ECG Heart Age Gap was associated with risk factors and outcomes [unadjusted hazard ratio (HR) (95% confidence interval) (5 year increments): 1.23 (1.13–1.34) and adjusted HR 1.11 (1.01–1.22)]. DNN-AI Heart Age Gap was associated with risk factors and outcomes after adjustments [HR (5 year increments): 1.11 (1.01–1.21)], but not in unadjusted analyses [HR 1.00 (0.93–1.08)], making it less easily applicable in clinical practice.
Conclusion
A-ECG Heart Age Gap is associated with cardiovascular risk factors and HF hospitalization or death. Explainable A-ECG Heart Age Gap has the potential for improving clinical adoption and prognostic performance compared with existing DNN-AI-type methods.
Keywords: Heart age, Vascular age, Risk prediction, Cardiovascular disease
Graphical Abstract
Graphical Abstract.
Background
Age is the strongest determinant of cardiovascular mortality, yet the ageing process, i.e. the complex physiological degeneration at molecular, cellular, or organ levels, occurs at a different pace in different individuals.1,2 Therefore, several attempts have been made to describe this process, i.e. to develop a measure that can adjust the chronological age for the individual rate of the ageing process, presenting it as vascular age or a Heart Age.1,3–5 The electrocardiogram (ECG) is an excellent tool for this purpose, since several electrocardiographic parameters change with age but also with cardiovascular disease.6–11 By estimating an individual’s biological age through ECG data, the resulting Heart Age can be contrasted to the individual’s chronological age. Beyond its potential prognostic value, it has great potential in being used to convey cardiovascular risk to a patient, a cornerstone of the preventive medicine conversation in the patient–physician relationship. Several deep neural network (DNN)-based Heart Age estimations have been presented, with the difference between the ECG estimate and chronological age (Heart Age Gap) in some of them associating with prognosis.12–16 Although the results of these studies are promising, artificial intelligence (AI) techniques based on DNNs lack transparency regarding which specific characteristics of the ECG contribute to the prediction models. This is sometimes frustratingly referred to as the ‘black box’ of AI. If similar or better risk predictions can be made with more transparent methods, this would increase physicians’ understanding of the results, yielding greater trust and an improved possibility of convincingly communicating the results to the patient.
An accurate and transparent ECG Heart Age has been developed using explainable advanced ECG (A-ECG) methods without DNNs.17 In healthy subjects, the A-ECG Heart Age Gap was zero. When secondarily applied to patients at risk of cardiovascular disease, the A-ECG Heart Age Gap was on average 7 years, and in patients with overt heart disease, it was 14 years. However, the prognostic value of the A-ECG Heart Age Gap and its association with traditional cardiovascular risk factors remains unknown. In this study, we therefore aimed to evaluate the prognostic value of the A-ECG Heart Age Gap and to study its association with several cardiovascular risk factors. We also aimed to compare the performance of a publicly available DNN-AI ECG Heart Age Gap applied to the same population.
Methods
Data were included from a prospectively acquired database of patients undergoing clinical cardiovascular magnetic resonance (CMR) imaging at University of Pittsburgh Medical Center (UPMC, Pittsburgh, PA, USA) who had an ECG recorded within 30 days of the CMR exam. The study was approved by the UPMC Institutional Review Board, and all participants provided written informed consent. The cohort has been presented in detail previously.18,19 For the purposes of this study, the following exclusion criteria were applied: missing follow-up data, heart rate ≥100/min, complete bundle branch blocks (QRS duration ≥130 ms), atrial fibrillation or flutter, or digoxin use. Of note, for the original cohort, patients with pacemakers or hypertrophic cardiomyopathy were excluded. Indications for the CMR exam are presented in Table S1, see Supplementary material online, Supplements.
Heart Age was determined for all patients by applying dedicated A-ECG software that has been described in detail elsewhere.19,20 When A-ECG is applied to standard 10 s 12-lead ECG recordings, multiple measures of ECG are considered including (i) conventional ECG measures, such as heart rate, waveform durations (P, PR, QRS, QT, JT, and TQ) including rate-corrected versions (QTc and JTc), as well as frontal plane QRS- and T-wave axes, and amplitude-based criteria such as the Cornell or Sokolow–Lyon indices19; (ii) spatial information from transformation of the 12-lead ECG to a derived vectorcardiogram,21 including the spatial mean and maximum QRS-T angles, spatial azimuths and elevations, and the spatial ventricular gradient and its components; and (iii) measures of waveform complexity of the QRS and T derived by singular value decomposition.
Beyond the patient’s age and sex, the following measures within a 10 s 12-lead ECG are included in the estimation of Heart Age by A-ECG, based on a previous study:17 conventional ECG measures including P-wave duration (ms), QT interval (ms), heart rate (min−1), and frontal plane QRS axis (in sine radians) and VCG measures including the maximum QRS amplitude in VCG lead Y (mV), spatial ventricular gradient minus spatial mean QRS (mV*s), root mean square of the QRS vector magnitude (mV), QRS average spatial velocity in the VCG vector magnitude (mV/s), the portion of the QRS loop in the posterior superior quadrant of the left sagittal plane by VCG (%), and T-wave complexity measures from singular value decomposition [ln (∑third to eighth Eigenvector)/(first T-wave Eigenvector minus second T-wave Eigenvector), unitless]. The intercept and coefficients for the respective measures are presented in Table 1. A-ECG Heart Age Gap was determined by subtracting the patient’s chronological age from the A-ECG Heart Age.
Table 1.
Measures included in the A-ECG Heart Age for males and females
| Males | Females | |
|---|---|---|
| Measure | Coefficient | Coefficient |
| Age, years | 0.820 | 0.850 |
| P-wave duration, ms | 0.288 | 0.331 |
| Spatial QT interval, ms | 0.081 | 0.095 |
| Heart rate, min−1 | 0.242 | 0.281 |
| QRS max amplitude in VCG lead Y, µV | −0.007 | −0.009 |
| Frontal plane QRS axis, sine radians | −5.834 | −6.354 |
| T-wave complexity, Ln ∑(EV3:8)/(EV1–EV2), unitless | 2.152 | 2.359 |
| Spatial ventricular gradient minus spatial mean QRS, mV*s | −55.841 | −67.642 |
| QRS RMS in VCG vector magnitude lead, mV | 10.315 | 11.701 |
| QRS average spatial velocity in VCG vector magnitude lead, mV/s | 0.106 | 0.116 |
| Portion of QRS loop in posterior superior quadrant of left sagittal plane by VCG, % | 0.033 | 0.034 |
| (Intercept) | −58.579 | −70.477 |
Ln, natural logarithm; EV, eigenvalues; VCG, vectocardiographic; RMS, root mean square.
Statistical analysis
Continuous variables were described using mean and standard deviation (SD) or median and interquartile range (IQR). Time-to-event analysis was performed using Kaplan–Meier curves with censoring at study end. The association between the Heart Age Gap and hospitalization for heart failure (HF) or death was analysed using multivariable Cox proportional hazard regression. We considered both models that were unadjusted and models adjusted for age, sex, hypertension, hyperlipidaemia, smoking, family history of ischaemic heart disease, body mass index, and diabetes. These covariates were chosen to be included in the final model, since they are often included in risk assessments.5 The effect of age on the association between Heart Age Gap and outcomes was evaluated by studying interaction effects of age by analysing the final model with and without an interaction term for age (Heart Age Gap × age). These models were then compared using the likelihood ratio test.
In addition to analysing Heart Age Gap as a continuous variable, we performed a Cox regression analysis after categorizing patients within three groups: (i) patients with an A-ECG Heart Age Gap ≤0; (ii) patients with an A-ECG Heart Age Gap between 0 and 10 years, approximating two standard deviations of the Heart Age Gap occurring in healthy volunteers (11.6 years);17 and (iii) patients with an A-ECG Heart Age Gap exceeding 10 years. Hazard ratios (HRs) are presented with 95% confidence intervals (CIs). The assumption of proportional hazards was confirmed using Schoenfeld’s residuals. We also explored the association between Heart Age Gap and cardiovascular risk factors or diseases and CMR-based imaging risk markers using logistic regression. Odds ratios (OR) are presented for each 5 year increment in Heart Age Gap with 95% CI.
Heart Age and Heart Age Gap estimation using a DNN have been published, and the code has been made openly available.13 This DNN-AI-based Heart Age was also applied to all ECGs in our study population, i.e. both the A-ECG Heart Age and the DNN-AI ECG Heart Age were tested in the same population, and none of the ECGs in this population were used to train either of the Heart Ages. The DNN-AI ECG Heart Age Gap was subsequently also evaluated regarding its association with risk factors and its prognostic strength using Cox regression both as a continuous variable and by the same stratification applied to the A-ECG Heart Age Gap (≤0, 0–10, and >10 years). Furthermore, two sensitivity analyses were performed. First, since heart rate is included in the Heart Age estimation, we also performed a survival analysis after additional adjustment for beta-blocker use. Second, a Kaplan–Meier graph was constructed using the stratification suggested by the authors and originators of the DNN (<−8, −8 to 8, and > 8 years) and is presented in Figure S1, see Supplementary material online, Supplements.13 In summary, neither of these sensitivity analyses changed the results in a meaningful way.
Sample size estimation
A power analysis was performed based on prior knowledge of the study population. The complete database consisted of 804 patients with complete follow-up data, with an overall event rate at 20% (death or hospitalization for HF). If 40% of the patients in this clinical cohort, with high prevalence of cardiovascular disease, were assumed to have an abnormal A-ECG Heart Age Gap (i.e. exceeding 10 years),17 678 subjects would be needed to detect a HR at 1.6, for those with an abnormal Heart Age Gap vs. normal A-ECG Heart Age Gap, given an expected event rate at 10% over 2 years in the reference group, with a power of 0.8 and a 95% level of significance.
A two-sided P-value of 0.05 was used to define statistical significance. Statistical analysis was performed using R version 3.5.3, packages: Survival v. 3.1-12 and Survminer v. 0.4.6, among others.
Findings
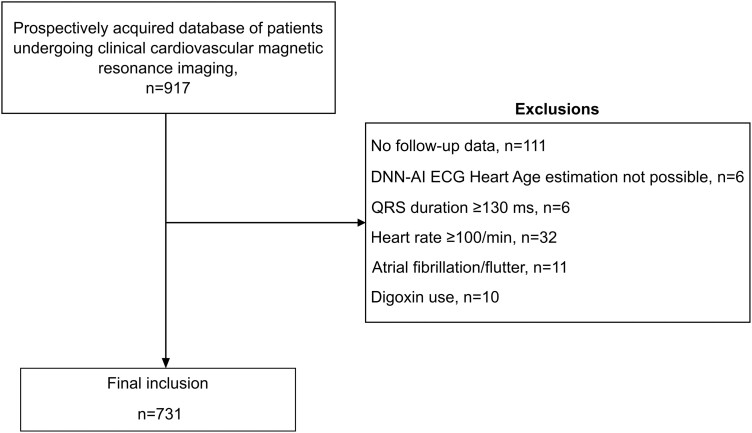
In total, 731 patients with a median follow-up of 5.7 (4.7–6.7) years were included [103 patients died (14.1%), and 52 patients (7.1%) were hospitalized due to HF]. A flowchart of patient inclusion and exclusion is presented in Figure 1. Baseline characteristics are presented in Table 2. For the entire population, A-ECG Heart Age Gap was 14.4 ± 9.5 years, DNN-AI ECG Heart Age Gap was 7.9 ± 11.9 years, and numerical results differed in head-to-head comparison (difference 6.3 ± 13.6 years, R2 = 0.03, P < 0.001). The relation between Heart Age and chronological age is presented in Figure 1.
Figure 1.
Flowchart of patient inclusion and exclusion. DNN-AI ECG, deep neural network artificial intelligence electrocardiogram.
Table 2.
Baseline characteristics
| A-ECG Heart Age Gap (years) | ||||
|---|---|---|---|---|
| Overall | ≤0 years | 0–10 | >10 | |
| n | 731 | 40 | 217 | 474 |
| Age, years | 53.4 ± 15.2 | 45.2 ± 15.5 | 48.9 ± 16.5 | 56.2 ± 13.9 |
| Male sex, n (%) | 401 (56.4) | 25 (62.5) | 132 (60.8) | 255 (53.8) |
| Body mass index, kg/m2 | 30.0 ± 7.8 | 26.0 ± 4.5 | 28.5 ± 7.5 | 31.1 ± 8.0 |
| Systolic BP, mmHg | 125 ± 19 | 122 ± 20 | 121 ± 18 | 128 ± 20 |
| Diastolic BP, mmHg | 75 ± 15 | 75 ± 12 | 72 ± 14 | 77 ± 15 |
| NT-proBNP, ng/L | 64 (26–184) | 36 (18–64) | 39 (19–116) | 85 (33–240) |
| eGFR, mL/min/1.73m2 | 89 ± 25 | 96 ± 19 | 94 ± 23 | 86 ± 26 |
| Hypertension, n (%) | 374 (51.2) | 10 (25.0) | 75 (34.6) | 289 (61.0) |
| Diabetes, n (%) | 156 (21.3) | 0 (0.0) | 28 (12.9) | 128 (27.0) |
| Hyperlipidaemia, n (%) | 294 (40.2) | 11 (27.5) | 70 (32.3) | 213 (44.9) |
| Smoker, n (%) | 116 (15.9) | 5 (12.5) | 37 (17.1) | 74 (15.6) |
| CABG, n (%) | 56 (7.7) | 0 (0.0) | 13 (6.0) | 43 (9.1) |
| PCI, n (%) | 94 (12.9) | 2 (5.0) | 21 (9.7) | 71 (15.0) |
| Family history of CAD, n (%) | 104 (14.2) | 5 (12.5) | 31 (14.3) | 68 (14.3) |
| Prior MI (known), n (%) | 110 (15.0) | 6 (15.0) | 24 (11.1) | 80 (16.9) |
| Heart failure, n (%) | 166 (22.7) | 3 (7.5) | 21 (9.7) | 142 (30.0) |
| HFpEF | 50 (6.8) | 2 (5.0) | 8 (3.7) | 40 (8.4) |
| HFrEF or HFmrEF | 116 (15.9) | 1 (2.5) | 13 (6.0) | 102 (21.5) |
| Cardiovascular magnetic resonance imaging measures | ||||
| LVEF, % | 55 ± 14 | 60 ± 7 | 58 ± 11 | 53 ± 16 |
| GLS, % | −16 ± 5 | −18 ± 2 | −17 ± 3 | −15 ± 5 |
| Presence of LGE, n (%) | 266 (36.4) | 11 (27.5) | 62 (28.6) | 193 (40.7) |
| MI, n (%) | 163 (22.3) | 4 (10.0) | 33 (15.2) | 124 (26.5) |
| Obstructive CAD, n (%) | 136 (18.6) | 1 (2.5) | 30 (13.8) | 105 (22.2) |
| Myocarditis, n (%) | 15 (2.1) | 1 (2.5) | 4 (1.8) | 10 (2.1) |
| Extracellular volume, % | 28 ± 4 | 27 ± 3 | 27 ± 4 | 28 ± 4 |
| End-diastolic LV volume, mL | 172 ± 70 | 156 ± 46 | 161 ± 52 | 178 ± 69 |
| End-systolic LV volume, mL | 83 ± 58 | 63 ± 25 | 70 ± 43 | 91 ± 65 |
| LV mass, g | 120 ± 46 | 103 ± 27 | 109 ± 37 | 127 ± 50 |
| Medications | ||||
| No medication, n (%) | 136 (19.0) | 15 (37.5) | 68 (31.3) | 53 (11.2) |
| Anti-platelets, n (%) | 380 (52.0) | 13 (32.5) | 99 (45.6) | 268 (56.5) |
| ACE inhibitors, n (%) | 295 (40.4) | 9 (22.5) | 63 (29.0) | 223 (47.0) |
| Beta-blockers, n (%) | 363 (49.7) | 9 (22.5) | 76 (35.0) | 278 (58.6) |
| Loop diuretics, n (%) | 142 (19.4) | 3 (7.5) | 22 (10.0) | 117 (24.7) |
| Calcium antagonist, n (%) | 54 (7.4) | 4 (10.0) | 10 (4.6) | 40 (8.4) |
| Hydrochlorthiazide, n (%) | 63 (8.6) | 2 (5.0) | 16 (7.1) | 45 (9.5) |
| Nitroglycerin, n (%) | 26 (3.7) | 0 (0.0) | 7 (3.2) | 19 (4.0) |
| Anti-arrhythmic, n (%) | 46 (6.3) | 3 (7.5) | 12 (5.5) | 31 (6.5) |
| Statins, n (%) | 304 (41.6) | 9 (22.5) | 74 (34.1) | 221 (46.6) |
| Insulin, n (%) | 107 (14.6) | 0 (0.0) | 19 (8.8) | 88 (18.6) |
| Oral hypoglycaemics, n (%) | 49 (6.7) | 0 (0.0) | 9 (4.6) | 40 (8.4) |
| Coumadin, n (%) | 44 (6.0) | 2 (5.0) | 14 (6.5) | 28 (5.9) |
Values are presented as mean ± standard deviation, median (interquartile range), or n (%).
ACE, angiotensin-converting enzyme; BP, blood pressure; CABG, coronary artery bypass grafting; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
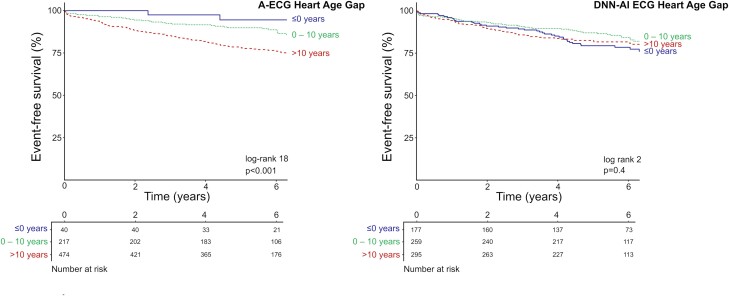
A-ECG Heart Age Gap was associated with incident HF hospitalization or death [unadjusted HR per 5 year increments 1.23 (1.13–1.34), C statistic 0.62, adjusted HR per 5 year increments 1.11 (1.01–1.22)] (Figure 2). Similar results were found after adjustment for beta-blocker use [adjusted HR per 5 year increments 1.09 (1.00–1.20)]. Patients with an abnormal A-ECG Heart Age Gap had higher risk of future HF hospitalization or death, in reference to those with a normal or low gap [unadjusted HR: 2.07 (1.38–3.11), adjusted HR: 1.64 (1.08–2.50)]. DNN-AI ECG Heart Age Gap was not associated with outcomes in unadjusted analysis [HR per 5 year increments 1.00 (0.93–1.08)] but was associated with outcomes in adjusted analysis [HR per 5 year increments 1.11 (1.01–1.21)] (Figure 2). Similar results were found after adjustment for beta-blocker use [adjusted HR per 5 year increments 1.09 (1.00–1.19)]. In the fully adjusted model, the interaction between Heart Age Gap and age for the prediction of future cardiovascular events was not statistically significant, neither for A-ECG Heart Age Gap (P = 0.13) nor for DNN-AI ECG Heart Age Gap (P = 0.15).
Figure 2.
Time-to-event analysis for A-ECG Heart Age Gap (left panel) and DNN-AI ECG Heart Age Gap (right panel) regarding death or heart failure hospitalization among 731 patients who were referred for a clinical CMR imaging study. For both the A-ECG Heart Age and for the DNN-AI ECG Heart Age, patients are divided into three groups based on the difference between the Heart Age and their chronological age. In blue solid lines, patients with a Heart Age Gap ≤0; in green dotted lines, patients with a Heart Age Gap within 0 to 10 years; and in red dashed lines, patients with a Heart Age Gap exceeding 10 years. The log rank and P-values presented refer to the difference between Heart Age Gap >10 years (red, dashed lines) and the other two groups combined (blue solid lines and green dotted lines). Note that the survival graph above shows the survival in different heart gap groups. Although the unadjusted DNN-AI ECG Heart Age Gap shows no association with survival as depicted above, it is significantly associated with survival after adjusting for age (see text). A-ECG, advanced electrocardiography; DNN-AI, deep neural network artificial intelligence.
A-ECG Heart Age Gap was associated with risk factors and cardiac MRI findings both unadjusted and after adjustment for age and sex. By comparison, DNN-AI ECG Heart Gap showed no association with hypertension, coronary artery disease, or diabetes unless adjusted for age and sex and was associated with cardiac MRI findings both unadjusted and after adjustment for age and sex (Tables 3 and 4).
Table 3.
Association between 5 year increments of A-ECG Heart Age Gap or DNN-AI ECG Heart Age Gap and presence of cardiovascular comorbidities expressed as odds ratios with 95% confidence limits
| Odds ratio (95% CI) | ||
|---|---|---|
| Unadjusted | Adjusted for age and sex | |
| Heart Age Gap | Hypertension | |
| A-ECG | 1.33 (1.22–1.45) | 1.22 (1.05–1.27) |
| DNN-AI | 0.97 (0.92–1.04) | 1.01 (1.02–102) |
| Diabetes | ||
| A-ECG | 1.34 (1.21–1.47) | 1.27 (1.15–1.41) |
| DNN-AI | 1.05 (0.98–1.13) | 1.20 (1.10–1.31) |
| Obstructive coronary heart disease | ||
| A-ECG | 1.19 (1.08–1.31) | 1.12 (1.00–1.25) |
| DNN-AI | 0.95 (0.88–1.03) | 1.14 (1.03–1.27) |
| Heart failure | ||
| A-ECG | 1.46 (1.33–1.62) | 1.45 (1.31–1.62) |
| DNN-AI | 1.10 (1.02–1.18) | 1.21 (1.11–1.32) |
Table 4.
Association between 5 year increments of A-ECG Heart Age Gap or DNN-AI ECG Heart Age Gap and presence of pathological findings on cardiovascular magnetic resonance imaging expressed as odds ratios with 95% confidence limits
| Odds ratio (95%CI) | ||
|---|---|---|
| Unadjusted | Adjusted for age and sex | |
| Heart Age Gap | Presence of late gadolinium enhancement | |
| A-ECG | 1.15 (1.07–1.26) | 1.16 (1.03–1.55) |
| DNN-AI | 1.08 (1.01–1.15) | 1.19 (1.10–1.28) |
| Reduced left ventricular ejection fraction (<50%) | ||
| A-ECG | 1.33 (1.22–1.46) | 1.39 (1.26–1.41) |
| DNN-AI | 1.15 (1.08–1.24) | 1.23 (1.14–1.34) |
| Left ventricular dilatation | ||
| A-ECG | 1.45 (1.28–1.66) | 1.54 (1.34–1.79) |
| DNN-AI | 1.11 (1.00–1.22) | 1.14 (1.03–1.27) |
| Increased left ventricular mass | ||
| A-ECG | 1.40 (1.26–1.57) | 1.42 (1.27–1.60) |
| DNN-AI | 1.14 (1.06–1.25) | 1.22 (1.11–1.35) |
Discussion
An increased A-ECG Heart Age Gap was associated with increased risk of future hospitalization for HF or death, even after adjusting for cardiovascular risk factors often incorporated into cardiovascular risk scores (sex, hypertension, hyperlipidaemia, diabetes, BMI, and family history of ischaemic heart disease). In addition, we showed that A-ECG Heart Age Gap was associated with increased risk of having hypertension, ischaemic heart disease, diabetes, or HF. Moreover, A-ECG Heart Age Gap was associated with increased likelihood of CMR-based pathological findings such as reduced left ventricular ejection fraction or presence of either increased extracellular volume fraction or late gadolinium enhancement. These results provide external validation of Heart Age estimation based on explainable A-ECG measures.
Conveying risk
Cardiovascular disease often develops and progresses silently for many years.22 Fortunately, several risk factors can be modified, and cardiovascular risk can therefore be reduced, for example, by dietary changes, increased physical activity, reduced alcohol consumption, or smoking cessation.23 Understanding the risk may incentivize lifestyle changes as well as adherence to any needed medications. Several pedagogical ways of expressing cardiovascular or cardiopulmonary risk to patients have been proposed, for example, by presenting a lung age,24 a vascular age,25 or a heart age.3,5 When smokers were presented with their lung age based on forced expiratory volumes at 1 s (FEV1), the chance of smoking cessation increased.24 Furthermore, by translating the Framingham risk score into the age corresponding to someone with the same risk score but without known modifiable risk factors, an improvement in metabolic parameters has been achieved.5 Apparently, years of healthy human ageing is an intuitive unit of measure in this setting.
In a randomized controlled study, pictorial presentation of atherosclerotic changes in carotid wall thickness to the patient and the physician also improved cardiovascular risk in patients.25 Such methods have the advantage of being easily understood by the patient and thereby provide strong incentives for lifestyle changes and/or increasing therapeutic compliance.26 Both FEV1 and carotid intima thickness provide an individualized assessment of risk and reflect actual pathological changes in the patient, moving one step beyond risk factor calculators, such as the Framingham risk score. However, both pulmonary function testing and carotid ultrasound depend on specialized equipment and staffing, such tools not being implemented in routine care for most patients with hypertension or diabetes. However, the opposite is true for the ECG. The presentation of an ECG-based Heart Age, or the Heart Age Gap, can be an intuitive way to express cardiovascular risk to a patient. This is important, since not adhering to needed medication, or not sustaining healthy lifestyle choices, may be caused by a discrepancy in the perceived and actual risk.27 For a patient, and possibly also for the physician, it may be easier to understand the risk expressed as an excessively aged heart in healthy human ageing years, rather than potentially more esoteric numerical values for blood pressure or cholesterol levels or scores expressed in risk for a particular event in percent per year.5 Our results show that A-ECG Heart Age Gap is strongly associated with cardiovascular risk factors and disease. Further, it also associated with outcomes in this dataset, including after adjusting for information often applied in traditional risk scores. This strengthens the potential of A-ECG Heart Age Gap to be used as an inexpensive, non-invasive complementary method to conventional advice and management using an instrument (ECG) that is readily available and commonly included in the routine assessment of patients. Indeed, A-ECG analysis can be performed on the digital raw data of a standard resting 12-lead ECG from any modern ECG machine that makes that data accessible, as is the case for the overwhelming majority of ECG machine vendors. Future studies are justified to evaluate whether the presentation of Heart Age Gap to patients can improve clinical outcome.
A-ECG vs. DNN-AI ECG
AI methods using neural networks have also been used to estimate Heart Age.5,12–14,16 Despite several studies describing AI-derived prediction models, there has yet to be any large-scale clinical implementation of DNN-based methods.14,15,28,29 One reason may be the lack of transparency of the steps between ECG input and clinical output of the DNN algorithms, often referred to as ‘black box’ models.30,31 Moreover, secondary use of so-called saliency methods or ‘heat maps’ might have limited effect in making these models more interpretable.32 Without the ability to have insight into which components of or measures from the ECG are most important when generating an output, it is effectively impossible to detect potential errors at human over-reading and difficult to detect any relevant bias when critically evaluating research on medical diagnostics based on such methods.33 In contrast, the measures included in the A-ECG Heart Age are fully transparent,17 and the individual values for each can be presented in conjunction with the estimated Heart Age. Therefore, A-ECG Heart Age Gap is explainable in the sense of allowing the physician to understand which ECG measures impacted the final result.34 A more profound explainability, however, would require an understanding of which physiological or biological changes gave rise to the ECG measures of interest. Some of the more important measures in the A-ECG Heart Age score, e.g. P-wave duration, QT interval, heart rate, and QRS axis, are intuitive in this matter, since they commonly change not only with age, but even more so in the presence of cardiac disease. Vectorcardiographic changes and measures of T-wave complexity, on the other hand, are somewhat less conventional, but are nonetheless, strongly associated with increased cardiovascular risk.35–39 Consequently, when compared with a DNN-AI ECG Heart Age, the A-ECG Heart Age is, inherently by design and through its included ECG measures, explainable to and by the clinician.
After adjusting for age and cardiovascular risk factors, the DNN-AI ECG Heart Age Gap was associated with future events. However, in contrast to the A-ECG Heart Age Gap, the unadjusted DNN-AI ECG Heart Age gap did not provide a prognostic association in the Kaplan–Meier survival curves (Figure 2). For the A-ECG Heart Age Gap, HRs were somewhat lower after adjustment for age and cardiovascular risk factors. Importantly, when applied in clinical practice, an unadjusted increased A-ECG Heart Age Gap is associated with increased cardiovascular risk without the need to take either age or other risk factors into account. A similar pattern was observed regarding association with risk factors. While A-ECG Heart Age Gap was associated with risk factors both unadjusted, and after adjustment for age and sex, the DNN-AI ECG Heart Gap showed no association with hypertension, coronary artery disease, or diabetes unless adjusted for age and sex.
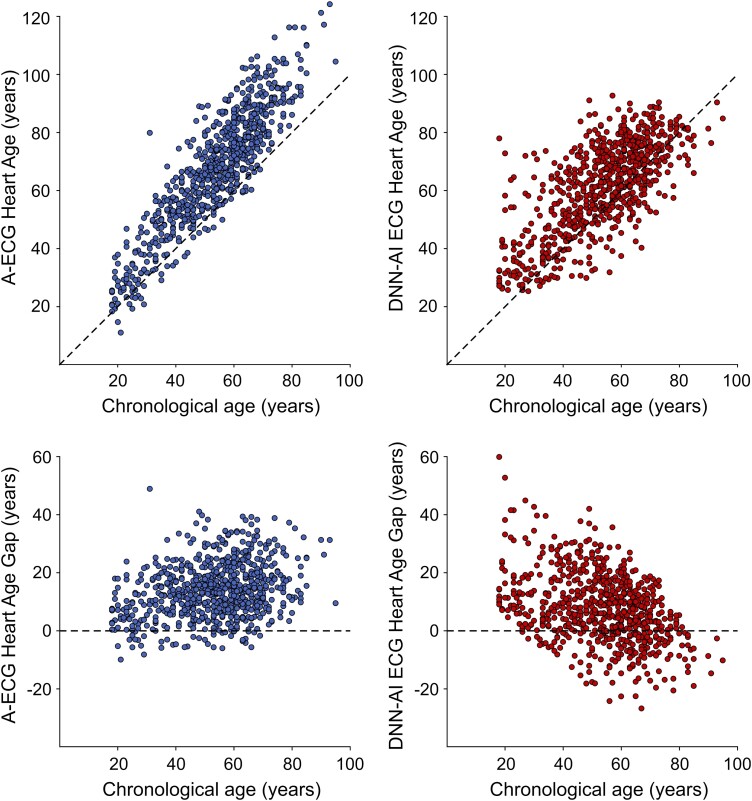
It is important to note that the patient’s chronological age is purposely included in the estimation of A-ECG Heart Age. A-ECG Heart Age estimations were originally derived from classical Bayesian statistical models incorporating results from ∼5 min ECG 12-lead ECGs.4 Such models were not developed to predict a patient’s chronological age but instead to estimate the ‘electrical age’ of the heart and specifically how the Heart Age differs from chronological age. The Bayesian statistical approach is thus focused on adjusting the known chronological age on the basis of electrocardiographic characteristics and sex with knowledge of the patient’s chronological age. Equally important, the A-ECG Heart Age was trained to accurately estimate Heart Age using only healthy individuals, who, indeed, had an average heart age gap of 0 among healthy individuals. This is in contrast to current DNN-AI ECG approaches, wherein no knowledge of the patient’s chronological age is assumed or included nor any rigorous acknowledgement of the patient’s health or disease status. But the task of estimating Heart Age without such knowledge is more challenging than commonly assumed, with its road to clinical implementation also being less clear, unless the higher prevalence of cardiovascular disease in older ages can be used as a factor within the given DNN’s prediction of chronological age. And attempting to predict chronological age, rather than Heart Age, also thwarts one main purpose of Heart Age estimations, which is to reflect any excess heart ageing across all age groups, something that must take the increased prevalence of cardiovascular disease with ageing into account. This difference in methodology is also evident from our results, in which the Heart Age estimations from the DNN-AI ECG Heart Age more closely followed the chronological age, as expected (Figure 3), while the A-ECG Heart Age deviated especially in older patients, who would also be expected to have a higher prevalence of disease. Further, as with other DNN-AI ECG Heart Age methods,5,12,14,16 the DNN method tested in this study13 was similarly not trained on purely heart-healthy individuals. This is important, since arguably, it is the ECG-based deviation from normal ageing that should be focused on in risk prediction. Notably, the A-ECG Heart Age was trained on healthy volunteers of a wide age range. This is a key difference in design compared with existing DNN-AI ECG Heart Age approaches, ensuring that the A-ECG Heart Age corresponds to years of healthy human ageing, without influence by cardiovascular risk factors or overt heart disease. Further, when very large numbers of ECGs (tens to hundreds of thousands or more) are included, as in the DNN-AI ECG Heart Age methods,5,12–14,16 performing quality control of each individual ECG is often not feasible, and findings influenced by reduced signal quality (noise, missing leads, lead reversals, etc.) may be incorporated into the algorithms. By comparison, when applied in a clinical situation, ECG quality is apparent to the reader and poor quality or invalid ECGs may be discarded and the recording repeated. Therefore, including any data for which it has been impossible to comprehensively verify data quality is unwise. In contrast to the DNN-AI ECG Heart Age, in the derivation of the A-ECG Heart Age, ECGs with tachycardia, bundle branch blocks, and atrial fibrillation were also excluded. Therefore, the comparison between the A-ECG and DNN-AI ECG methods in the current study is only applicable to patients without these ECG findings. In future studies, development of dedicated A-ECG Heart Age scores specific to the settings of bundle branch blocks and/or atrial fibrillation may be of value.
Figure 3.
Scatter plots showing the relation between Heart Age (upper panels; left: A-ECG, right: DNN-AI) and Heart Age Gap (lower panels) and chronological age. The dashed lines denote the line of identity, i.e. no difference between Heart Age and chronological age. In most cases, in this clinical cohort with a high prevalence of disease, A-ECG Heart Age was higher than chronological age. DNN-AI ECG Heart Age Gap was lower in older patients than in younger (lower right panel), while an opposite trend could be observed for A-ECG Heart Age Gap.
Although A-ECG Heart Age Gap predicted increased risk for mortality and HF hospitalization in this study, other A-ECG scores more specifically tailored to predict events rather than Heart Age per se can also be constructed to further optimize predictive performance. And several well-validated electrocardiographic and other predictors of future cardiovascular events in patients with known cardiovascular disease already exist.18,40–44 Moreover, other A-ECG scores dedicated towards predicting events in the context of any given clinical (e.g. HF and post-infarction) or ECG (e.g. bundle branch block and atrial fibrillation) scenario wherein prediction is specifically desired will be better tailored for such a task. That said, A-ECG Heart Age Gap is arguably most suited for use in patients with no or few known risk factors or symptoms of heart disease, for whom lifestyle or other changes may lessen progression to overt cardiovascular disease. Nonetheless, in order for the A-ECG Heart Age Gap to be useful for conveying risk to patients, it needs to be a measure that is ideally also closely associated with future adverse events, as demonstrated in the current study.
Study limitations
The patients included in this study came from a clinical cohort with a relatively high prevalence of cardiovascular disease. This is a limitation since these findings may not be generalizable to populations with a lower prevalence of established cardiovascular disease, i.e. populations in which Heart Age Gap is likely most optimally used. Future studies are justified to determine the prognostic value, as well as the clinical impact, of Heart Age Gap in such populations. Nonetheless, the cardiovascular conditions present in the cohort of the current study are typical examples of conditions that early preventive measures aim to avoid. Since both the A-ECG and DNN-AI ECG Heart Ages were applied to the same cohort, this limitation does not affect the comparison of the methods.
Another limitation is the retrospective nature of this analysis. However, one purpose of the current study was to pursue further external validation of both the A-ECG and DNN-AI ECG Heart Age Gaps. Lastly, few patients had zero or a negative Heart Age Gap, which may affect the predictions made in this study. However, this can also be considered further evidence of the strong association between an elevated A-ECG Heart Age Gap and future risk, given the high prevalence of disease in this cohort.
Conclusion
A-ECG Heart Age Gap is a transparent, explainable, and intuitive measure associated with increased risk of having cardiovascular risk factors or disease as well as incident HF hospitalization and death. Explainable A-ECG methods for Heart Age Gap have the potential for improving clinical adoption and prognostic performance compared with existing DNN-AI-type methods.
Authors' contributions
T.L. contributed to the study design, performed the statistical analysis, wrote the first manuscript drafts, and prepared the figures and tables. M.M. and E.B.S. curated the data, contributed to the study design, and critically revised the manuscript, and M.M. also contributed to the analysis of the data. A.H.R. and A.L.P.R. contributed to the study design and analysis of the data and critically revised the manuscript. T.T.S. contributed to the study design and analysis of the data and critically revised the manuscript. M.U. designed the study, provided supervision, contributed to analysis of the data, and critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Supplementary Material
Contributor Information
Thomas Lindow, Kolling Institute, Royal North Shore Hospital, University of Sydney, Sydney, Australia; Department of Clinical Physiology, Research and Development, Växjö Central Hospital, Region Kronoberg, Sweden; Clinical Physiology, Clinical Sciences, Lund University, Sweden.
Maren Maanja, Department of Clinical Physiology, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden.
Erik B Schelbert, Minneapolis Heart Institute East, United Hospital, MN, USA.
Antônio H Ribeiro, Department of Information Technology, Uppsala University, Uppsala, Sweden.
Antonio Luiz P Ribeiro, Telehealth Center, Hospital das Clínicas, and Internal Medicine Department, Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
Todd T Schlegel, Department of Clinical Physiology, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden; Nicollier-Schlegel SARL, Trélex, Switzerland.
Martin Ugander, Kolling Institute, Royal North Shore Hospital, University of Sydney, Sydney, Australia; Department of Clinical Physiology, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden.
Funding
The Swedish Heart-Lung Foundation, the Swedish Cardiac Society, the Royal Swedish Academy of Sciences, Women and Health Foundation, Region Kronoberg, The Swedish Heart and Lung Association, New South Wales Health, Heart Research Australia, University of Sydney, and Brazilian research agencies CNPq and FAPEMIG.
Data availability
Deidentified participant data can be made available upon requests directed to the corresponding author. Proposals will be reviewed on the basis of the scientific objective. A data use agreement will be required before the release of participant data.
References
- 1. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol 2020;75:919–930. [DOI] [PubMed] [Google Scholar]
- 2. Kucharska-Newton AM, Stoner L, Meyer ML. Determinants of vascular age: an epidemiological perspective. Clin Chem 2019;65:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groenewegen KA, den Ruijter HM, Pasterkamp G, Polak JF, Bots ML, Peters SA. Vascular age to determine cardiovascular disease risk: a systematic review of its concepts, definitions, and clinical applications. Eur J Prev Cardiol 2016;23:264–274. [DOI] [PubMed] [Google Scholar]
- 4. Ball RL, Feiveson AH, Schlegel TT, Starc V, Dabney AR. Predicting “heart age” using electrocardiography. J Pers Med 2014;4:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez-Gonzalez AA, Aguilo A, Frontera M, Bennasar-Veny M, Campos I, Vicente-Herrero T, et al. Effectiveness of the heart age tool for improving modifiable cardiovascular risk factors in a Southern European population: a randomized trial. Eur J Prev Cardiol 2015;22:389–396. [DOI] [PubMed] [Google Scholar]
- 6. Goorakani Y, Sedigh Rahimabadi M, Dehghan A, Kazemi M, Chijan MR, Bijani M, et al. Correlation of resting heart rate with anthropometric factors and serum biomarkers in a population-based study: Fasa PERSIAN cohort study. BMC Cardiovasc Disord 2020;20:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogliari G, Mahinrad S, Stott DJ, Jukema JW, Mooijaart SP, Macfarlane PW, et al. Resting heart rate, heart rate variability and functional decline in old age. Can Med Assoc J 2015;187:E442–E449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morcet JF, Safar M, Thomas F, Guize L, Benetos A. Associations between heart rate and other risk factors in a large French population. J Hypertens 1999;17:1671–1676. [DOI] [PubMed] [Google Scholar]
- 9. Evans JG, Prior IA, Tunbridge WM. Age-associated change in QRS axis: intrinsic or extrinsic ageing? Gerontology 1982;28:132–137. [DOI] [PubMed] [Google Scholar]
- 10. Rautaharju PM, Mason JW, Akiyama T. New age- and sex-specific criteria for QT prolongation based on rate correction formulas that minimize bias at the upper normal limits. Int J Cardiol 2014;174:535–540. [DOI] [PubMed] [Google Scholar]
- 11. Chhabra L, Devadoss R, Chaubey VK, Spodick DH. Interatrial block in the modern era. Curr Cardiol Rev 2014;10:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attia ZI, Friedman PA, Noseworthy PA, Lopez-Jimenez F, Ladewig DJ, Satam G, et al. Age and sex estimation using artificial intelligence from standard 12-lead ECGs. Circ Arrhythm Electrophysiol 2019;12:e007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lima EM, Ribeiro AH, Paixão GMM, Ribeiro MH, Pinto-Filho MM, Gomes PR, et al. Deep neural network-estimated electrocardiographic age as a mortality predictor. Nat Commun 2021;12:5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang CH, Lin CS, Luo YS, Lee YT, Lin C. Electrocardiogram-based heart age estimation by a deep learning model provides more information on the incidence of cardiovascular disorders. Front Cardiovasc Med 2022;9:754909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirota N, Suzuki S, Arita T, Yagi N, Otsuka T, Yamashita T. Prediction of biological age and all-cause mortality by 12-lead electrocardiogram in patients without structural heart disease. BMC Geriatr 2021;21:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ladejobi AO, Medina-Inojosa JR, Shelly Cohen M, Attia ZI, Scott CG, LeBrasseur NK, et al. The 12-lead electrocardiogram as a biomarker of biological age. Eur Heart J Digit Health 2021;2:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindow T, Palencia-Lamela I, Schlegel TT, Ugander M. Heart age estimated using explainable advanced electrocardiography. Sci Rep 2022;12:9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue H, Artico J, Davies RH, Adam R, Shetye A, Augusto JB, et al. Automated in-line artificial intelligence measured global longitudinal shortening and mitral annular plane systolic excursion: reproducibility and prognostic significance. J Am Heart Assoc 2022;11:e023849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maanja M, Schlegel TT, Kozor R, Lundin M, Wieslander B, Wong TC, et al. The electrical determinants of increased wall thickness and mass in left ventricular hypertrophy. J Electrocardiol 2020;58:80–86. [DOI] [PubMed] [Google Scholar]
- 20. Schlegel TT, Kulecz WB, Feiveson AH, Greco EC, DePalma JL, Starc V, et al. Accuracy of advanced versus strictly conventional 12-lead ECG for detection and screening of coronary artery disease, left ventricular hypertrophy and left ventricular systolic dysfunction. BMC Cardiovasc Disord 2010;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kors JA, van Herpen G, Sittig AC, van Bemmel JH. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J 1990;11:1083–1092. [DOI] [PubMed] [Google Scholar]
- 22. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 23. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 24. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008;336:598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Näslund U, Ng N, Lundgren A, Fhärm E, Grönlund C, Johansson H, et al. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open-label, randomised controlled trial. Lancet 2019;393:133–142. [DOI] [PubMed] [Google Scholar]
- 26. Soureti A, Hurling R, Murray P, van Mechelen W, Cobain M. Evaluation of a cardiovascular disease risk assessment tool for the promotion of healthier lifestyles. Eur J Cardiovasc Prev Rehab 2010;17:519–523. [DOI] [PubMed] [Google Scholar]
- 27. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 28. Attia IZ, Tseng AS, Benavente ED, Medina-Inojosa JR, Clark TG, Malyutina S, et al. External validation of a deep learning electrocardiogram algorithm to detect ventricular dysfunction. Int J Cardiol 2021;329:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bos JM, Attia ZI, Albert DE, Noseworthy PA, Friedman PA, Ackerman MJ. Use of artificial intelligence and deep neural networks in evaluation of patients with electrocardiographically concealed long QT syndrome from the surface 12-lead electrocardiogram. JAMA Cardiol 2021;6:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The Lancet Respiratory Medicine LRM . Opening the black box of machine learning. Lancet Respir Med 2018;6:801–801. [DOI] [PubMed] [Google Scholar]
- 31. Gladding PA, Hewitt W, Schlegel TT. Going deep with ECG and aortic stenosis: touchdown or incomplete pass? J Am Heart Assoc 2020;9:e016193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhatt A, Ganatra A, Kotecha K. COVID-19 pulmonary consolidations detection in chest X-ray using progressive resizing and transfer learning techniques. Heliyon 2021;7:e07211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quinn TP, Jacobs S, Senadeera M, Le V, Coghlan S. The three ghosts of medical AI: can the black-box present deliver? Artif Intel Med 2021;124:102158. [DOI] [PubMed] [Google Scholar]
- 34. Amann J, Blasimme A, Vayena E, Frey D, Madai VI. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak 2020;20:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm 2005;2:73–78. [DOI] [PubMed] [Google Scholar]
- 36. Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J 2003;24:1357–1364. [DOI] [PubMed] [Google Scholar]
- 37. Horinaka S, Yamamoto H, Tabuchi T, Takada M, Akabane T, Onoda M, et al. Ventricular gradient variability. New ECG method for detection of ischemic heart disease. J Electrocardiol 1995;28:177–183. [DOI] [PubMed] [Google Scholar]
- 38. Okin PM, Malik M, Hnatkova K, Lee ET, Galloway JM, Best LG, et al. Repolarization abnormality for prediction of all-cause and cardiovascular mortality in American Indians: the Strong Heart Study. J Cardiovasc Electrophysiol 2005;16:945–951. [DOI] [PubMed] [Google Scholar]
- 39. Zabel M, Malik M, Hnatkova K, Papademetriou V, Pittaras A, Fletcher RD, et al. Analysis of T-wave morphology from the 12-lead electrocardiogram for prediction of long-term prognosis in male US veterans. Circulation 2002;105:1066–1070. [DOI] [PubMed] [Google Scholar]
- 40. Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L, et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol 2009;2:548–554. [DOI] [PubMed] [Google Scholar]
- 41. Baumert M, Porta A, Vos MA, Malik M, Couderc JP, Laguna P, et al. QT interval variability in body surface ECG: measurement, physiological basis, and clinical value: position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016;18:925–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dang Y, Hou Y. The prognostic value of late gadolinium enhancement in heart diseases: an umbrella review of meta-analyses of observational studies. Eur Radiol 2021;31:4528–4537. [DOI] [PubMed] [Google Scholar]
- 43. Savarese G, Vedin O, D'Amario D, Uijl A, Dahlström U, Rosano G, et al. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail 2019;7:306–317. [DOI] [PubMed] [Google Scholar]
- 44. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data can be made available upon requests directed to the corresponding author. Proposals will be reviewed on the basis of the scientific objective. A data use agreement will be required before the release of participant data.