Abstract
Intramyocellular lipid content (IMCL) is elevated in insulin-resistant humans, but it changes over time, and relationships with comorbidities remain unclear. We examined IMCL during the initial course of diabetes and its associations with complications. Participants of the German Diabetes Study (GDS) with recent-onset type 1 (n = 132) or type 2 diabetes (n = 139) and glucose-tolerant control subjects (n = 128) underwent 1H-MRS to measure IMCL and muscle volume, whole-body insulin sensitivity (hyperinsulinemic-euglycemic clamps; M-value), and cycling spiroergometry (VO2max). Subgroups underwent the same measurements after 5 years. At baseline, IMCL was ∼30% higher in type 2 diabetes than in other groups independently of age, sex, BMI, and muscle volume. In type 2 diabetes, the M-value was ∼36% and ∼62% lower compared with type 1 diabetes and control subjects, respectively. After 5 years, the M-value decreased by ∼29% in type 1 and ∼13% in type 2 diabetes, whereas IMCL remained unchanged. The correlation between IMCL and M-value in type 2 diabetes at baseline was modulated by VO2max. IMCL also associated with microalbuminuria, the Framingham risk score for cardiovascular disease, and cardiac autonomic neuropathy. Changes in IMCL within 5 years after diagnosis do not mirror the progression of insulin resistance in type 2 diabetes but associate with early diabetes-related complications.
Article Highlights
Intramyocellular lipid content (IMCL) can be elevated in insulin-resistant humans, but its dynamics and association with comorbidities remain unclear.
Independently of age, sex, body mass, and skeletal muscle volume, IMCL is higher in recent-onset type 2, but not type 1 diabetes, and remains unchanged within 5 years, despite worsening insulin resistance.
A degree of physical fitness modulates the association between IMCL and insulin sensitivity in type 2 diabetes. Whereas higher IMCL associates with lower insulin sensitivity in people with lower physical fitness, there is no association between IMCL and insulin sensitivity in those with higher degree of physical fitness.
IMCL associates with progression of microalbuminuria, cardiovascular disease risk, and cardiac autonomic neuropathy.
Introduction
During the development of obesity, triglycerides are stored not only in adipose tissue but also ectopically in other tissues such as skeletal muscle. Intramyocellular lipid content (IMCL) correlates with whole-body and skeletal muscle insulin resistance in sedentary individuals without diabetes (1) as well as in humans with a family history of type 2 diabetes (2), prediabetes (3), obesity (4) and even in overt type 1 diabetes (5). However, IMCL is not generally increased in diabetes (e.g., in lean people with metabolically well-controlled type 2 diabetes or in those with type 1 diabetes) (6,7). This can be explained by the observation that intracellular lipid metabolites (i.e., lipotoxins, rather than neutral lipids and triglycerides) are involved in the pathogenesis of insulin resistance (8,9). In addition, most studies of the role of IMCL in diabetes comprised humans with unclear or long disease duration, when chronic glucose toxicity worsens insulin resistance (10). Thus, the initial dynamics of IMCL and its relationship with changes in insulin resistance in newly diagnosed diabetes are still unknown.
Strong evidence supports beneficial effects of physical activity on the risk of type 2 diabetes (11). Indeed, aerobic fitness modifies the relationship between IMCL and insulin sensitivity in healthy humans (12), which is likely related to muscle mitochondrial function (13). Nevertheless, the impact of moderate differences in habitual physical activity and aerobic fitness on ectopic fat accumulation and insulin resistance in overt type 2 diabetes is not fully understood.
Whole-body insulin resistance also associates with the development of diabetes-related complications, such as cardiovascular disease, cardiac autonomic neuropathy (CAN), and chronic kidney disease (14–16). In line, the severe insulin-resistant diabetes endotype exhibits the highest prevalence of cardiovascular and nephropathy risk already within the first year after the diabetes diagnosis (17,18). Nevertheless, the impact of insulin resistance on diabetes-related complications during the early course of the disease is yet unclear.
We hypothesized that higher IMCL and its changes during the early course of type 2, but not type 1 diabetes, are key contributors to the association of whole-body insulin resistance with diabetes-related comorbidities. Furthermore, we hypothesized that physical fitness differently affects the relationship between IMCL and whole-body insulin sensitivity in type 1 and type 2 diabetes. To this end, we examined a comprehensively phenotyped cohort of individuals with diabetes during the first year after diagnosis, when the impact of long-term glucose toxicity is negligible, and 5 years later to determine the impact of IMCL on changes of insulin resistance and diabetes-related comorbidities.
Research Design and Methods
Volunteers
Individuals with type 1 (n = 132) or type 2 diabetes (n = 139) and glucose-tolerant control subjects (n = 128) were recruited from the ongoing prospective observational German Diabetes Study (GDS), which has been described in detail before (19). Briefly, GDS enrolls individuals aged 18–69 years and diagnosed with diabetes within the last 12 months based on the criteria of the American Diabetes Association (20). Individuals with no first-degree relatives having diabetes undergo a 75-g oral glucose tolerance test (AccuCheck Dextro O.G-T.; Roche, Basel, Switzerland) to confirm normal glucose tolerance serving as a control group. Exclusion criteria comprise diabetes due to other causes, other chronic diseases, and immunosuppressive treatment. The study was approved by the Heinrich Heine University of Düsseldorf Medical Faculty Ethics Committee (reference number 4508), registered at Clinicaltrials.gov (NCT01055093), and performed according to the Declaration of Helsinki. Written informed consent was obtained from all participants upon study inclusion.
Three days prior to each study visit, participants had to refrain from strenuous physical activity and to adhere to a balanced isocaloric alcohol-free nutrition. They discontinued any glucose-lowering medication for 3 days prior to all metabolic examinations, which were performed after a 10-h overnight fast. Supplementary Table 1 shows the glucose-lowering medication of individuals with type 2 diabetes at baseline and at the 5-year follow-up. In addition, data from the 5-year follow-up visits were analyzed in subgroups of individuals with type 1 (n = 27) or type 2 diabetes (n = 38) and glucose-tolerant control subjects (n = 20).
MRI and 1H-MRS
All measurements were performed after overnight fasting in a 3-T whole-body magnetic resonance scanner (Achieva X-series; Philips Healthcare, Best, the Netherlands).
Total, subcutaneous, and visceral adipose tissue volumes were quantified by whole-body MRI by using T1-weighted fast spin-echo and postprocessing by a trained operator using SliceOmatic 5.0 software (Tomovision, Montréal, Québec, Canada) (21). An in-house image processing pipeline was developed on MATLAB R2021a (The MathWorks, Natick, MA) for the segmentation of the skeletal muscle volume of the upper leg between the gluteus maximus muscle and the knee from whole-body MRIs. As a preliminary step, the image slices were manually selected, following separation of background and foreground image signal, using a Fuzzy-C means clustering algorithm and removal of motion artifacts from the background signal. Segmentation of skeletal muscle, background, and subcutaneous adipose/bone tissues was performed using a Gaussian mixture model with three clusters to enable the quantification of skeletal muscle volume.
For assessment of IMCL, water-suppressed and nonsuppressed 1H-MRS was performed in the fasted state in a voxel (10 ∗ 10 ∗ 20 mm3) placed within the tibialis anterior muscle on the left leg using point resolved spectroscopy sequence (repetition time/echo time = 2,000/29 ms). The tibialis anterior muscle, a mainly glycolytic muscle predominantly composed of type IIB and IIX fibers, which reflects the fast twitch muscle type (22), was chosen because of its uniform fiber orientation along the muscle axis, allowing for good 1H-MRS separation of IMCL from extramyocellular lipid signals (23).
IMCL content was calculated from the peak areas of IMCL-CH2 at 1.3 ppm with respect to the water peak area and was corrected for T1 and T2 relaxation effects, as reported previously (24). Supplementary Table 2 provides a more detailed description of the 1H-MRS data acquisition, while Supplementary Fig. 1 depicts representative point resolved spectroscopy sequence spectra and voxel location.
Whole-Body Insulin Sensitivity
The modified Botnia clamp combines an intravenous glucose tolerance test with a hyperinsulinemic-euglycemic clamp test (25). First, the intravenous glucose tolerance test started with an administration of a 30% glucose infusion bolus (1 mg/kg body wt) with frequent blood sampling for 60 min to measure blood glucose, C-peptide, and insulin. The subsequent hyperinsulinemic-euglycemic clamp started with a priming dose of short-acting human insulin (Insuman Rapid; Sanofi; Frankfurt, Germany) for 10 min (10 mU ∗ kg [body wt]−1 ∗ min−1) continued by constant infusion (1.5 mU ∗ kg [body wt]−1 ∗ min−1) for 3 h. Whole-body insulin sensitivity is given as the M-value as calculated from mean glucose infusion rates with glucose space correction during the steady-state period (19). In addition, the fasting adipose-tissue insulin resistance index (ADIPO-IR) was computed from fasting concentrations of free fatty acids (FFA; mmol/L) and insulin (pmol/L) (21).
Indirect Calorimetry
Measurements of the respiratory quotient (RQ) and resting energy expenditure (REE) were performed in the fasted state and during clamp steady state using the canopy mode and a Vmax Encore 29n (CareFusion, Höchberg, Germany), as described (26).
Physical Fitness
Physical fitness was assessed on a cycle ergometer by an incremental exhaustive exercise test (Ergometrics 900; Ergoline, Blitz, Germany) with continuous monitoring of respiratory gas exchange, blood pressure, heart rate, and electrocardiogram. The load rate was increased by 16 W/min until the maximal exhaustion was reached, and maximal aerobic capacity (VO2max) was recorded (19).
Cardiovascular Parameters and Risk Scores
Arterial blood pressure and heart rate were measured in supine position on both arms, together with an electrocardiogram in resting conditions (19). Established cardiovascular risk scores computed from routinely collected variables were used to estimate cardiovascular risk. The Framingham Risk Scores for Coronary Heart Disease (FRS-CHD) and for Cardiovascular Disease (FRS-CVD) were calculated based on age, sex, systolic blood pressure (SBP), total and HDL-cholesterol, smoking status, treatment for hypertension, and diabetes to estimate the relative 10-year risks (27). The Atherosclerotic Cardiovascular Disease (ASCVD) risk score estimates 10-year risk for atherosclerotic cardiovascular events and is based on age, sex, SBP, total and HDL-cholesterol, smoking status, treatment for hypertension, diabetes, and race. The Systematic COronary Risk Evaluation (SCORE) estimates fatal cardiovascular risk in populations at high risk and is calculated based on age, sex, SBP, total cholesterol, and smoking status (27).
Diabetic Neuropathy
This study comprises assessment of both CAN and diabetic sensorimotor polyneuropathy (DSPN).
CAN was defined by the presence of at least three abnormal results among seven heart rate variability tests during spontaneous breathing over 5 min (very low and low-frequency power spectrum, coefficient of R-R interval variation), at deep breathing (expiration-to-inspiration ratio), in response to a Valsalva maneuver (Valsalva ratio), and during a postural change from supine to standing position (maximum-to-minimum 30:15 ratio, SBP change) using a VariaCardio TF5 system (MIE Medical Research, Leeds, U.K.) (28).
DSPN was diagnosed according to Toronto Consensus criteria, including subclinical and confirmed DSPN stages as previously described (29). Peripheral nerve function was assessed by electrophysiological testing and clinical neuropathy score surveys for signs and symptoms of DSPN. Motor nerve conduction velocity was measured in the peroneal, median, and ulnar nerves, while sensory nerve conduction velocity and sensory nerve action potentials were measured in the sural, median, and ulnar nerves (29).
Chronic Kidney Disease
Microalbuminuria was defined by urinary albumin levels of 20–200 mg/L and macroalbuminuria by levels >200 mg/L. The estimated glomerular filtration rate (eGFR) was calculated based on cystatin C and creatinine (normal kidney function: stage 1, eGFR >90 mL/min/1.73 m2; mildly impaired: stage 2, eGFR 60–90 mL/min/1.73 m2; and moderately impaired: stage 3, eGFR <60 mL/min/1.73 m2) (30).
Laboratory Analyses
Analyses of metabolic and routine laboratory parameters were performed as described (19).
Statistics
Results are given as mean (SD) or median (interquartile range) for continuous variables and percentages for categorical variables, as appropriate. Skewed data were log-transformed before analysis. Changes in variables of interest were evaluated by paired t tests. The sample size allowed detection of a change of IMCL between baseline and 5-year follow-up in type 2 diabetes (the primary outcome) of at least 9.5% with a power of (1−β) of 90% at an α level of 5%. Associations between parameters were evaluated with Spearman correlation coefficients, regression analyses adjusted for baseline value, and corresponding P values. P values < 0.05 indicated significant differences or correlations. Multiple linear regression models were used to analyze the effect of the interaction between VO2max, IMCL, and whole-body insulin sensitivity. Regression models estimated a predictive value of IMCL as an independent variable for the presence (baseline) and development (5-year follow-up adjusted to baseline) of diabetes-related complications, as dependent variables. Statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC), and graphs were generated using GraphPad Prism 7.01 software (GraphPad Software, La Jolla, CA).
Data and Resource Availability
To ensure data privacy of the participants, the generated data sets of the currently still ongoing GDS are not publicly available, especially because they are subject to national data protection laws and restrictions by the ethics committee. However, pseudonymized data can be requested through an individual project agreement within the GDS.
Results
Characteristics of the Study Population
At baseline, participants with type 2 diabetes had higher age, BMI, fasting C-peptide, and REE under fasting conditions, but lower HDL-cholesterol, than both control subjects and the type 1 diabetes group (Table 1). Participants with type 1 diabetes had lower total and LDL-cholesterol, as well as insulin-stimulated REE than control subjects and the type 2 diabetes group. People with type 2 diabetes had markedly lower whole-body insulin sensitivity (62% vs. control subjects, 36% vs. type 1 diabetes) (Fig. 1A) and higher ADIPO-IR (P < 0.001). In both diabetes groups, whole-body insulin sensitivity was higher in men compared with women (Fig. 1B). They also featured lower Vo2max (vs. control subjects: P < 0.001, vs. type 1 diabetes: P = 0.006) and insulin-stimulated RQ, but higher whole-body, subcutaneous, and visceral adipose tissue volume (Table 2) as well as hsCRP. Skeletal muscle volume was similar in type 1 and type 2 diabetes groups, but lower than in control subjects (Table 2). At baseline, criteria of borderline or confirmed diagnosis of CAN and DSPN were met by 13 and 27 participants with type 2 diabetes, respectively.
Table 1.
Characteristics of the study population at baseline
| Glucose-tolerant control subjects | Type 1 diabetes | Type 2 diabetes | |
|---|---|---|---|
| (n = 128) | (n = 132) | (n = 139) | |
| Age (years) | 43 ± 14 | 37 ± 11a | 52 ± 9b,c |
| Sex (male/female) | 87/41 | 72/60 | 95/44 |
| BMI (kg/m2) | 26.2 ± 4.4 | 25.0 ± 4.3a | 30.5 ± 5.1b,c |
| Plasma glucose (mg/dL) | 88 ± 12 | 132 ± 28a | 129 ± 32b |
| C-peptide (ng/dL) | 1.65 ± 0.85 | 1.07 ± 1.06a | 3.14 ± 1.23b,c |
| HbA1c (NGSP, %) | 5.2 ± 0.3 | 6.5 ± 0.9a | 6.3 ± 0.8b,c |
| HbA1c (mmol/mol) | 33 ± 3 | 47 ± 10a | 46 ± 9b,c |
| Total cholesterol (mg/dL) | 193 ± 39 | 184 ± 39a | 196 ± 43c |
| LDL-cholesterol (mg/dL) | 120 ± 36 | 110 ± 32a | 126 ± 36c |
| HDL-cholesterol (mg/dL) | 62 ± 18 | 63 ± 16 | 46 ± 12b,c |
| Triglycerides (mg/dL) | 97 ± 46 | 84 ± 34 | 140 ± 62b,c |
| FFA (µmol/L) | 506 ± 215 | 650 ± 276a | 626 ± 250b |
| hsCRP (mg/L) | 0.15 ± 0.24 | 0.19 ± 0.24 | 0.30 ± 0.30b,c |
| ADIPO-IR (a.u.) | 8.0 ± 0.9 | 8.6 ± 1.0a | 9.0 ± 0.9b,c |
| VO2max (mL ∗ min−1) | 2,447 ± 781 | 2,183 ± 658a | 2,000 ± 537b,c |
| RQbasal (a.u.) | 0.80 ± 0.07 | 0.79 ± 0.05 | 0.80 ± 0.07 |
| RQclamp (a.u.) | 0.96 ± 0.07 | 0.93 ± 0.06a | 0.91 ± 0.07b,c |
| REEbasal (kcal/day) | 1,660 ± 290 | 1,651 ± 245 | 1,836 ± 293b,c |
| REE clamp (kcal/day) | 1,937 ± 326 | 1,785 ± 279a | 1,918 ± 298c |
Data are shown as absolute numbers or mean ± SD, as appropriate. P values are based on ANOVA. Blood sampling was done in overnight fasted state participants. a.u., arbitrary units. RQbasal, RQ in fasting conditions; RQclamp, RQ during clamp; REEbasal, REEin fasting conditions; REEclamp, REE during clamp.
P < 0.05 type 1 diabetes vs. control subjects.
P < 0.05 type 2 diabetes vs. control subjects.
P < 0.05 type 1 diabetes vs. type 2 diabetes.
Figure 1.
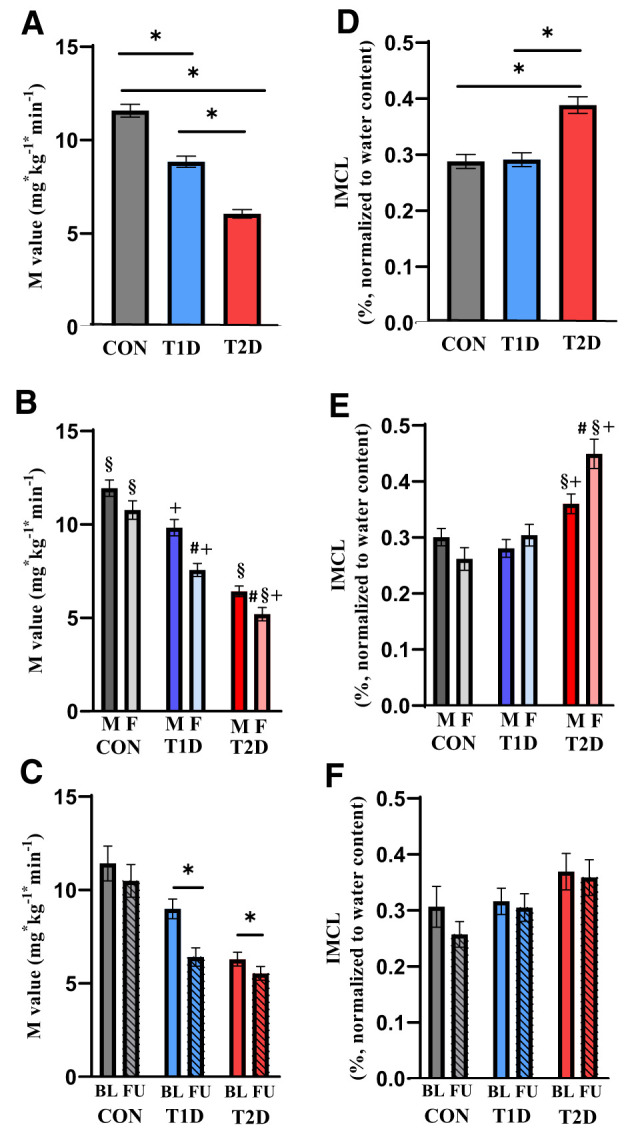
Whole-body insulin sensitivity from M-value (A–C) and IMCL triglyceride content (D–F) from 1H MRS at baseline and at the 5-year follow-up. A, B, D, and E represent the whole cohort at baseline (glucose-tolerant control [CON] subjects, n = 128; type 1 diabetes [T1D], n = 132; type 2 diabetes [T2D], n = 139), and C and F represent the follow-up cohort (glucose-tolerant control subjects, n = 20; type 1 diabetes, n = 27; type 2 diabetes, n = 38). Sex-specific differences within groups at baseline are shown in B and E (males [M] in darker color, females [F] in bright color). In C and F, hatched columns represent 5-year follow-up (FU) data. Data are presented as mean and SD. P values based on ANOVA by paired or unpaired t tests. BL, baseline; *P < 0.05. #P < 0.05 vs. men within the same group. §P < 0.05 vs. same sex of the T1D group. +P < 0.05 vs. same sex of the control subjects group.
Table 2.
Parameters of body composition of the study population at baseline
| Glucose-tolerant control subjects | Type 1 diabetes | Type 2 diabetes | |
|---|---|---|---|
| (n = 117) | (n = 74) | (n = 75) | |
| Adipose tissue volume (cm3) | |||
| Whole-body | 19,931 ± 9,468 | 19,268 ± 9,311 | 27,614 ± 9,822b,c |
| Subcutaneous | 18,108 ± 8,218 | 17,829 ± 8,834 | 23,637 ± 9,398b,c |
| Visceral | 2,106 ± 1,978 | 1,400 ± 1,223a | 3,974 ± 1,892b,c |
| Skeletal muscle volume (cm3) | 2,720 ± 709 | 2,427 ± 654a | 2,486 ± 562b |
Data are shown as mean ± SD. P values are based on ANOVA.
P < 0.05 type 1 diabetes vs. controls.
P < 0.05 type 2 diabetes vs. controls.
P < 0.05 type 1 diabetes vs. type 2 diabetes.
Five years later, glycemia and BMI increased in both diabetes groups (Table 3), while whole-body insulin sensitivity decreased (29% in type 1 diabetes, 13% in type 2 diabetes) (Fig. 1C) compared with baseline. Glycemia remained higher and whole-body insulin sensitivity was lower in both diabetes groups than in control groups (all P < 0.001). BMI was higher in individuals with type 2 diabetes compared with the other groups (both P < 0.01). Fasting C-peptide, REE under fasting conditions, and insulin-stimulated REE as well as RQ decreased in type 1 diabetes but not in type 2 diabetes. Total cholesterol decreased in type 1 diabetes, while triglycerides increased in type 2 diabetes, whereas LDL- and HDL-cholesterol, FFA, and ADIPO-IR did not change in any group over 5 years (Table 3).
Table 3.
Characteristics of subgroups examined at baseline and 5-year follow-up
| Glucose-tolerant control subjects (n = 20) | Type 1 diabetes (n = 27) | Type 2 diabetes (n = 38) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Age (years) | 47 ± 15 | 53 ± 15* | 36 ± 11 | 41 ± 11* | 51 ± 9 | 57 ± 9* |
| Sex (male/female) | 16/4 | 12/15 | 28/10 | |||
| BMI (kg/m2) | 26.2 ± 3.5 | 26.7 ± 3.6 | 24.4 ± 4.4 | 26.7 ± 5.2* | 29.6 ± 4.9 | 30.1 ± 4.5* |
| Plasma glucose (mg/dL) | 92 ± 7 | 91 ± 8 | 119 ± 26 | 143 ± 50* | 127 ± 29 | 146 ± 35* |
| C-peptide (ng/dL) | 1.86 ± 1.09 | 1.91 ± 1.01 | 0.96 ± 0.69 | 0.69 ± 0.87* | 3.03 ± 1.25 | 3.33 ± 1.54 |
| HbA1c (NGSP, %) | 5.3 ± 0.3 | 5.2 ± 0.4 | 6.2 ± 0.8 | 7.0 ± 0.9* | 6.2 ± 0.6 | 6.7 ± 0.8* |
| HbA1c (mmol/mol) | 34 ± 3 | 34 ± 4 | 45 ± 9 | 54 ± 10* | 44 ± 7 | 50 ± 9* |
| Total cholesterol (mg/dL) | 200 ± 37 | 194 ± 39 | 198 ± 35 | 179 ± 27* | 193 ± 36 | 190 ± 34 |
| LDL-cholesterol (mg/dL) | 125 ± 35 | 125 ± 35 | 114 ± 30 | 106 ± 25 | 120 ± 31 | 120 ± 33 |
| HDL-cholesterol (mg/dL) | 63 ± 16 | 61 ± 13 | 72 ± 17 | 68 ± 17 | 46 ± 13 | 48 ± 15 |
| Triglycerides (mg/dL) | 103 ± 60 | 96 ± 38 | 79 ± 28 | 84 ± 43 | 138 ± 70 | 171 ± 100* |
| FFA (µmol/L) | 563 ± 212 | 479 ± 206 | 706 ± 289 | 680 ± 424 | 623 ± 219 | 598 ± 188 |
| hsCRP (mg/L) | 0.10 ± 0.09 | 0.11 ± 0.10 | 0.14 ± 0.13 | 0.17 ± 0.14 | 0.30 ± 0.21 | 0.20 ± 0.14* |
| ADIPO-IR (a.u.) | 8.3 ± 0.8 | 8.0 ± 0.8 | 8.7 ± 0.9 | 8.6 ± 1.2 | 9.0 ± 0.7 | 9.1 ± 0.9 |
| RQbasal (a.u.) | 0.81 ± 0.04 | 0.80 ± 0.07 | 0.80 ± 0.05 | 0.80 ± 0.09 | 0.79 ± 0.06 | 0.79 ± 0.17 |
| RQclamp (a.u.) | 0.96 ± 0.07 | 0.94 ± 0.07 | 0.94 ± 0.07 | 0.91 ± 0.06* | 0.89 ± 0.05 | 0.93 ± 0.05 |
| REEbasal (kcal/day) | 1,653 ± 273 | 1,726 ± 254 | 1,604 ± 236 | 1,568 ± 257* | 1,811 ± 299 | 1,827 ± 307 |
| REEclamp (kcal/day) | 1,943 ± 275 | 1,993 ± 294 | 1,731 ± 253 | 1,708 ± 294* | 1,939 ± 306 | 1,911 ± 321 |
Data are shown as absolute numbers or mean ± SD, as applicable. P values based on paired t tests. Blood sampling was done in overnight fasted state participants. a.u., arbitrary units; RQbasal, RQ in fasting conditions; RQclamp, RQ during clamp; REEbasal, REE in fasting conditions; REEclamp, REE during clamp.
P < 0.05 vs. baseline.
IMCL at Baseline and 5 Years Later
Even after adjustment for age, sex, BMI, and skeletal muscle volume, IMCL at baseline was higher in people with type 2 diabetes than in those with type 1 diabetes (+29%, P < 0.001) and control subjects (+30%, P < 0.001), without difference between individuals with type 1 diabetes and control subjects (Fig. 1D). IMCL was higher in women than in men with type 2 diabetes, but not different between men and women in the glucose-tolerant and type 1 diabetes groups (Fig. 1E). At 5 years, IMCL had not significantly changed in any group (Fig. 1F), but remained higher in type 2 diabetes compared with control subjects (+33%, P = 0.028), regardless the glucose-lowering treatment at baseline.
Associations of IMCL With VO2max, Metabolic Parameters, and Body Composition
At baseline, IMCL negatively associated with whole-body insulin sensitivity in both diabetes groups (Supplementary Table 3), even after adjustment for age, sex, and BMI. Regression models revealed that neither baseline IMCL nor changes in IMCL related to changes in whole-body insulin sensitivity over 5 years after diagnosis of diabetes.
In type 2 diabetes, the level of Vo2max modulated the association between IMCL and whole-body insulin sensitivity. Elevated IMCL associated with reduced whole-body insulin sensitivity in adults with type 2 diabetes and a lower degree of Vo2max, but not in those with a higher degree of Vo2max (P = 0.022 for the interaction between Vo2max, IMCL, and M-value) (Fig. 2). The interaction remained significant even after adjustments for age, sex, and BMI but was not found in type 1 diabetes or in individuals without diabetes (Supplementary Table 4). Moreover, no interaction was found between adipose tissue mass, IMCL, and VO2max in either group. Of note, adjusting insulin sensitivity (M) for insulin levels during the steady state (M/I) did not affect the interaction of IMCL and VO2max, and M and M/I were strongly correlated across all groups (Supplementary Fig. 2).
Figure 2.
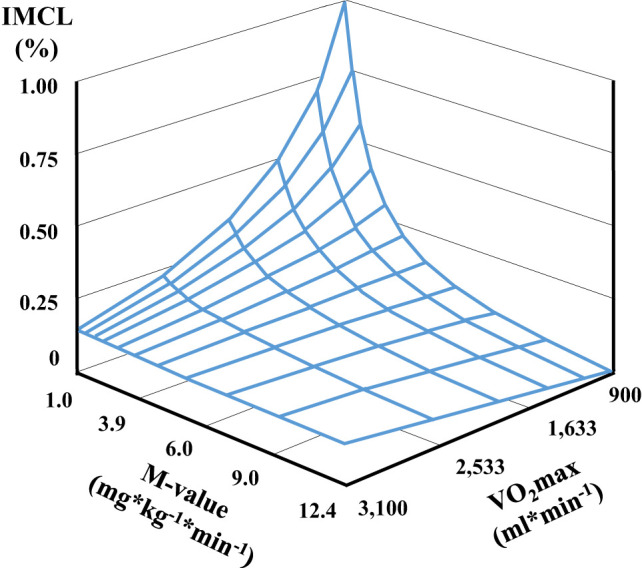
Tridimensional regression describing the impact of physical fitness (VO2max) on the relationship between IMCL content and whole-body insulin sensitivity (M-value) in recent-onset type 2 diabetes at baseline (n = 139).
In type 2 diabetes, baseline IMCL associated with fasting blood glucose, ADIPO-IR, whole-body adipose tissue (Supplementary Table 3), fasting C-peptide (β = 0.22, P = 0.002), and hsCRP (β = 0.43, P = 0.006). In type 1 diabetes, baseline IMCL associated positively with total cholesterol and negatively with insulin-stimulated RQ (Supplementary Table 3). There were no associations between IMCL and glycated hemoglobin (HbA1c), FFA, subcutaneous and visceral adipose tissue volume, or skeletal muscle volume in any group (Supplementary Table 3).
Associations of IMCL With Cardiovascular Risk, Diabetes-Related Chronic Kidney Disease, and Neuropathy in Type 2 Diabetes
Regression analyses with baseline IMCL as an independent variable and established cardiovascular risk scores as a dependent variable revealed a positive association between IMCL and FRS-CVD at baseline (Table 4). On the other hand, IMCL at 5 years associated with FRS-CHD, ASCVD, and SCORE, but not with FRS-CVD. However, these associations were significant only before adjustment for age, sex, BMI, and whole-body insulin sensitivity. Baseline IMCL predicted accelerated increase for all cardiovascular risk scores (FRS-CHD, FRS-CVD, ASCVD, and SCORE), with association for an accelerated 10-year risk for cardiovascular disease (FRS-CVD), which remained significant after adjustments (Table 4).
Table 4.
Association between IMCL triglyceride contents and comorbidities at baseline and 5-year follow-up of type 2 diabetes
| IMCL vs. complication status at baseline (n = 139) | IMCL vs. complication status at 5 years (n = 38) | Baseline IMCL vs. complication status at 5 years (n = 38) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β* | P * | β** | P ** | β | P | β* | P * | β** | P ** | β | P | β* | P * | β** | P ** | |
| FRS-CHD | −0.20 | 0.410 | −0.07 | 0.695 | −0.13 | 0.466 | 0.90 | 0.006 | 0.26 | 0.303 | 0.18 | 0.475 | 0.59 | 0.046 | 0.52 | 0.070 | 0.52 | 0.211 |
| FRS-CVD | 0.47 | 0.036 | −0.07 | 0.521 | −0.12 | 0.289 | 0.18 | 0.643 | 0.30 | 0.105 | 0.29 | 0.089 | 0.61 | 0.009 | 0.60 | 0.005 | 0.67 | 0.002 |
| ASCVD score | −0.31 | 0.130 | −0.10 | 0.453 | −0.16 | 0.232 | 0.89 | 0.002 | 0.22 | 0.223 | 0.18 | 0.258 | 0.58 | 0.011 | 0.48 | 0.021 | 0.53 | 0.012 |
| SCORE | −0.68 | 0.023 | −0.04 | 0.752 | −0.02 | 0.882 | 1.01 | 0.001 | 0.04 | 0.839 | 0.08 | 0.685 | 0.43 | 0.029 | 0.48 | 0.011 | 0.53 | 0.005 |
| CAN | 1.52 | 0.039 | 1.46 | 0.059 | 1.15 | 0.143 | −0.41 | 0.569 | 0.45 | 0.644 | 1.12 | 0.338 | −0.83 | 0.577 | −1.58 | 0.480 | −1.73 | 0.498 |
| DSPN | 0.49 | 0.302 | 0.71 | 0.163 | 0.50 | 0.353 | −1.47 | 0.166 | −2.13 | 0.232 | −0.40 | 0.834 | 3.65 | 0.368 | 1.54 | 0.659 | 2.01 | 0.777 |
| Microalbuminuria | 0.67 | 0.018 | 0.58 | 0.045 | 0.69 | 0.024 | 1.09 | 0.045 | 1.04 | 0.059 | 1.29 | 0.009 | 2.17 | 0.026 | 2.14 | 0.033 | 2.05 | 0.042 |
P values based on logistic or linear regression models, as appropriate. Bold values are statistically significant. β, correlation coefficient.
P adjusted for sex, age, and BMI.
P adjusted for age, sex, BMI, and whole-body insulin sensitivity.
Regression models further revealed an association of IMCL with presence of CAN at baseline only, which was lost after adjustments, and IMCL was neither related to DSPN at baseline nor at 5 years after the diabetes diagnosis (Table 4).
IMCL also associated with microalbuminuria at baseline (Table 4) and at 5 years after the diabetes diagnosis, and baseline IMCL predicted accelerated progression of microalbuminuria within 5 years (Table 4). Of note, all associations between IMCL and microalbuminuria were independent of age, sex, BMI, and whole-body insulin sensitivity. However, there was no relationship between IMCL and eGFR.
No associations between IMCL and cardiovascular risk, chronic kidney disease, DSPN, or CAN were observed in type 1 diabetes (data not shown).
Discussion
This study shows that intramyocellular triglyceride content is 1) already elevated in recent-onset type 2 diabetes, which occurs independent of age, sex, body mass, and skeletal muscle volume, 2) correlated with whole-body insulin resistance, which is modulated by aerobic fitness, and, most importantly, 3) associated with higher cardiovascular risk, prevalent CAN, and microalbuminuria. However, IMCL remains largely unchanged despite increasing whole-body insulin resistance during the initial course of type 2 diabetes.
IMCL is a well-recognized biomarker of muscle insulin resistance (31); however, a relationship between IMCL and diabetes-specific complications has been less clear. This study now provides direct evidence for a link between IMCL and accelerated risk of cardiovascular risk, CAN, and microalbuminuria in type 2 but not type 1 diabetes. The positive association of IMCL with 10-year risk for cardiovascular disease (FRS-CVD) was attenuated after adjustment for key confounders (age, sex, body mass, and whole-body insulin sensitivity). Nonetheless, IMCL was strongly associated with FRS-CHD, ASCVD, and SCORE after 5 years of known type 2 diabetes, and baseline IMCL related to higher 10-year risk of cardiovascular disease even after adjustment for confounders. These data indicate that IMCL might not directly affect risk of macrovascular complications at diagnosis, but predict accelerated increase during the early course of type 2 diabetes. Although the association between insulin resistance and cardiovascular disease is well established (32), most of the evidence for a modulating role of ectopic fat contents comes from adipose tissue, liver, and endothelium (15). The current findings suggest that IMCL could also serve as a noninvasive biomarker, characterized by good stability (33) and reproducibility (34,35), for the risk of diabetes-related cardiovascular complications.
Insulin resistance may further contribute to development of CAN in type 2 diabetes (16), and lower cardiac vagal activity and baroreflex sensitivity associate with higher hepatocellular lipid content, suggesting a role of ectopic fat also for parasympathetic CAN (36). The current study extends this concept by indicating a direct link between CAN and IMCL at the onset of type 2 diabetes. However, adjustment for other possible confounders attenuates this correlation, which was also lost at 5 years after diabetes diagnosis.
Finally, insulin resistance is an independent risk factor for chronic kidney disease (14), so that IMCL may also relate to this diabetes-related complication. Indeed, IMCL strongly associates with microalbuminuria at baseline and 5 years later, independent of age, sex, and body mass. This association remained even after adjustment for whole-body insulin resistance, and baseline IMCL independently predicted progression of microalbuminuria over the first 5 years of known type 2 diabetes. This suggests an important role of systemic lipid metabolism and ectopic fat for the pathogenesis of abnormal kidney function, as suggested by higher renal adipose tissue content and its relationship to microalbuminuria in early diabetic kidney disease (37). The absence of a similar correlation with eGFR underlines that the higher IMCL rather reflects the early damage of renal glomeruli.
In the type 1 diabetes group, whole-body insulin sensitivity was higher than in people with type 2 diabetes, despite similar fasting glycemia, but lower than in glucose-tolerant control subjects. The intravenous glucose bolus may lead to different degrees of hyperglycemia and portal insulinemia between type 1 and type 2 diabetes, which could affect the glucose infusion rates during the subsequent clamp period. However, the high-dose insulin clamp and the time lag until the clamp steady-state renders is unlikely. In support, whole-body insulin sensitivity (M-value) strongly correlated with M-value normalized to insulinemia during clamp steady state (M/I) across all groups.
Baseline and follow-up IMCL was only higher in type 2, but not type 1 diabetes compared with glucose-tolerant humans. In contrast, previous studies reported elevated IMCL in individuals with type 1 diabetes (5,38), which may be due to their poor metabolic control (average HbA1c of 8.6% and 7.7%, respectively) compared with the current study of mostly well-controlled diabetes (75% had HbA1c <7%). In line, previous studies in people with well-controlled type 1 diabetes (average HbA1c of 6.8%, 6.3%, and 7.4%, respectively) found no IMCL elevation, independent of disease duration ranging from 13 to 25 years (7,39,40). Also, chronic peripheral hyperinsulinemia did not affect IMCL in type 1 diabetes after pancreas-kidney transplantation (41). Likewise, neither fed nor fasted muscle triglyceride content was elevated in a rodent model of type 1 diabetes (42). Nonetheless, differences in reporting the level of IMCL in type 1 diabetes might be also affected by methodology. For example, higher levels in samples obtained by biopsy might be due to incomplete separation of intra- from extracellular lipids and/or ex vivo lipolysis. Indeed, a recent systematic review supported this view in that the analytical approach can influence the levels of IMCL at least in response to exercise (43). Differences were also found in studies reporting IMCL in sedentary individuals, with some reporting higher IMCL compared with matched control subjects using 1H-MRS (5) or biopsy (38) and others reporting similar IMCL in type 1 diabetes and glucose-tolerant humans (7,40,41). Overall, IMCL content in type 1 diabetes seems to depend rather on glycemic control than diabetes duration or insulinemia.
Higher IMCL tightly relates to lower levels of whole-body insulin sensitivity and physical fitness in sedentary humans (22), whereas a more complex tridirectional relationship has been proposed for exercise-trained people, by which higher IMCL relates to higher insulin sensitivity (12,44). This phenomenon, known as the athletés paradox, was described in observational studies of glucose-tolerant individuals comprising participants with higher physical fitness, such as endurance-trained athletes or triathletes (44,45), or in exercise intervention studies in elderly sedentary people with overweight or obesity (46). In the current study, we did not observe any interaction between physical fitness, insulin sensitivity, and IMCL in glucose-tolerant control subjects or people with type 1 diabetes, probably because of their only moderate degree of physical fitness (mean Vo2max 31 mL ∗ min−1 ∗ kg−1). However, the degree of physical fitness modulated the interaction between whole-body insulin sensitivity and IMCL in type 2 diabetes. Interestingly, the interaction between IMCL and insulin sensitivity was not reversed from a negative to positive relationship in individuals with type 2 diabetes and a higher degree of physical fitness. This is likely due to the overall (∼20%) lower physical fitness in participants with type 2 diabetes compared with control subjects, which is in line with the physical fitness in other studies (47) and across all diabetes endotypes (27). The physical performance and its dynamics over time are influenced by several factors. Approximately 36% of people with recent-onset type 2 diabetes fail to improve their insulin sensitivity despite increased habitual physical activity and physical fitness, which is at least in part genetically mediated (48). Further, impairment of metabolic flexibility and mitochondrial respiration (49) may compromise the ability of diabetic muscle to use IMCL as an energetic substrate in contrast to rapid adaptation of skeletal muscle in insulin-sensitive trained individuals (12). Nonetheless, the link between elevated IMCL and insulin resistance in sedentary individuals in populations without diabetes (12) or with recent type 2 diabetes in the current study supports the paradigm of a protective role of higher physical fitness in type 2 diabetes in preventing excessive IMCL accumulation and in turn insulin resistance.
At baseline, IMCLs were higher in women than in men with recent-onset type 2 diabetes, but not in the other groups. This is partly in line with the observation that men may preferentially store triglycerides in the liver, whereas women rather accumulate triglycerides in skeletal muscles (50). Nonetheless, neither age, sex, nor BMI modulated the interaction between IMCL, insulin sensitivity, and physical fitness in the type 2 diabetes group of the current study.
Of note, IMCL did not change in both diabetes groups during the initial 5 years, indicating a stable behavior as a biomarker, as also suggested from the only minimal changes in IMCL in diabetic rodents (33) despite rising muscle lipid metabolites in insulin-resistant mice (51). This differs from the typical increase in liver fat content observed during the early progression of human type 2 diabetes (21) and murine insulin resistance (51). Taken together, these findings support a concept of tissue-specific roles of ectopic lipid storage and cellular lipid metabolites during the pathogenesis of diabetes.
The strengths of the current study include the prospective design of GDS, the relatively large sample size of comprehensively phenotyped individuals recently diagnosed with type 1 or type 2 diabetes, and the use of state-of-the-art methodology (1H-MRS, Botnia clamp, respiratory gas exchange measurement during cycling ergometry, and nerve conduction studies).
While the recruitment strategy of GDS allows individuals with a defined known disease duration to be examined, the results cannot be generalized to the general population with diabetes and to other regions of the world. Another limitation of the study is a relatively lower sample size of the follow-up cohort as well as lower number of paired measurements of body composition caused by the positioning for MRI scans, which was considered inconvenient by several participants. Finally, IMCL reflects total intramyocellular triglycerides and does not allow conclusions about lipid contents in type I or type II myocytes and subcellular distribution of lipids (4), which, however, does jeopardize the possible value of IMCL as a noninvasive clinical biomarker.
In conclusion, these findings demonstrate that intramyocellular triglyceride content is elevated in recent-onset type 2 diabetes, which—modified by aerobic fitness—correlates with whole-body insulin resistance but does not change during the initial course of diabetes. Of note, IMCL associates with higher risk of both microvascular and macrovascular complications early in type 2 diabetes, suggesting that IMCL may serve as a stable noninvasive biomarker of comorbidities and complications related to type 2 diabetes.
Article Information
Acknowledgments. The authors would like to thank the staff of the Clinical Research Center, Institute for Clinical Diabetology at the German Diabetes Center, Düsseldorf, Germany, for excellent help with the experiments. The authors thank the participants for their invaluable contributions to the study. The GDS Study Group consists of M. Roden (speaker), H. Al-Hasani, B. Belgardt, G.J. Bönhof, V. Burkart, A.E. Buyken, G. Geerling, C. Herder, A. Icks, K. Jandeleit-Dahm, J. Kotzka, O. Kuß, E. Lammert, W. Rathmann, V. Schrauwen-Hinderling, J. Szendroedi, S. Trenkamp, R. Wagner and their coworkers who contributed to the design and conduct of the GDS.
Funding. The research of M.R. is supported in part by grants from the German Federal Ministry of Health (BMG), the Ministry of Culture and Science of the State North Rhine-Westphalia and the German Federal Ministry of Education and Research both to German Center for Diabetes Research (DZD e.V.), as well as by grants from the European Community (HORIZON-HLTH-2022-STAYHLTH-02-01: Panel A) to the INTERCEPT-T2D consortium, EUREKA Eurostars-2 (E! 113230 DIA-PEP), German Science Foundation (CRC/SFB 1116/2 B12, RTG/GRK 2576 vivid, Project 3), Schmutzler Stiftung, and the program “Profilbildung 2020,” an initiative of the Ministry of Culture and Science of the State of North Rhine-Westphalia.
The sole responsibility for the content of this publication lies with the authors.
Duality of Interest. M.R. has received personal fees for consulting or serving on advisory boards from Astra-Zeneca, Eli Lilly, Novo Nordisk, Boehringer Ingelheim, and Sanofi US, and has been supported for investigator-initiated trials by Boehringer Ingelheim, Novartis Pharma, and Nutricia/Danone. R.W. reports lecture fees from Novo Nordisk, Sanofi, and Eli Lilly and served on an advisory board for Akcea Therapeutics, Daiichi Sankyo, Sanofi, Eli Lilly, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S. wrote the article and researched the data. M.S., O.P.Z., Y.K., K.B., G.H., A.S., G.J.B., F.M., I.Y., C.M., M.H., and M.B. performed the examinations and researched the data. K.S. performed the statistical analyses. O.P.Z., M.K., V.B., V.B.S.-H., R.W., and M.R. contributed to the discussion and reviewed and edited the article. All authors gave final approval of this version to be published. M.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the 58th Annual Meeting of European Association for the Study of Diabetes, Stockholm, Sweden, 19–23 September 2022.
Footnotes
Clinical trial reg. no. NCT01055093, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.23710308.
A complete list of members of the GDS Group can be found in the supplementary material online.
Contributor Information
GDS Group:
M. Roden, H. Al-Hasani, B. Belgardt, G.J. Bönhof, V. Burkart, A.E. Buyken, G. Geerling, C. Herder, A. Icks, K. Jandeleit-Dahm, J. Kotzka, O. Kuß, E. Lammert, W. Rathmann, V. Schrauwen-Hinderling, J. Szendroedi, S. Trenkamp, and R. Wagner
References
- 1. Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999;42:113–116 [DOI] [PubMed] [Google Scholar]
- 2. Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999;48:1600–1606 [DOI] [PubMed] [Google Scholar]
- 3. Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 1999;48:1113–1119 [DOI] [PubMed] [Google Scholar]
- 4. Coen PM, Dubé JJ, Amati F, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 2010;59:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 2003;285:E1174–E1181 [DOI] [PubMed] [Google Scholar]
- 6. Szendroedi J, Schmid AI, Chmelik M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007;4:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kacerovsky M, Brehm A, Chmelik M, et al. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J Intern Med 2011;269:189–199 [DOI] [PubMed] [Google Scholar]
- 8. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature 2019;576:51–60 [DOI] [PubMed] [Google Scholar]
- 9. Krebs M, Roden M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes Obes Metab 2005;7:621–632 [DOI] [PubMed] [Google Scholar]
- 10. Kahn D, Perreault L, Macias E, et al. Subcellular localisation and composition of intramuscular triacylglycerol influence insulin sensitivity in humans. Diabetologia 2021;64:168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 2015;30:529–542 [DOI] [PubMed] [Google Scholar]
- 12. Thamer C, Machann J, Bachmann O, et al. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 2003;88:1785–1791 [DOI] [PubMed] [Google Scholar]
- 13. Koliaki C, Roden M. Alterations of mitochondrial function and insulin sensitivity in human obesity and diabetes mellitus. Annu Rev Nutr 2016;36:337–367 [DOI] [PubMed] [Google Scholar]
- 14. Koppe L, Pelletier CC, Alix PM, et al. Insulin resistance in chronic kidney disease: new lessons from experimental models. Nephrol Dial Transplant 2014;29:1666–1674 [DOI] [PubMed] [Google Scholar]
- 15. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014;10:293–302 [DOI] [PubMed] [Google Scholar]
- 16. Manzella D, Paolisso G. Cardiac autonomic activity and Type II diabetes mellitus. Clin Sci (Lond) 2005;108:93–99 [DOI] [PubMed] [Google Scholar]
- 17. Zaharia OP, Strassburger K, Strom A, et al.; German Diabetes Study Group . Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol 2019;7:684–694 [DOI] [PubMed] [Google Scholar]
- 18. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 19. Szendroedi J, Saxena A, Weber KS, et al.; GDS Group . Cohort profile: the German Diabetes Study (GDS). Cardiovasc Diabetol 2016;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 21. Kupriyanova Y, Zaharia OP, Bobrov P, et al.; GDS group . Early changes in hepatic energy metabolism and lipid content in recent-onset type 1 and 2 diabetes mellitus. J Hepatol 2021;74:1028–1037 [DOI] [PubMed] [Google Scholar]
- 22. Roden M. Muscle triglycerides and mitochondrial function: possible mechanisms for the development of type 2 diabetes. Int J Obes 2005;29(Suppl. 2):S111–S115 [DOI] [PubMed] [Google Scholar]
- 23. Krššák M, Lindeboom L, Schrauwen-Hinderling V, et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed 2021;34:e4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krssák M, Mlynárik V, Meyerspeer M, Moser E, Roden M. 1H NMR relaxation times of skeletal muscle metabolites at 3 T. MAGMA 2004;16:155–159 [DOI] [PubMed] [Google Scholar]
- 25. Kahl S, Nowotny B, Piepel S, et al. Estimates of insulin sensitivity from the intravenous-glucose-modified-clamp test depend on suppression of lipolysis in type 2 diabetes: a randomised controlled trial. Diabetologia 2014;57:2094–2102 [DOI] [PubMed] [Google Scholar]
- 26. Nowotny B, Zahiragic L, Krog D, et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 2013;62:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saatmann N, Zaharia OP, Strassburger K, et al. Physical fitness and cardiovascular risk factors in novel diabetes subgroups. J Clin Endocrinol Metab 2022;107:1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ziegler D, Strom A, Bönhof G, et al.; GDS group . Differential associations of lower cardiac vagal tone with insulin resistance and insulin secretion in recently diagnosed type 1 and type 2 diabetes. Metabolism 2018;79:1–9 [DOI] [PubMed] [Google Scholar]
- 29. Ziegler D, Bönhof GJ, Strom A, et al. Progression and regression of nerve fibre pathology and dysfunction early in diabetes over 5 years. Brain 2021;144:3251–3263 [DOI] [PubMed] [Google Scholar]
- 30. Levey AS, Coresh J, Balk E, et al.; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 31. Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 2007;104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuhlmann J, Neumann-Haefelin C, Belz U, et al. Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes 2003;52:138–144 [DOI] [PubMed] [Google Scholar]
- 34. Kautzky-Willer A, Krssak M, Winzer C, et al. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes 2003;52:244–251 [DOI] [PubMed] [Google Scholar]
- 35. Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol 2008;67:275–284 [DOI] [PubMed] [Google Scholar]
- 36. Ziegler D, Strom A, Kupriyanova Y, et al.; GDS Group . Association of lower cardiovagal tone and baroreflex sensitivity with higher liver fat content early in type 2 diabetes. J Clin Endocrinol Metab 2018;103:1130–1138 [DOI] [PubMed] [Google Scholar]
- 37. Wang YC, Feng Y, Lu CQ, Ju S. Renal fat fraction and diffusion tensor imaging in patients with early-stage diabetic nephropathy. Eur Radiol 2018;28:3326–3334 [DOI] [PubMed] [Google Scholar]
- 38. Ebeling P, Essén-Gustavsson B, Tuominen JA, Koivisto VA. Intramuscular triglyceride content is increased in IDDM. Diabetologia 1998;41:111–115 [DOI] [PubMed] [Google Scholar]
- 39. Bernroider E, Brehm A, Krssak M, et al. The role of intramyocellular lipids during hypoglycemia in patients with intensively treated type 1 diabetes. J Clin Endocrinol Metab 2005;90:5559–5565 [DOI] [PubMed] [Google Scholar]
- 40. Wolf P, Fellinger P, Pfleger L, et al. Reduced hepatocellular lipid accumulation and energy metabolism in patients with long standing type 1 diabetes mellitus. Sci Rep 2019;9:2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stadler M, Anderwald C, Pacini G, et al. Chronic peripheral hyperinsulinemia in type 1 diabetic patients after successful combined pancreas-kidney transplantation does not affect ectopic lipid accumulation in skeletal muscle and liver. Diabetes 2010;59:215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jelenik T, Séquaris G, Kaul K, et al. Tissue-specific differences in the development of insulin resistance in a mouse model for type 1 diabetes. Diabetes 2014;63:3856–3867 [DOI] [PubMed] [Google Scholar]
- 43. Stokie JR, Abbott G, Howlett KF, Hamilton DL, Shaw CS. Intramuscular lipid utilization during exercise: a systematic review, meta-analysis, and meta-regression. J Appl Physiol (1985). 2023;134:581–592 [DOI] [PubMed] [Google Scholar]
- 44. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 45. Klepochová R, Valkovič L, Hochwartner T, et al. Differences in muscle metabolism between triathletes and normally active volunteers investigated using multinuclear magnetic resonance spectroscopy at 7T. Front Physiol 2018;9:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 2008;294:E882–E888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nesti L, Pugliese NR, Sciuto P, Natali A. Type 2 diabetes and reduced exercise tolerance: a review of the literature through an integrated physiology approach. Cardiovasc Diabetol 2020;19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pesta D, Jelenik T, Zaharia OP, et al. NDUFB6 polymorphism is associated with physical activity-mediated metabolic changes in type 2 diabetes. Front Endocrinol (Lausanne) 2021;12:693683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2011;8:92–103 [DOI] [PubMed] [Google Scholar]
- 50. Beaudry KM, Devries MC. Sex-based differences in hepatic and skeletal muscle triglyceride storage and metabolism. Appl Physiol Nutr Metab 2019;44:805–813 [DOI] [PubMed] [Google Scholar]
- 51. Jelenik T, Kaul K, Séquaris G, et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes 2017;66:2241–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]


