Abstract
Ceramides are lipid molecules involved in inflammation-related signaling. Recent studies have shown that higher amounts of specific circulating ceramides and their ratios are associated with future development of cardiovascular (CV) disease (CVD). We examined the associations between serum ceramide levels with CVD, kidney failure, and all-cause mortality in individuals with long-standing type 1 diabetes (T1D). We included 662 participants with T1D and 6-year follow-up, with a mean age of 55 years and mean diabetes duration of 33 years. Baseline serum samples were analyzed using liquid chromatography–mass spectrometry. Six predefined ceramide levels were measured, and predefined ratios were calculated. Adjusted Cox regression analyses on ceramide levels in relation to future CV events (CVE), kidney failure, and all-cause mortality were performed, with and without adjustment for age, sex, BMI, LDL, triglycerides, systolic blood pressure, HbA1c, history of CVD, smoking status, statin use, estimated glomerular filtration rate (eGFR), and urinary albumin excretion rate (UAER). The ceramide ratio cer(d18:1/18:0)/cer(d18:1/24:0) was significantly associated with risk of CVE (hazard ratio [HR] = 1.33, P = 0.01) and all-cause mortality (HR = 1.48, P = 0.01) before and after adjustments. All five investigated ceramide ratios were associated with kidney failure, before adjusting for the kidney markers eGFR and UAER. In this study, we demonstrate specific ceramides and ratios associated with 6-year cardiovascular risk and all-cause mortality in a T1D cohort. This highlights the strength of ceramide association with vascular complications and presents a new potential tool for early risk assessment if validated in other cohorts.
Article Highlights
Improved tools for assessing risk for diabetes complication before onset will help in complication prevention.
We investigated a set of six predefined ceramides and their ratios versus 6-year outcomes of cardiovascular events, kidney failure, and all-cause mortality in people with long-standing type 1 diabetes, using Cox regression with and without adjustment for potential confounders.
We found that several ceramides and ceramide ratios associated with cardiovascular events and all-cause mortality. The ratio of cer(d18:1/18:0)/cer(d18:1/24:0) was an especially robust marker.
These finding show that ceramides can be biomarkers of cardiovascular disease and all-cause mortality in individuals with long-standing type 1 diabetes.
Introduction
The number of people living with type 1 diabetes (T1D) is rising, and estimates foresee further increase in the coming years (1). Two major complications to T1D are cardiovascular (CV) disease (CVD) and chronic kidney disease, the former being the leading cause of death in individuals with T1D (2,3). Individuals with T1D and diabetic kidney disease are subjected to an increased mortality risk (4). Diabetes, CVD, and kidney disease are closely interlinked, sharing risk factors and molecular mechanisms (5,6).
Identification of biomarkers that stratify those at highest risk for these complications would allow early intervention with optimization of risk factors and potentially organ protective therapies, as has been seen in type 2 diabetes (T2D) with nonsteroidal mineralocorticoid receptor antagonists, glucagon-like peptide 1 receptor agonists (GLP-1RA), and sodium glucose cotransporter 2 inhibitors (SGLT2i) (7,8).
Ceramides are lipid molecules implicated in inflammation and apoptosis signaling (9). Recent studies have found six specific ceramides associated with CV outcomes and mortality years before onset of the clinical disease. Tarasov et al. (10) were among the first to show a link between ceramides and CV risk, when they showed that the amounts of the ceramides cer(d18:1/16:0), cer(d18:1/18:0), cer(d18:1/20:0), and cer(d18:1/24:1), here referred to as cer16, cer18, cer20, and cer24:1, respectively, were significantly higher and cer(d18:1/24:0), referred to as cer24:0, was significantly lower in people who developed coronary artery disease. Tarasov et al. (10) also suggested using the ceramide ratio to cer24:0 to mitigate variations of individual ceramides; these ratios resulted in even stronger association and have been adapted by many subsequent studies. Later studies found cer(d18:1/22:0) (cer22) to associate to CVD an all-cause mortality. Together, these six ceramides and their ratio to cer24:0 have been reported as biomarkers of CV outcomes in multiple large studies (10–16). The levels of these specific ceramides are also increased in individuals with T2D, and have been linked to insulin resistance (17–20) as well as to an increased risk of developing T2D (21–23). However, evidence for ceramide levels as risk predictors for CVD in T1D cohorts is lacking. In this study, we set out to evaluate these six prespecified ceramides in relation to CVD, kidney failure, and all-cause mortality in a prospective study of 662 individuals with T1D.
Research Design and Methods
Participants
This study is based on a prospective cohort study of participants with T1D recruited at the Steno Diabetes Center Copenhagen outpatient clinic between 2009 and 2011, described in full by Theilade et al. (24). A follow-up study was carried out in 2017 obtaining information about hospitalization and death from Danish national registries, as well as carrying out mass spectrometry–based lipidomics analysis using serum samples collected at the start of the original study; details of the follow-up protocol have been reported by Tofte et al. (25). In the current study, a total of 662 participants were included, comprising 296 females (45%) and 366 males (55%).
The original study is registered at ClinicalTrials.gov under the identifier NCT01171248; it holds ethical approval from the Danish National Committee on Biomedical Research Ethics, Copenhagen, Denmark (2009-056). The follow-up protocol was approved The Ethics Committee E, Region Hovedstaden, Hillerød, Denmark. Both the original study and the follow-up protocol adhere to principles of the Declaration of Helsinki. Written and informed consent was given by the participants before inclusion in the original study, which included consent for inclusion in a follow-up study.
Clinical Outcomes
Clinical outcomes at follow-up, followed until 31 December 2016, were assessed using information from the Danish National Death Register and the Danish National Health Register (25–27). Data on hospital admission, diagnoses according to the International Classification of Diseases, Tenth Revision codes, and procedural codes according to the Nordic Classification of Surgical Procedures were obtained from the Danish National Health Register; the specific codes used can be found in Supplementary Table 1. Biochemical measurements including estimated glomerular filtration rate (eGFR) and urinary albumin excretion rate (UAER) were obtained from the local electronic laboratory records.
CV events (CVE) are a composite of several outcomes, as previously defined (28), namely, CV mortality, coronary artery disease including nonfatal myocardial infarction and coronary revascularization (percutaneous arterial intervention or coronary bypass grafting), nonfatal stroke, and peripheral arterial interventions including amputations. For the analyses of any CVE, only the first event was included, even if participants experienced consecutive end points. All deaths, except when an unambiguous non-CV cause of death was reported, were defined as CV mortality. Kidney failure was defined as either receiving dialysis or kidney transplantation or having an eGFR ≤15 mL/min/1.73 m2 (25). Albuminuria status was defined by urinary albumin excretion rate (UAER), normoalbuminuria was defined as having a UAER below 30 mg/g, moderate increase in albuminuria as UAER between 30 and 299 mg/g, and severe increase in albuminuria as UAER ≥300 mg/g.
Lipid Analysis
Untargeted lipidomics analysis of baseline serum was carried out using a protocol as previously described in detail (25,29). The analysis was carried out as follows. Serum samples were stored at −80°C and were handled on ice whenever possible. A lipid fraction was created and extracted using a Folch extraction with minor modifications (30). The samples were analyzed using ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry, a detailed description can be found in the Supplementary Material.
The raw mass spectrometry data were analyzed for ceramide amounts as follows: preprocessing was carried out with MZmine 2 v.2.28 (31). In this study, we focused on six prespecified ceramides, namely, cer16, cer18, cer20, cer22, cer24:0, and cer24:1, that were selected based on their consistent association to CV risk as found in the literature. To measure these ceramides, we used their respective water loss adduct [M-H2O+H]+ as the quantifier ion and the protonated adduct [M+H]+ as a qualifier ion with matching retention time (32). Final preprocessing was performed in R v.4.2.0 (33). Semiquantification was achieved by comparing ceramide peak areas to the peak area of an exogenous pure standard cer(d18:1/17:0) spiked in all samples for a final concentration of 2 μg/mL. Outliers were defined as measures more than 3 SD away from the median. R code for preprocessing can be found on GitHub: https://github.com/Asger-W/Profil-Ceramides.
Statistics
All statistics and visualizations were produced in R, and the code for the statistical analysis can be found on GitHub. Clinical characteristics presented as n (%), mean (SD), or median (interquartile range [IQR]) were compared between individuals developing CVE or those who did not, using Welch t test for continuous variables and χ2 test for categorical variables and compiled into a table with the tableone package (34). Survival analysis was performed with the survival and survminer packages. Cox proportional hazards regression analyses were carried out for each ceramide and ratio to cer24:0, using three levels of adjustments: a crude model without adjustments; a model with level 1 adjustment; and adjusted for age, sex, BMI, LDL, triglycerides, systolic blood pressure, glycated hemoglobin A1c (HbA1c), history of CVD, smoking status, and statin use. The model with final adjustments (level 2 adjustments) included eGFR and log-transformed UAER in addition to the confounders in the level 1 adjusted model. The variables used for adjustment were themselves modeled with Cox regression, in one model without adjustment and in one model with the same adjustments as the level 2 adjusted model except for the interrogated variable. Multiple testing correction was carried out for the six ceramides, with Bonferroni correction P value = 0.05/6. Sex was considered as a factor in the statistical analysis of the data, and a separate Cox regression analysis was carried out stratified by sex, for all ceramides, ratios, and outcomes. Correlation matrix of Pearson correlations was produced and drawn as heatmaps with the ggcorrplot package.
Data and Resource Availability
The data set analyzed here is not publicly available, for the privacy of the participants, in compliance with EU and Danish data protection law. The data can be accessed upon request; relevant legal permission from the data protection agency is required. Data access request should be directed to P.R., peter.rossing@regionh.dk. The code used for data analysis is available on gitHub: https://github.com/Asger-W/Profil-Ceramides.
Results
Baseline Characteristics
Here we investigated a prospective cohort of 662 participants with long-standing T1D, baseline lipid measurements, and follow-up data regarding disease and death with median (IQR) follow-up time of 6.3 [5.9–6.7] years. The mean (SD) age was 55 (13) years, the diabetes duration was 33 (16) years, and 45% were female. A total of 94 participants experienced a CVE, 23 progressed to kidney failure, and 58 died; there were 24 people that overlapped between CVE and all-cause mortality, 7 people experienced both a CVE and kidney failure, and 6 people with kidney failure died (Supplementary Fig. 1). Clinical characteristics of the total and subpopulations with and without CVE are summarized in Table 1, and ceramide measures are summarized in Table 2. Ceramide quartiles can be found in Supplementary Table 3. Participants experiencing a CVE had generally higher lipid levels typically associated with CV risk. Total cholesterol, LDL, and VLDL were higher compared with the groups without CVE, but not significantly so, while triglycerides and cer16 and cer18 levels were significantly higher at baseline in the CVE group compared with the non-CVE group (mean [SD] = 2.32 [0.41] ng/mL vs. 2.19 [0.45] ng/mL and 1.36 [0.41] ng/mol vs. 1.24 [0.35] ng/mL, respectively; P = 0.007 and 0.008, respectively).
Table 1.
Clinical characteristics
| Overall | No CVE | CVE | Groupwise comparison | |
|---|---|---|---|---|
| N | 662 | 568 | 94 | |
| Age (years) | 54.6 (12.7) | 53.6 (12.9) | 60.7 (9.5) | <0.001 |
| Diabetes duration (years) | 32.7 (15.9) | 31.3 (15.8) | 41.3 (13.1) | <0.001 |
| Female | 296 (45%) | 264 (47%) | 32 (34%) | 0.038 |
| Smoking | 137 (21%) | 116 (20%) | 21 (22%) | 0.774 |
| BMI (kg/m2) | 25.4 (5.8) | 25.4 (6.0) | 25.7 (4.2) | 0.519 |
| Previous CVD | 139 (21%) | 85 (15%) | 54 (57%) | <0.001 |
| Retinopathy: no/mild-moderate nonproliferative/proliferative | 140 (21%)/275 (42%)/120 (18%) | 133 (23%)/246 (43%)/98 (17%) | 7 (7%)/29 (31%)/22 (23%) | <0.001 |
| UAER (mg/24 h) | 17 [8–65] | 14 [8–52] | 48 [20–219] | <0.001 |
| HbA1c (mmol/mol) | 64.3 (12.7) | 63.8 (12.6) | 67.7 (12.5) | 0.006 |
| HbA1c (%) | 8.0 (1.2) | 8.0 (1.2) | 8.3 (1.1) | 0.006 |
| Hemoglobin (mmol/L) | 8.5 (0.9) | 8.5 (0.8) | 8.2 (1.0) | 0.012 |
| Total cholesterol (mmol/L) | 4.7 (0.9) | 4.7 (0.8) | 4.8 (1.0) | 0.193 |
| HDL (mmol/L) | 1.7 (0.5) | 1.7 (0.5) | 1.6 (0.6) | 0.089 |
| LDL (mmol/L) | 2.5 (0.8) | 2.4 (0.7) | 2.6 (0.9) | 0.138 |
| VLDL (mmol/L) | 0.5 (0.3) | 0.5 (0.3) | 0.6 (0.3) | 0.03 |
| Triglyceride (mmol/L) | 1.13 (0.66) | 1.09 (0.58) | 1.35 (1.01) | 0.015 |
| eGFR (mL/min/1.73m2) | 81.53 (25.51) | 83.95 (24.69) | 67.00 (25.68) | <0.001 |
| hsCRP (mg/L) | 3.41 (7.02) | 3.36 (7.20) | 3.70 (5.83) | 0.616 |
| Systolic blood pressure (mmHg) | 132 (17) | 131 (17) | 137 (19) | 0.004 |
| Diastolic blood pressure (mmHg) | 74 (9) | 75 (9) | 73 (10) | 0.089 |
| Below albuminuria/moderately increased/severely increased (<30/30–299/≥300 mg/g) | 308 (47%)/165 (25%)/189 (29%) | 290 (51%)/133 (23%)/145 (26%) | 18 (19%)/32 (34%)/44 (47%) | <0.001 |
| RAAS treatment* | 445 (67%) | 358 (63%) | 87 (93%) | <0.001 |
| Antihypertensive treatment | 475 (72%) | 382 (67%) | 93 (99%) | <0.001 |
| Beta blocker treatment | 85 (13%) | 58 (10%) | 27 (29%) | <0.001 |
| Calcium channel blocker treatment | 202 (31%) | 155 (27%) | 47 (50%) | <0.001 |
| Insulin pump user | 57 (9%) | 52 (9%) | 5 (5%) | 0.303 |
| Daily insulin dose (IU) | 48.7 (35.0) | 48.8 (36.2) | 47.9 (26.3) | 0.781 |
| Statin treatment | 397 (60%) | 321 (57%) | 76 (81%) | <0.001 |
| Aspirin or clopidogrel treatment | 349 (53%) | 273 (48%) | 76 (81%) | <0.001 |
| Diuretic treatment | 334 (51%) | 260 (46%) | 74 (79%) | <0.001 |
| Thiazide treatment | 186 (28%) | 159 (28%) | 27 (29%) | 0.982 |
| Loop diuretic treatment | 149 (23%) | 105 (19%) | 44 (47%) | <0.001 |
| MRA treatment | 28 (4%) | 17 (3%) | 11 (12%) | <0.001 |
Data are presented as n (%), mean (SD), or median [IQR]. Groupwise comparisons between the two treatments were tested using a Welch two-sample t test for continuous variables and χ2 test for categorical variables. MRA, mineralocorticoid receptor antagonist; RAAS, Renin angiotensin aldosterone system.
Spironolactone that blocks aldosterone is given separately.
Table 2.
Ceramide measures
| Overall | Individuals without CVE | Individuals who developed CVE | Groupwise comparison | |
|---|---|---|---|---|
| n | 662 | 568 | 94 | |
| Cer16 ng/mL | 2.21 (0.44) | 2.19 (0.45) | 2.32 (0.41) | 0.007* |
| Cer18 ng/mL | 1.26 (0.36) | 1.24 (0.35) | 1.36 (0.41) | 0.008* |
| Cer20 ng/mL | 1.78 (0.66) | 1.78 (0.66) | 1.83 (0.71) | 0.523 |
| Cer22 ng/mL | 10.26 (4.28) | 10.24 (4.22) | 10.43 (4.65) | 0.707 |
| Cer24:0 ng/mL | 39.99 (16.29) | 39.94 (16.00) | 40.30 (18.06) | 0.855 |
| Cer24:1 ng/mL | 19.55 (7.44) | 19.32 (7.20) | 20.93 (8.66) | 0.09 |
| Ratio cer16/cer24:0 | 0.06 (0.02) | 0.06 (0.02) | 0.07 (0.03) | 0.137 |
| Ratio cer18/cer24:0 | 0.03 (0.01) | 0.03 (0.01) | 0.04 (0.01) | 0.019 |
| Ratio cer20/cer24:0 | 0.05 (0.01) | 0.05 (0.01) | 0.05 (0.01) | 0.261 |
| Ratio cer22/cer24:0 | 0.26 (0.04) | 0.26 (0.04) | 0.26 (0.04) | 0.461 |
| Ratio cer24:1/cer24:0 | 0.51 (0.11) | 0.50 (0.11) | 0.54 (0.12) | 0.005* |
Data are presented as mean (SD). Groupwise comparisons between the two groups were tested using a Welch two-sample t test.
Indicate passing Bonferroni correction.
Ceramide Levels and Longitudinal End Points
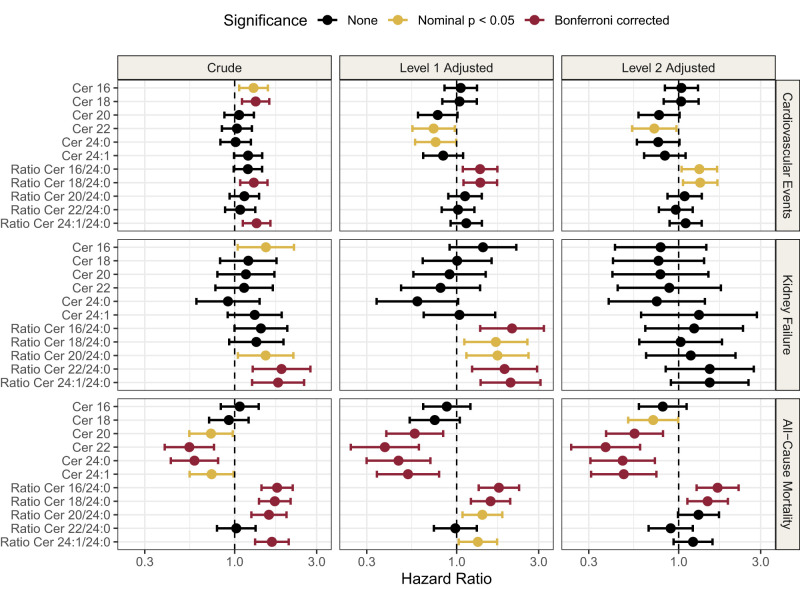
Cox regression analysis was carried out on the six investigated ceramides to test their association with CVE, kidney failure, and all-cause mortality (Fig. 1).
Figure 1.
Forest plot of HRs for ceramide and ratios for outcomes of CVE, kidney failure, and mortality. The crude models are unadjusted; level 1 adjusted for age, sex, BMI, LDL, triglycerides, systolic blood pressure, HbA1c, history of CVD, smoking status and statin use; and level 2 adjusted for all the same variables as level 1, but also including eGFR and UAER. HRs are reported per doubling of the log10 ceramide.
For CV outcomes, the ratio of cer18/cer24:0 was the only measure that was significantly associated at all levels of adjustments (level 2 adjusted model: hazard ratio [HR] = 1.33, 95% CI = 1.06–1.68, P = 0.01). Cer16, cer18, and the ratio cer24:1/cer24:0 were significantly associated with CVE in crude models, but not in adjusted models. Cer22 (HR = 0.72, 95% CI = 0.54–0.97, P = 0.03) and the ratio cer16/cer24:0 (HR = 1.32, 95% CI = 1.04–1.67, P = 0.02) were statistically significant in the fully adjusted models, but not in the crude model.
In the model with kidney failure as outcome, cer16 and ratios cer20/cer24:0, cer22/cer24:0, and cer24:1/cer24:0 were significantly associated in crude models. In the level 1 adjusted model, all the ratios to cer24:0 were significantly associated with kidney failure (HR = 2.1, 95% CI = 1.37–3.22, P = 6.56*10−4), (HR = 1.69, 95% CI = 1.11–2.58, P = 0.01), (HR = 1.73, 95% CI = 1.14–2.62, P = 0.01), (HR = 1.9, 95% CI = 1.23–2.94, P = 3.83*10−3), and (HR = 2.06, 95% CI = 1.38–3.07, P = 4.14*10−4) for cer16/cer24:0, cer18/cer24:0, cer20/cer24:0, cer22/cer24:0, and cer24:1/cer24:0, respectively; however, none of these results persisted after inclusion of eGFR and UAER in the model. A full list of model estimates for all the models and adjustment levels can be found in Supplementary Table 4, with a visual summary in Fig. 1.
Cer20, cer22, cer24:0, and cer24:1 were all significantly inversely associated with all-cause mortality, and this finding persisted through all three levels of adjustments. The strongest metabolite associations to all-cause mortality in the adjusted models were cer22 (HR = 0.38, 95% CI = 0.24–0.60, P = 3.3*10−5) and cer24:0 (HR = 0.47, 95% CI = 0.31–0.73, P = 6.6*10−4). The ratios cer16/cer24:0 and cer18/cer24:0 were also associated with all-cause mortality throughout all levels of adjustments(HR = 1.69, 95% CI = 1.27–2.23, P = 2.8*10−4 and HR = 1.48, 95% CI = 1.12–1.94, P = 5*10−3, respectively, in the fully adjusted model).
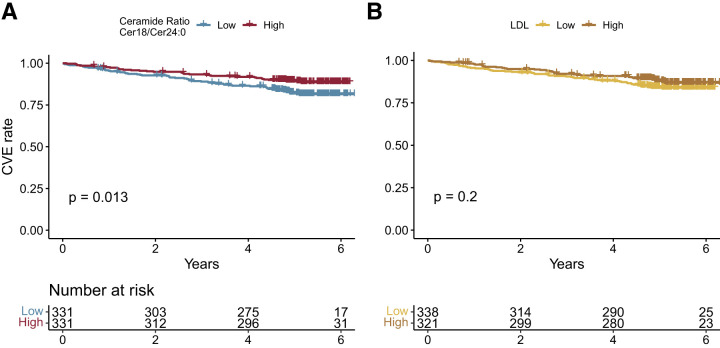
The ratio of cer18/cer24:0 was the only measure that was significantly associated with CVE and all-cause mortality at all levels of adjustments. Kaplan-Meier curves (Fig. 2) show that participants above the median of ratio of cer18/cer24:0 were at higher risk (81% survival rate, 95% CI = 77–85, 6 years after measuring) than participants below the median (89% survival rate, 95% CI = 85–92, 6 years after measuring, P = 0.013) for CVE. The ceramide ratio cer18/cer24:0 was better at differentiating CVE from non-CVE than LDL, based on which participants above the median of LDL were at a similar risk (84% survival rate, 95% CI = 0.80–0.88, 6 years after measuring) to the participants with LDL lower than the median (87% survival rate, 95% CI = 0.83–0.91, 6 years after measuring, P = 0.2).
Figure 2.
Kaplan-Meier plot of (A) cer18/cer24:0 ratio and (B) LDL against CVE. High is metabolite level greater than or equal to the median; low is individuals with a metabolite level below the median.
Examining the variables used for adjustment, we found that CVD history was the variable with the strongest association with CVE (HR = 3.72, 95% CI = 2.34–5.92, P = 2.84*10−8), Supplementary Fig. 2 and Supplementary Table 5. The ceramides showed strongest correlation with each other and with the other lipid measures like LDL and triglycerides correlation coefficients between 0.3 and 45 and between 0.27 and 0.5, respectively, Fig. 3, with extended heatmap in Supplementary Fig. 3. In addition, cer24:0 had a small positive correlation with eGFR (0.08), compared with the other ceramides that had negative or no correlation with eGFR.
Figure 3.
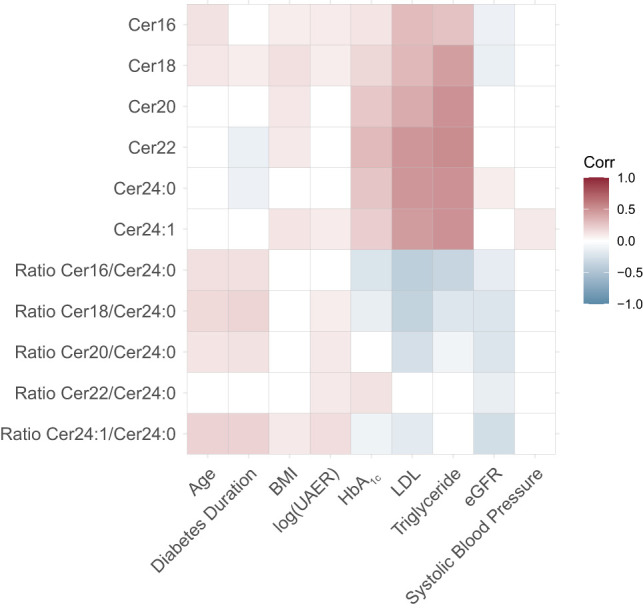
Heatmap of ceramides correlation to possible CVE confounders, presented as correlations coefficients from Pearson correlation.
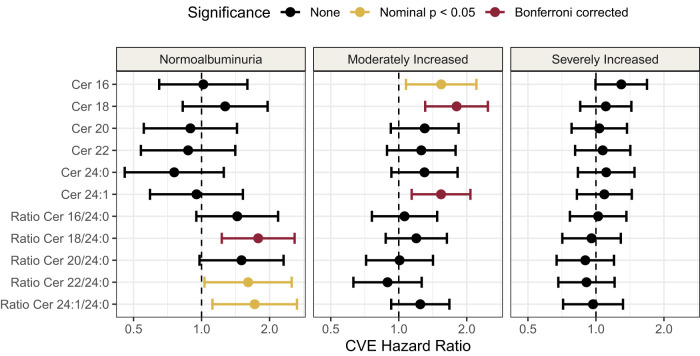
We investigated how the HRs for CVE were affected by albuminuria and found that, from normoalbuminuria to moderate increase in albuminuria, cer16 and cer18 were significantly associated with CVE, but, in the subpopulation with severely increased albuminuria, all significant associations were lost (Fig. 4). The levels of cer18 and cer24:0 and the ratio of cer18/cer24:0 were plotted for each albuminuria group and for CVE (Supplementary Fig. 4 and Supplementary Table 6), which shows that the difference in ceramide level between individuals with and without a CVE is gradually smaller with worsening albuminuria.
Figure 4.
Forest plot of HRs for ceramide and ratios for CVE separated by albuminuria status. Normoalbuminuria is defined as <30 mg/g, moderately increased is between 30 and 299 mg/g, and severely increased is ≥300 mg/g. These models are unadjusted crude models. HRs are reported per doubling of the log10 ceramide.
Finally, sensitivity analysis was carried out, investigating the effect of antihypertensive treatment, antiplatelet drugs, Renin-angiotensin-aldosterone system (RAAS) treatment, and HDL; adjusting for these variables had little or no influence on the models and is therefore not included. A Cox regression analysis carried out in females and males separately (Supplementary Fig. 5) showed that the ceramides and the ceramide ratios associated to CVE in males but not in females. All the ceramide ratios associated to kidney failure in males, while, in females, only cer22, cer24:1, and ratio cer22/24:0 associated to kidney failure. The same ceramide and ceramide ratios associate to all-cause mortality in both sexes, but the associations were a bit stronger in males. We also found that the association between ceramides and kidney failure was stronger in people who had not previously had a CVD; similarly, the association between ceramides and all-cause mortality was strongest in people without previous CVD (Supplementary Fig. 6). A t test of ceramide amount, comparing people with and without previous CVD, showed that ratios cer18/cer24:0 and cer18/cer24:0 was significantly higher in people with previous CVD than in those without (Supplementary Table 7).
Discussion
We investigated the potential of a small set of specific circulating ceramides as biomarkers for CV risk, kidney failure, and all-cause mortality in individuals with T1D. In brief, the key findings are as follows. 1) Several of the investigated ceramides associated with CVE risk and all-cause mortality in individuals with T1D. 2) The cer18/cer24:0 ratio was the most consistent measure and showed constant, significant association with CVE and all-cause mortality across all confounder adjustment levels. The cer18/cer24:0 ratio furthermore outperformed LDL cholesterol in separating individuals who progressed to a CVE from the group who did not. 3) Ratios of the ceramide cer24:0 associated, albeit more weakly, to kidney failure, until the adjustment for UAER and eGFR. The loss of association after adjustment to UAER and eGFR may be explained by collinearity between ceramide ratios and eGFR (Fig. 3). 4) We observed that the ceramides’ ability to differentiate between individuals with and without future CVE depended on albuminuria status; whether this is a direct effect or an indicator of compounding illness is not clear, though it should be noted that these ceramides have previously been associated with albuminuria and eGFR (35–37). Interestingly, the ceramides are not correlated with traditional risk factors such as BMI or blood pressure (Supplementary Fig. 3). Previous studies found that including ceramides measures improved upon the ability of traditional lipid measurements like LDL and TG to predict major adverse cardiac events and suggested ceramides associate to CVD independently of established clinical risk factors (12,38).
There is compelling evidence that ceramides can predict CVD risk. Tarasov et al. (10) were among the first to report increased levels of cer16, cer18, cer20, and cer24:1 as risk markers of coronary artery disease, together with a reduced risk associated with cer24:0. Several subsequent studies found associations for ceramide levels and CV risk (39–41) and severity (42); however, few have studied these biomarkers in relation to diabetes. Alshehry et al. (43) investigated lipids associated with CVE and CV death in a T2D cohort and found that, among other lipids, cer24:1 was positively associated with CVE and CV death. We did not observe an association between cer24:1 and CVE; however, we did observe a protective association to all-cause mortality. We have not been able to find any studies investigating the CV risk of these ceramides in people with T1D. Lipid measures are well-established measures of CVD risk (44,45). We found that the ratio of cer18/cer24:0 could be a comparable measure to LDL. Havulinna et al. (12) showed that adding cer18 measures to LDL levels improved their ability to predict incident rate of CVE, while adding LDL levels to cer18 level did not improve prediction. Several mechanisms have been suggested for the observed link between ceramides and CVD. One plausible explanation is that ceramides are recruited during inflammation to induce apoptosis in dysregulated cells such as foam cells (46). It has been suggested that ceramides have a causative role in promoting CVD (47), for instance, through mitochondria disruption, promoting reactive oxygen species and cell death (48–50).
The association between ceramides and kidney disease in the literature is less clear. One study in T1D found that individuals with lower ceramide levels of cer20 and cer24:1 at baseline were more likely to progress to macroalbuminuria, with an average 6.5-years follow-up (35). The people included in that study were both younger, with a mean age of 27 years, and had a shorter diabetes duration, 6 years on average, compared with our study, where the participants were middle aged, a mean age of 55 years, and had long-standing T1D, 33 years on average, which could explain why we do not find the same associations for cer20 and cer24:1. On the other hand, other studies have found an increase in ceramide levels in people with chronic kidney disease (37) and diabetic kidney disease (36) compared with controls without kidney disease. The increase of cer16 found in these two studies is consistent with the association between cer16 and risk of kidney failure found in this study (Fig. 1). The relationship between ceramides and kidney disease is complex and likely bidirectional. Elevated ceramide levels could contribute to the development and progression of kidney disease, while kidney dysfunction may also contribute to changes in ceramide metabolism. Considering these reports and our study, it is possible that ceramide levels increase with kidney/endothelial damage up until a tipping point, where the levels then drop; a similar metabolic tipping point has been suggested in individuals with severe liver fibrosis (51). The influence of albuminuria status on ceramides’ ability to associate with CVE should be investigated further and be considered when using these ceramides as risk predictors.
This study has some limitations. We were not able to control for diet, exercise, and change in medication in this post hoc analysis, which could have influenced ceramide levels (15,19). It should be noted that the participants had long-standing T1D and therefore were likely to adhere to a low-carb diet without major changes. Information on race was not available but was expected to represent the Danish population. Another limitation was that we did not have information on the use of medication targeting triglycerides, which potentially could affect ceramide levels. Despite the possible variability introduced by uncontrolled factors, we found robust associations between ceramides, CVE, and all-cause mortality. Validating our finding in an independent replication cohort would help cement our results further; however, we were not able to identify a similar cohort. For the strengths, the levels of the ceramides were measured in a semiquantitative manner allowing for comparison with other future studies. Additionally, investigating few specific molecular targets measured in concentrations reduces the risk of incidental discoveries. It was also a strength that this analysis was carried out in a large, well-characterized prospective cohort.
In this study, we investigated a small set of specific ceramides in relation to CVE, kidney failure, and all-cause mortality. We found that these ceramides have potential as susceptibility biomarkers of CVD and all-cause mortality in individuals with long-standing T1D. The ratio cer18/cer24:0 appears to be the strongest independent risk marker for CVD and all-cause mortality, and future work should focus on validating it in clinical practice.
Article Information
Funding. Funding was provided by Steno Diabetes Center Copenhagen, Gentoſte, Denmark. We acknowledge support from Novo Nordisk Foundation grant NNF14OC0013659 “PROTON Personalizing treatment of diabetic nephropathy.”
Duality of Interest. P.R. has received honoraria for consultancy to Steno Diabetes Center Copenhagen from Astellas, Astra Zeneca, Boehringer Ingelheim, Bayer, Merck, Gilead, Novo Nordisk, and Sanofi Aventis. N.T. and S.A.W. are full-time employees of Novo Nordisk A/S. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.W. performed data analysis and drafted the manuscript. A.W., V.R.C., T.S., S.T., N.T., S.A.W., T.V., H.V., P.R., and C.L.-Q. contributed to the conceptualization and interpretation of this study. S.T. and P.R. conducted the original study. V.R.C., T.S., N.T., and S.A.W. carried out the follow-up study and provided material and clinical data for this study. All authors approved the final version of the manuscript. A.W. and C.L.-Q. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. An early non-peer-reviewed version of this article was submitted to the MedRxiv preprint server (https://www.medrxiv.org/content/10.1101/2022.12.09.22283278v1) on 13 December 2022. Parts of this work were presented at the 58th Annual Meeting of the European Association for the Study of Diabetes (EASD), Stockholm, Sweden, 19–23 September 2022.
Footnotes
Clinical trial reg. no. NCT01171248, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.23712915.
References
- 1. Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107842. [DOI] [PubMed] [Google Scholar]
- 2. Jørgensen ME, Almdal TP, Carstensen B. Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia 2013;56:2401–2404 [DOI] [PubMed] [Google Scholar]
- 3. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bjerg L, Hulman A, Carstensen B, Charles M, Witte DR, Jørgensen ME. Effect of duration and burden of microvascular complications on mortality rate in type 1 diabetes: an observational clinical cohort study. Diabetologia 2019;62:633–643 [DOI] [PubMed] [Google Scholar]
- 5. de Boer IH, Gao X, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clin J Am Soc Nephrol 2016;11:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lees JS, Welsh CE, Celis-Morales CA, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med 2019;25:1753–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dennis JM. Precision medicine in type 2 diabetes: using individualized prediction models to optimize selection of treatment. Diabetes 2020;69:2075–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaurasia B, Summers SA. Ceramides in metabolism: key lipotoxic players. Annu Rev Physiol 2021;83:303–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarasov K, Ekroos K, Suoniemi M, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 2014;99:E45–E52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Havulinna AS, Sysi-Aho M, Hilvo M, et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016;36:2424–2430 [DOI] [PubMed] [Google Scholar]
- 13. Peterson LR, Xanthakis V, Duncan MS, et al. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc 2018;7:e007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemaitre RN, Jensen PN, Hoofnagle A, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk: the Cardiovascular Health Study. Circ Heart Fail 2019;12:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang DD, Toledo E, Hruby A, et al. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevención con Dieta Mediterránea). Circulation 2017;135:2028–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mantovani A, Bonapace S, Lunardi G, et al. Association between plasma ceramides and inducible myocardial ischemia in patients with established or suspected coronary artery disease undergoing myocardial perfusion scintigraphy. Metabolism 2018;85:305–312 [DOI] [PubMed] [Google Scholar]
- 17. Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009;58:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang H, Kasumov T, Gatmaitan P, et al. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity (Silver Spring) 2011;19:2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergman BC, Brozinick JT, Strauss A, et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 2015;309:E398–E408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lemaitre RN, Yu C, Hoofnagle A, et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes 2018;67:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hilvo M, Salonurmi T, Havulinna AS, et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia 2018;61:1424–1434 [DOI] [PubMed] [Google Scholar]
- 22. Chew WS, Torta F, Ji S, et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight 2019;5:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fretts AM, Jensen PN, Hoofnagle A, et al. Plasma ceramide species are associated with diabetes risk in participants of the strong heart study. J Nutr 2020;150:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theilade S, Lajer M, Hansen TW, et al. 24-hour central aortic systolic pressure and 24-hour central pulse pressure are related to diabetic complications in type 1 diabetes - a cross-sectional study. Cardiovasc Diabetol 2013;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tofte N, Suvitaival T, Ahonen L, et al. Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci Rep 2019;9:16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(Suppl.):30–33 [DOI] [PubMed] [Google Scholar]
- 27. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39(Suppl.):26–29 [DOI] [PubMed] [Google Scholar]
- 28. Ferreira-Divino LF, Suvitaival T, Rotbain Curovic V, et al. Circulating metabolites and molecular lipid species are associated with future cardiovascular morbidity and mortality in type 1 diabetes. Cardiovasc Diabetol 2022;21:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curovic VR, Suvitaival T, Mattila I, et al. Circulating metabolites and lipids are associated to diabetic retinopathy in individuals with type 1 diabetes. Diabetes 2020;69:2217–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 31. Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 2010;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim M, Nevado-Holgado A, Whiley L, et al. Association between plasma ceramides and phosphatidylcholines and hippocampal brain volume in late onset Alzheimer’s disease. J Alzheimers Dis 2017;60:809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. R Core Team . R: A Language and Environment for Statistical Computing, 2018. R Foundation for Statistical Computing. Accessed 15 February 2022. Available from https://www.r-project.org/
- 34. Yoshida K, Bartel A, Chipman JJ, et al. R Package ‘tableone’: create “Table 1” to describe baseline characteristics with or without propensity score weights. 2022; Accessed 15 February 2022. Available from https://cran.rproject.org/web/packages/tableone/index.html
- 35. Klein RL, Hammad SM, Baker NL, et al.; DCCT/EDIC Research Group . Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metabolism 2014;63:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu JJ, Ghosh S, Kovalik JP, et al. Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney Int Rep 2016;2:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mantovani A, Lunardi G, Bonapace S, et al. Association between increased plasma ceramides and chronic kidney disease in patients with and without ischemic heart disease. Diabetes Metab 2021;47:101152. [DOI] [PubMed] [Google Scholar]
- 38. Gencer B, Morrow DA, Braunwald E, et al. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. Eur J Prev Cardiol 2022;29:895–902 [DOI] [PubMed] [Google Scholar]
- 39. Cheng JM, Suoniemi M, Kardys I, et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 2015;243:560–566 [DOI] [PubMed] [Google Scholar]
- 40. Anroedh S, Hilvo M, Akkerhuis KM, et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J Lipid Res 2018;59:1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides a novel predictor of major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol 2018;38:1933–1939 [DOI] [PubMed] [Google Scholar]
- 42. Mantovani A, Bonapace S, Lunardi G, et al. Associations between specific plasma ceramides and severity of coronary-artery stenosis assessed by coronary angiography. Diabetes Metab 2020;46:150–157 [DOI] [PubMed] [Google Scholar]
- 43. Alshehry ZH, Mundra PA, Barlow CK, et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation 2016;134:1637–1650 [DOI] [PubMed] [Google Scholar]
- 44. Iso H, Jacobs DR Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 1989;320:904–910 [DOI] [PubMed] [Google Scholar]
- 45. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dolfi B, Gallerand A, Haschemi A, Guinamard RR, Ivanov S. Macrophage metabolic regulation in atherosclerotic plaque. Atherosclerosis 2021;334:1–8 [DOI] [PubMed] [Google Scholar]
- 47. Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 2021;18:701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Law BA, Liao X, Moore KS, et al. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J 2018;32:1403–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dadsena S, Bockelmann S, Mina JGM, et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun 2019;10:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akawi N, Checa A, Antonopoulos AS, et al. Fat-secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. J Am Coll Cardiol 2021;77:2494–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McGlinchey AJ, Govaere O, Geng D, et al. Metabolic signatures across the full spectrum of non-alcoholic fatty liver disease. JHEP Rep 2022;4:100477. [DOI] [PMC free article] [PubMed] [Google Scholar]