Abstract
Purpose
Although Klebsiella aerogenes (formerly Enterobacter aerogenes) and Enterobacter cloacae share many phenotypic characteristics, controversy exists as to whether they cause clinically distinguishable infections. The objective of this study was to determine the comparative incidence, determinants, and outcomes of K. aerogenes and E. cloacae bloodstream infections (BSI).
Methods
Population-based surveillance was conducted among residents aged ≥ 15 years of Queensland, Australia during 2000–2019.
Results
Overall 695 and 2879 incident K. aerogenes and E. cloacae BSIs were identified for incidence rates of 1.1 and 4.4 per 100,000 population, respectively. There was a marked increase in incidence associated with older age and with males with both species. Patients with K. aerogenes BSIs were older, were more likely male, to have community-associated disease, and to have a genitourinary source of infection. In contrast, E. cloacae were more likely to have co-morbid diagnoses of liver disease and malignancy and be associated with antimicrobial resistance. Enterobacter cloacae were significantly more likely to have repeat episodes of BSI as compared to K. aerogenes. However, no differences in length of stay or all cause 30-day case-fatality were observed.
Conclusion
Although significant demographic and clinical differences exist between K. aerogenes and E. cloacae BSI, they share similar outcomes.
Keywords: Klebsiella, Enterobacter, Incidence, Epidemiology
Introduction
Enterobacter species are important causes of infections both in community and institutional settings [1, 2]. Enterobacter cloacae is the most common species causing human disease among the more than twenty species belonging to the genus [1]. As a result of chromosomally encoded AmpC b-lactamases and a propensity to acquire other genes, multi-drug resistant Enterobacter species are of significant clinical importance due to their risk for treatment failure and relapse [3–6]. Traditionally, the second most common species within the genus causing human disease was Enterobacter aerogenes [1]. However, based on genetic relatedness studies, this organism has been reclassified and renamed as Klebsiella aerogenes.
Despite the phenotypic similarities between Enterobacter cloacae and Klebsiella aerogenes, controversy exists as to whether they cause clinically distinguishable infections and/or result in different outcomes. A number of investigations have been undertaken to examine this question in North America, Europe, and Asia [7–10]. However, these studies were underpowered to detect significant differences due to small sample sizes. In addition, prior investigations were limited by conduct at selected hospital(s) such that they were at risk for several important biases [11]. The objective of this study was therefore to determine the comparative incidence, clinical determinants, and outcomes of Klebsiella aerogenes and Enterobacter cloacae bloodstream infections (BSI) in a large Australian population.
Patients and methods
A retrospective population-based laboratory surveillance cohort design was utilized. All residents aged 15 years and older who had BSI due to K. aerogenes or E. cloacae identified within the publicly funded healthcare system in Queensland, Australia during January 1, 2000, and December 31, 2019, were included. The human research ethics committee at Royal Brisbane and Women’s Hospital approved this study and granted a waiver of individual consent (LNR/2020/QRBW/62494).
Pathology Queensland first identified all blood cultures for K. aerogenes (recorded as E. aerogenes) or E. cloacae during the surveillance period. These included all cultures submitted from community and institutional collection sites state wide within the publicly funded system. Pathology Queensland used the BACT/ALERT® 3D system (bioMérieux, Durham, NC) for blood culture testing throughout the study period except for use of the BACT/ALERT® VIRTUO® system (bioMérieux, Durham, NC) from 2018 and thereafter at the main central laboratory that services Greater Brisbane area and some rural Queensland sites. At Pathology Queensland blood cultures are routinely incubated for a minimum of 5 days. BacT/ALERT FA plus (aerobic) and FN plus (anaerobic) media bottles were used as standard. Methods for species identification through the study period included VITEK®2 GN ID (bioMérieux), API 20E (bioMérieux) and MALDI-TOF MS (VITEK MS; bioMérieux). Antibiotic susceptibility testing was performed using both an automated method (i.e., VITEK® AST card) and disc diffusion according to recognized standards (CLSI or EUCAST) at the time of testing.
Admission, clinical, and outcome information was obtained through linkages to state-wide hospital admissions and death registries. Previously validated definitions were applied to classify episodes of BSI [12, 13]. Incident episodes were defined by the first isolation of K. aerogenes or E. cloacae per patient per 30 days and isolation of these organisms with at least one other species within 48 h defined a polymicrobial infection. Admissions to any private or public institutions within the state were identified and discharge diagnostic codes (ICD-10AM) were obtained. Contiguous admissions (i.e., transfer between institutions) were deemed to represent a single admission episode. Deaths occurring in any location within the state on or before December 31, 2020, were identified using the Registry of General Deaths. Hospital-onset, healthcare-associated, and community-associated BSI were classified as per the method of Friedman et al.[14], and comorbidities were defined as per Charlson et al.[15, 16]. A clinical focus was assigned based on review of diagnosis-related group and primary diagnosis hospital discharge codes.
Analysis was performed using Stata 17 (StataCorp, College Station, USA). Incident BSI episodes were age- and sex-standardized (to 2019 Queensland population) using 5-year strata and reported as rates per 100,000 residents [17]. Incidence rates were compared using incidence rate ratios (IRR) with exact 95% confidence intervals (CI). Skewed continuous variables were described using medians with interquartile ranges (IQR) and groups were compared using the Mann–Whitney–Wilcoxon test. Categorical data were compared using Fisher’s exact test. A multivariable logistic regression model was developed to examine factors associated with all cause 30-days case fatality. Age, sex, onset classification, antimicrobial resistance, Charlson Comorbidity Index, polymicrobial infection, and focus of infection were included in the initial model. Stepwise backward variable elimination was performed to develop the most parsimonious model. Calibration and discrimination were assessed using the Hosmer–Lemeshow test and the area under the receiver operator characteristic curve, respectively. P values < 0.05 were deemed to represent statistical significance.
Results
During the two decades of surveillance, 695 and 2879 incident K. aerogenes and E. cloacae BSIs were identified for annual sex- and age-standardized incidence rates of 1.1 and 4.4 per 100,000 population, respectively. No significant differences were observed between K. aerogenes and E. cloacae in the distribution of incident cases by month or year of study or by region within the state.
Demographic determinants
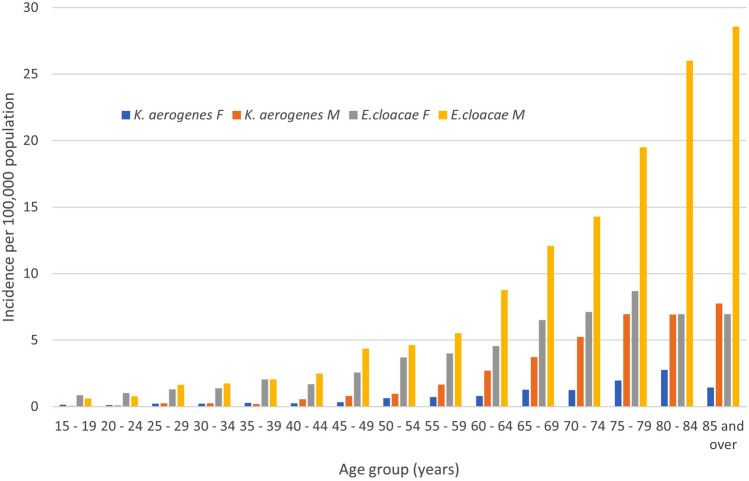
The median age (IQR) of K. aerogenes cases was 67.1 (56.6–76.7) years and this was significantly older than that of patients with E. cloacae BSI (median 63.8, IQR 49.5–75.0; p < 0.001). A higher proportion of K. aerogenes BSI cases were male (493/695; 70.9%) as compared to those with E. cloacae (1805/2879; 62.7%; p < 0.001). The age and sex distribution of BSI episodes showed a marked increase in incidence associated with older age with both species as shown in Fig. 1. Overall males were at increased risk for BSI both with K. aerogenes (IRR 2.49; 95% CI 2.11–2.95; p < 0.001) and E. cloacae (IRR 1.71; 95% CI 1.59–1.85; p < 0.001). However, this significant sex-related excess risk was observed only in those aged 40 years and older for both K. aerogenes (IRR 2.84; 95% CI 2.38–3.40; p < 0.001) and E. cloacae (IRR 1.91; 95% CI 1.76–2.08; p < 0.001). Furthermore, while the excess risk in males was relatively constant with increasing age among those with K. aerogenes BSI, the excess risk continued to increase in magnitude through to the oldest group of those with E. cloacae BSI (Fig. 1).
Fig. 1.
Age- and sex-specific incidence rates of Klebsiella aerogenes and Enterobacter cloacae bloodstream infections (F = female; M = male)
Clinical determinants and microbiology
There were several clinical determinants that differed among the two species, and these are displayed in Table 1. While the proportion of community-onset cases was not different between species, exposure to healthcare was significantly (p < 0.001) more common among E. cloacae cases with 2311 (80.3%) being either hospital onset or healthcare associated as compared to 505 (72.6%) for K. aerogenes. The presence of co-morbid diseases was similar with the exception that both liver disease and malignancy was higher among E. cloacae as compared to K. aerogenes BSI (Table 1). The distribution of focus of infection was different between the species and this was attributable to a higher proportion of non-focal (p = 0.004) and a lower proportion of pelvic/genitourinary foci (p < 0.001) observed among E. cloacae as compared to K. aerogenes BSI. Resistance to antimicrobials was significantly higher among E. cloacae isolates (Table 1).
Table 1.
Clinical factors and microbiology associated with Klebsiella aerogenes and Enterobacter cloacae bloodstream infection
| Factor |
Klebsiella aerogenes N = 695 |
Enterobacter cloacae N = 2879 |
p value |
|---|---|---|---|
| Onset classification | < 0.001 | ||
| Hospital | 308 (44.3%) | 1346 (46.8%) | |
| Healthcare associated | 197 (28.3%) | 965 (33.5%) | |
| Community associated | 190 (27.3%) | 568 (19.7%) | |
| Median Charlson (IQR) score | 2 (1–5) | 2 (1–5) | 0.079 |
| Charlson variables | |||
| Myocardial infarction | 79 (11.4%) | 277 (9.6%) | 0.2 |
| Congestive heart failure | 119 (17.1%) | 491 (17.1%) | 1.0 |
| Peripheral vascular disease | 57 (8.2%) | 249 (8.7%) | 0.8 |
| Cerebrovascular disease | 48 (6.9%) | 219 (7.6%) | 0.6 |
| Dementia | 30 (4.3%) | 97 (3.3%) | 0.3 |
| Chronic pulmonary | 87 (12.5%) | 316 (11.0%) | 0.2 |
| Rheumatic | 7 (1.0%) | 45 (1.6%) | 0.4 |
| Peptic ulcer disease | 21 (3.0%) | 101 (3.5%) | 0.6 |
| Liver disease | 59 (8.5%) | 354 (12.3%) | 0.004 |
| Diabetes mellitus | 205 (29.5%) | 765 (26.6%) | 0.1 |
| Plegia | 29 (4.2%) | 164 (5.7%) | 0.1 |
| Renal disease | 150 (21.6%) | 675 (23.5%) | 0.3 |
| Malignancy | 193 (27.7%) | 983 (34.1%) | 0.001 |
| HIV | 1 (0.1%) | 9 (0.3%) | 0.7 |
| Focus of infection | < 0.001 | ||
| No focus | 393 (56.6%) | 1800 (62.5%) | |
| Soft tissue | 18 (2.6%) | 126 (4.4%) | |
| Bone and joint | 5 (0.7%) | 59 (2.1%) | |
| Upper respiratory | 1 (0.1%) | 7 (0.2%) | |
| Lower respiratory | 26 (3.7%) | 99 (3.4%) | |
| Endovascular | 6 (0.8%) | 42 (1.5%) | |
| Central nervous | 3 (0.4%) | 10 (0.4%) | |
| Intrabdominal | 130 (18.7%) | 470 (16.3%) | |
| Genitourinary | 113 (16.3%) | 266 (9.2%) | |
| Polymicrobial etiology | 154 (22.2%) | 712 (24.7%) | 0.2 |
| Antimicrobial resistance | |||
| Ciprofloxacin | 12/680 (1.8%) | 107/2759 (3.9%) | 0.005 |
| Co-trimoxazole | 8/681 (1.1%) | 492/2790 (17.6%) | < 0.001 |
| Gentamicin | 2/695 (0.3%) | 228/2879 (8.1%) | < 0.001 |
| Tobramycin | 2/679 (0.3%) | 202/2781 (7.3%) | < 0.001 |
| Ceftriaxone | 140/601 (23.3%) | 605/2487 (24.3%) | 0.6 |
| Meropenem | 1/638 (0.1%) | 23/2555 (0.9%) | 0.07 |
| Piperacillin-tazobactam | 396/533 (25.3%) | 446/2193 (20.3%) | < 0.001 |
| Cefepime | 4/544 (0.7%) | 166/2246 (7.4%) | < 0.001 |
Hospital course and outcome
The overall lengths of stay among 687 (98.8%) and 2838 (98.6%) patients admitted to hospital with K. aerogenes and E. cloacae BSI were not significantly different (p = 0.07) with medians of 13 (IQR 7–31) and 15 (IQR 8–37) days, respectively.
During the surveillance period, seven (1.0%) patients with K. aerogenes had a second episode of incident BSI and this occurred a median of 376 (IQR 42–434) days after the index case. In contrast, among patients with E. cloacae BSI, 101 (3.5%) had second, 10 (0.4%) had third, and one had fourth incident episodes; as compared to K. aerogenes, E. cloacae were almost four-fold higher risk for one or more recurrences (relative risk 3.59; 95% CI 1.68–7.68; p < 0.001). The median time between episodes was 106 (56–298) days overall, and 106 (58–297) for first repeat episodes which was not significantly (p = 0.5) different for that of K. aerogenes.
A total of 94 and 392 patients died within 30-days of K. aerogenes and E. cloacae BSIs death for all cause case-fatality rates of 13.5% and 13.6% (p = 1.0), respectively. All cause 30-days case-fatality was not significantly different between species when limited to mono-microbial infections (57/541; 10.5% versus 284/2167; 13.1%; p = 0.1) or among first episodes (93/688; 13.5% versus 373/2767; 13.5%; p = 1.0). After adjustment for confounding variables in a logistic regression model (n = 3574, goodness of fit p = 1.0, area under receiver operator characteristic = 0.7392), no difference between K. aerogenes and E. cloacae was observed in risk for death as shown in Table 2.
Table 2.
Logistic regression modeling of factors associated with 30-day all cause case fatality
| Factor | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Enterobacter cloacae (versus Klebsiella aerogenes) | 1.01 | 0.78–1.30 | 0.95 |
| Charlson comorbidity index (per point) | 1.23 | 1.18–1.28 | < 0.001 |
| Onset classification | |||
| Hospital-onset | 1 (ref) | – | |
| Healthcare-associated | 0.76 | 0.61–0.95 | 0.18 |
| Community-associated | 0.58 | 0.42–0.80 | 0.001 |
| Age (per year) | 1.03 | 1.02–1.04 | < 0.001 |
| Ceftriaxone resistance | 1.55 | 1.23–1.94 | < 0.001 |
| Focus of infection | |||
| No focus | 1 (ref) | – | |
| Lower respiratory | 1.80 | 1.13–2.88 | 0.014 |
| Genitourinary | 0.46 | 0.30–0.70 | < 0.001 |
| Other | 0.80 | 0.62–1.03 | 0.08 |
Discussion
In this study, we report novel data on the comparative incidence, determinants, and outcome associated with K. aerogenes and E. cloacae BSIs in a large Australian population. We find that E. cloacae had a fourfold higher incidence, and that demographic and clinical features and resistance rates are significantly different between these species. However, despite these important differences, these species share similar case-fatality. This study provides compelling evidence that K. aerogenes and E. cloacae are epidemiologically distinct and result in a different spectrum of clinical illness.
There is a paucity of studies that have reported on the population epidemiology of Enterobacter species infections. Al-Hasan et al. examined temporal trends among 38 mono-microbial Enterobacter species BSI in Olmsted County, USA, during 1998–2007 and found an incidence of 3.3 per 100,000 population [18]. Of these, 26 and 10 BSI cases were due to E. cloacae and K. aerogenes for respective incidence rates of 2.2 and 0.9 per 100,000 population [18]. Stokes et al. reported on E. cloacae complex BSI in Calgary, Canada, during 2015–2017 and identified 154 isolates corresponding to an annual incidence of 1.2–1.5 per 100,000 population [2]. Other population-based studies investigating a range of pathogens have identified Enterobacter species ranking among the top 10 most frequent causes of BSI [19, 20]. We are unaware of prior population-based studies that have specifically examined the epidemiology of K. aerogenes BSI.
Studies that have compared the clinical determinants and outcome between E. cloacae and K. aerogenes BSIs have reported conflicting results [7–10]. Jeon et al. conducted a retrospective, single centre, matched study of 194 patients at a tertiary centre in the Republic of Korea and found that E. cloacae complex BSI were at twice the risk for 30-day case-fatality as compared to K. aerogenes [7]. Alvarez-Marin conducted a 3-years study in five Spanish hospitals including a total of 285 BSI cases and found that E. cloacae BSI (n = 196) was associated with a higher co-morbid illness burden than with K. aerogenes (n = 89) [8]. However, they observed no differences in demographics, acquisition type, source, antimicrobial resistance, or case fatality [8]. Wesevich identified 150 BSI cases over a 14-year period at an academic tertiary care centre in the USA and found no differences in hospital case-fatality between species [9]. However, K. aerogenes BSI had a worse outcome as compared to E. cloacae when a composite outcome measure of hospital case-fatality, recurrence, or complication was analyzed [9]. Song et al. examined 239 BSI cases in the Republic of Korea and found higher rates of resistance with E. cloacae (n = 172) infections, although K aerogenes (n = 67) was associated with more severe disease and a worse outcome [10].
We did not observe any differences in all cause case fatality among BSIs due to K. aerogenes and E. cloacae., and this was true for both crude and adjusted analyses. It is notable that ceftriaxone resistance was a significant independent variable associated with death (Table 2). We previously conducted a study in one Queensland and three New South Wales hospitals and found that relapsing or persistent Enterobacter species bacteremia between 3 and 28 days post index culture was infrequent and that emergence of resistance to third generation cephalosporins was low [3]. There remains considerable debate as to whether cephalosporins or b-lactam b-lactamase inhibitor combination agents may be used to treat Enterobacter species infections [3, 21–23].
There are some strengths and limitations of our study that merit discussion. Our study cohort was approximately tenfold larger than any previous investigations comparing K. aerogenes and E. cloacae BSI [7–10]. As a result, we had higher statistical power to detect differences between these species. Another design advantage is that we included all cases identified within the publicly funded system state-wide, such that biases associated with study of selected hospital(s), including referral bias, were minimized [11, 24]. However, it is a limitation that we did not include private laboratories in our surveillance. While we suspect that these represent a limited proportion of cases, we are unable to quantify this potential bias. Our study was retrospective, and as a result we were limited to previously recorded data available in existing databases. Variables such as antimicrobial treatments and severity of illness scores were not available.
In summary, this study is a major addition to the body of literature. We highlight the different clinical features and epidemiology between E. cloacae and K. aerogenes BSI, and by study of a large cohort demonstrate that these species share a similar outcome.
Author contributions
Conception and design (KBL, DLP, PNAH), acquisition of data (KBL, FE, PNAH, DLP) analysis and interpretation of data (KBL), and manuscript preparation (KBL, FE, PNAH, DLP).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No external funding was obtained to support this study.
Data availability
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data release for the purposes of research under section 280 of the Public Health Act 2005 requires application to the Director General (PHA@health.qld.gov.au).
Declarations
Conflict of interest
P.H. participated as an advisory board member for both MSD and Sandoz, payment was paid to the University of Queensland. The other authors declare they have no conflicts of interest.
Ethical approval and consent to participate
The human research ethics committee at Royal Brisbane and Women’s Hospital approved this study and granted a waiver of individual consent (LNR/2020/QRBW/62494).
References
- 1.Davin-Regli A, Lavigne JP, Pagès JM. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev. 2019 doi: 10.1128/CMR.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stokes W, Peirano G, Matsumara Y, Nobrega D, Pitout JDD. Population-based surveillance of Enterobacter cloacae complex causing blood stream infections in a centralized Canadian region. Eur J Clin Microbiol Infect Dis. 2022;41(1):119–125. doi: 10.1007/s10096-021-04309-z. [DOI] [PubMed] [Google Scholar]
- 3.Harris PNA, Peri AM, Pelecanos AM, Hughes CM, Paterson DL, Ferguson JK. Risk factors for relapse or persistence of bacteraemia caused by Enterobacter spp.: a case-control study. Antimicrob Resist Infect Control. 2017;6:14. doi: 10.1186/s13756-017-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020 doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babiker A, Bower C, Lutgring JD, Petit RA, 3rd, Howard-Anderson J, Ansari U, et al. Clinical and genomic epidemiology of mcr-9-carrying carbapenem-resistant Enterobacterales isolates in metropolitan Atlanta, 2012 to 2017. Microbiol Spectrum. 2022;10(4):e0252221. doi: 10.1128/spectrum.02522-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell JM, Lubian AF, Partridge S, Gottlieb T, Iredell J, Daley DA, et al. Australian Group on Antimicrobial Resistance (AGAR) Australian Gram-negative Sepsis Outcome Programme (GNSOP) annual report 2019. Commun Dis Intell. 2018;2020:44. doi: 10.33321/cdi.2020.44.80. [DOI] [PubMed] [Google Scholar]
- 7.Jeon M, Huh K, Ko JH, Cho SY, Huh HJ, Lee NY, et al. Difference in the clinical outcome of bloodstream infections caused by Klebsiella aerogenes and Enterobacter cloacae complex. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Álvarez-Marín R, Lepe JA, Gasch-Blasi O, Rodríguez-Martínez JM, Calvo-Montes J, Lara-Contreras R, et al. Clinical characteristics and outcome of bacteraemia caused by Enterobacter cloacae and Klebsiella aerogenes: more similarities than differences. J Glob Antimicrob Resist. 2021;25:351–358. doi: 10.1016/j.jgar.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Wesevich A, Sutton G, Ruffin F, Park LP, Fouts DE, Fowler VG, Jr, et al. Newly named Klebsiella aerogenes (formerly Enterobacter aerogenes) is associated with poor clinical outcomes relative to other enterobacter species in patients with bloodstream infection. J Clin Microbiol. 2020 doi: 10.1128/JCM.00582-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song EH, Park KH, Jang EY, Lee EJ, Chong YP, Cho OH, et al. Comparison of the clinical and microbiologic characteristics of patients with Enterobacter cloacae and Enterobacter aerogenes bacteremia: a prospective observation study. Diagn Microbiol Infect Dis. 2010;66(4):436–440. doi: 10.1016/j.diagmicrobio.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Laupland KB. Defining the epidemiology of bloodstream infections: the 'gold standard' of population-based assessment. Epidemiol Infect. 2013;141(10):2149–2157. doi: 10.1017/S0950268812002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leal JR, Gregson DB, Church DL, Henderson EA, Ross T, Laupland KB. The validation of a novel surveillance system for monitoring bloodstream infections in the Calgary zone. Can J Infect Dis Med Microbiol. 2016;2016:2935870. doi: 10.1155/2016/2935870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis. 2012;12:85. doi: 10.1186/1471-2334-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 17.Queensland population projections 2002 to 2026. Queensland Government. Available at: https://public.tableau.com/views/HHSpopulationprojections. Accessed Sep 22, 2021.
- 18.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Temporal trends in Enterobacter species bloodstream infection: a population-based study from 1998–2007. Clin Microbiol Infect. 2011;17(4):539–545. doi: 10.1111/j.1469-0691.2010.03277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gubbels S, Nielsen J, Voldstedlund M, Kristensen B, Schonheyder HC, Vandenbroucke-Grauls CM, et al. Utilization of blood cultures in Danish hospitals: a population-based descriptive analysis. Clin Microbiol Infect. 2015;21(4):344. doi: 10.1016/j.cmi.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Verway M, Brown KA, Marchand-Austin A, Diong C, Lee S, Langford B, et al. Prevalence and mortality associated with bloodstream organisms: a population-wide retrospective cohort study. J Clin Microbiol. 2022;60(4):e0242921. doi: 10.1128/jcm.02429-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaubey VP, Pitout JD, Dalton B, Gregson DB, Ross T, Laupland KB. Clinical and microbiological characteristics of bloodstream infections due to AmpC beta-lactamase producing Enterobacteriaceae: an active surveillance cohort in a large centralized Canadian region. BMC Infect Dis. 2014;14:647. doi: 10.1186/s12879-014-0647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart AG, Paterson DL, Young B, Lye DC, Davis JS, Schneider K, et al. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections caused by AmpC β-lactamase-producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: a pilot multicenter randomized controlled trial (MERINO-2) Open Forum Infect Dis. 2021;8(8):ofab387. doi: 10.1093/ofid/ofab387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious diseases society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74(12):2089–2114. doi: 10.1093/cid/ciab1013. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Influence of referral bias on the clinical characteristics of patients with Gram-negative bloodstream infection. Epidemiol Infect. 2011;139(11):1750–1756. doi: 10.1017/S095026881100001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data release for the purposes of research under section 280 of the Public Health Act 2005 requires application to the Director General (PHA@health.qld.gov.au).