Abstract
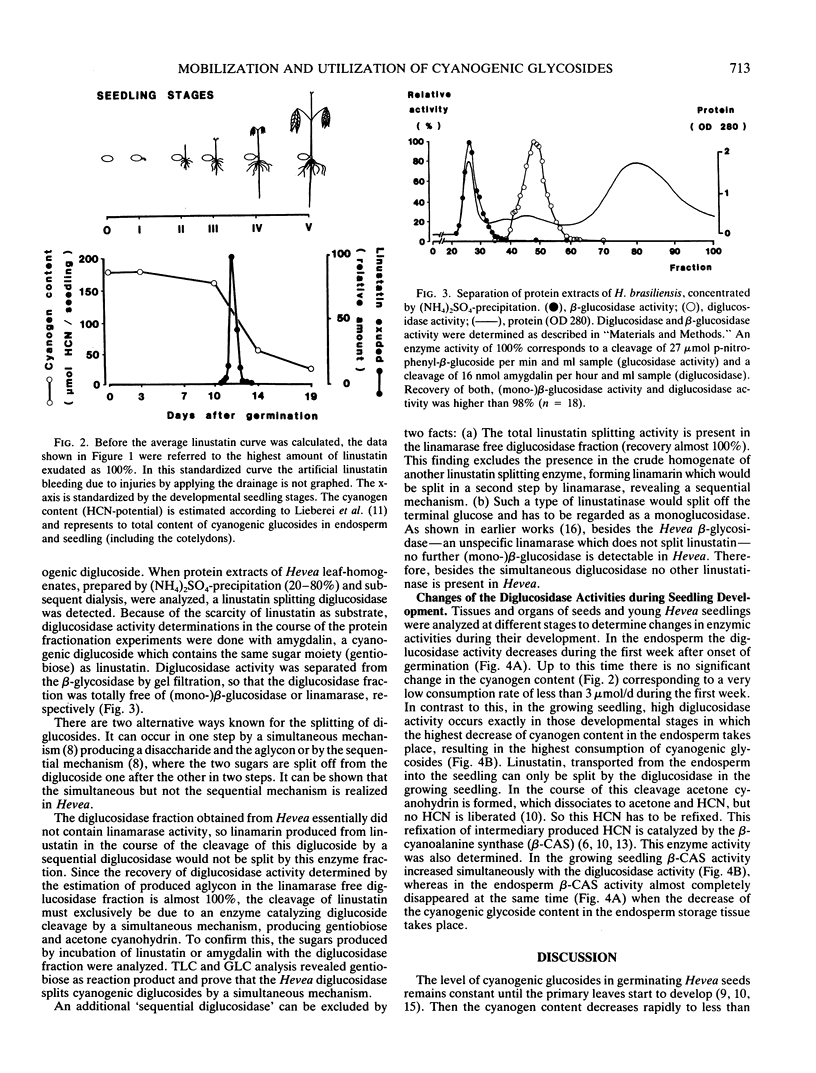
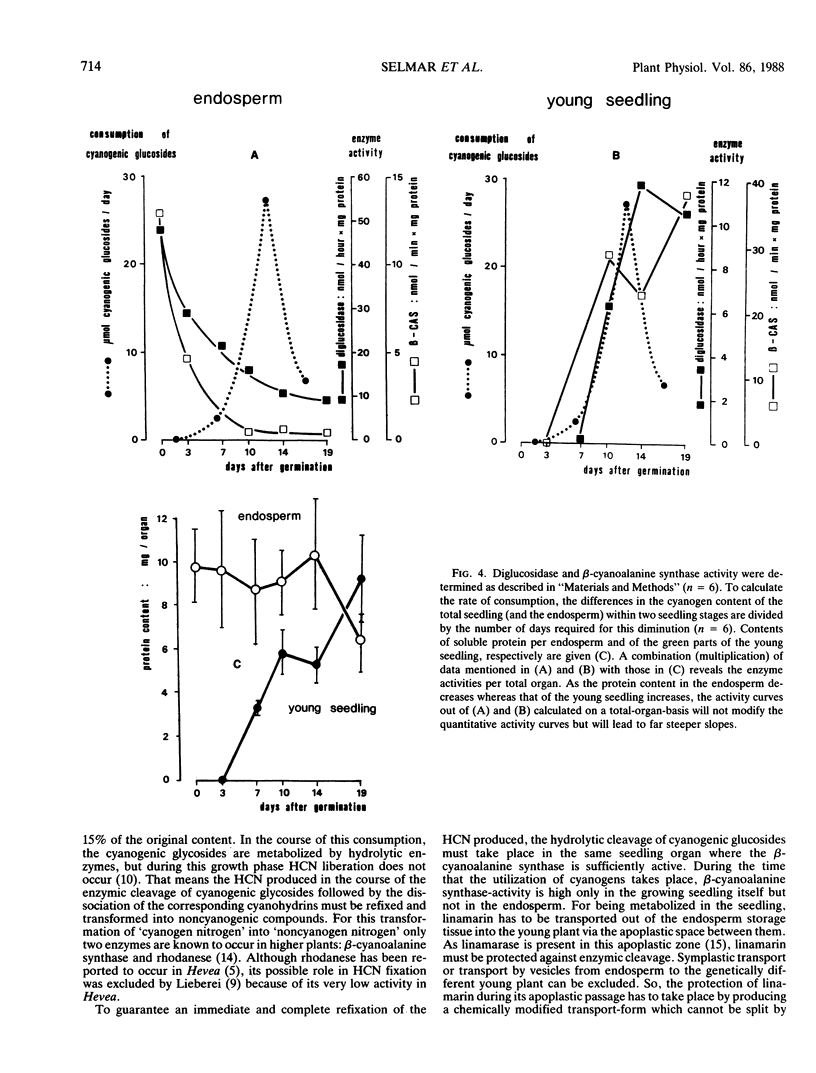
In the seeds of Hevea brasiliensis, the cyanogenic monoglucoside linamarin (2-β-d-glucopyranosyloxy-2-methylpropionitrile) is accumulated in the endosperm. After onset of germination, the cyanogenic diglucoside linustatin (2-[6-β-d-glucosyl-β-d-glucopyranosyloxy]-2- methylpropionitrile) is formed and exuded from the endosperm of Hevea seedlings. At the same time the content of cyanogenic monoglucosides decreases. The linustatin-splitting diglucosidase and the β-cyanoalanine synthase that assimilates HCN, exhibit their highest activities in the young seedling at this time. Based on these observations the following pathway for the in vivo mobilization and metabolism of cyanogenic glucosides is proposed: storage of monoglucosides (in the endosperm)—glucosylation—transport of the diglucoside (out of the endosperm into the seedling)—cleavage by diglucosidase—reassimilation of HCN to noncyanogenic compounds. The presence of this pathway demonstrates that cyanogenic glucosides, typical secondary plant products serve in the metabolism of developing plants as N-storage compounds and do not exclusively exhibit protective functions due to their repellent effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Castric P. A., Farnden K. J., Conn E. E. Cyanide metabolism in higher plants. V. The formation of asparagine from -cyanoalanine. Arch Biochem Biophys. 1972 Sep;152(1):62–69. doi: 10.1016/0003-9861(72)90193-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. R., Conn E. E. Cyanide metabolism in higher plants. IV. Purification and properties of the beta-cyanolanine synthase of blue lupine. J Biol Chem. 1969 May 25;244(10):2632–2640. [PubMed] [Google Scholar]
- Hösel W., Nahrstedt A. Spezifische Glucosidasen für das Cyanglucosid Triglochinin Reinigung und Charakterisierung von beta-Glucosidasen aus Alocasia macrorrhiza Schott. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1265–1275. [PubMed] [Google Scholar]
- Miller J. M., Conn E. E. Metabolism of hydrogen cyanide by higher plants. Plant Physiol. 1980 Jun;65(6):1199–1202. doi: 10.1104/pp.65.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D., Lieberei R., Biehl B., Voigt J. Hevea Linamarase-A Nonspecific beta-Glycosidase. Plant Physiol. 1987 Mar;83(3):557–563. doi: 10.1104/pp.83.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]


