Abstract
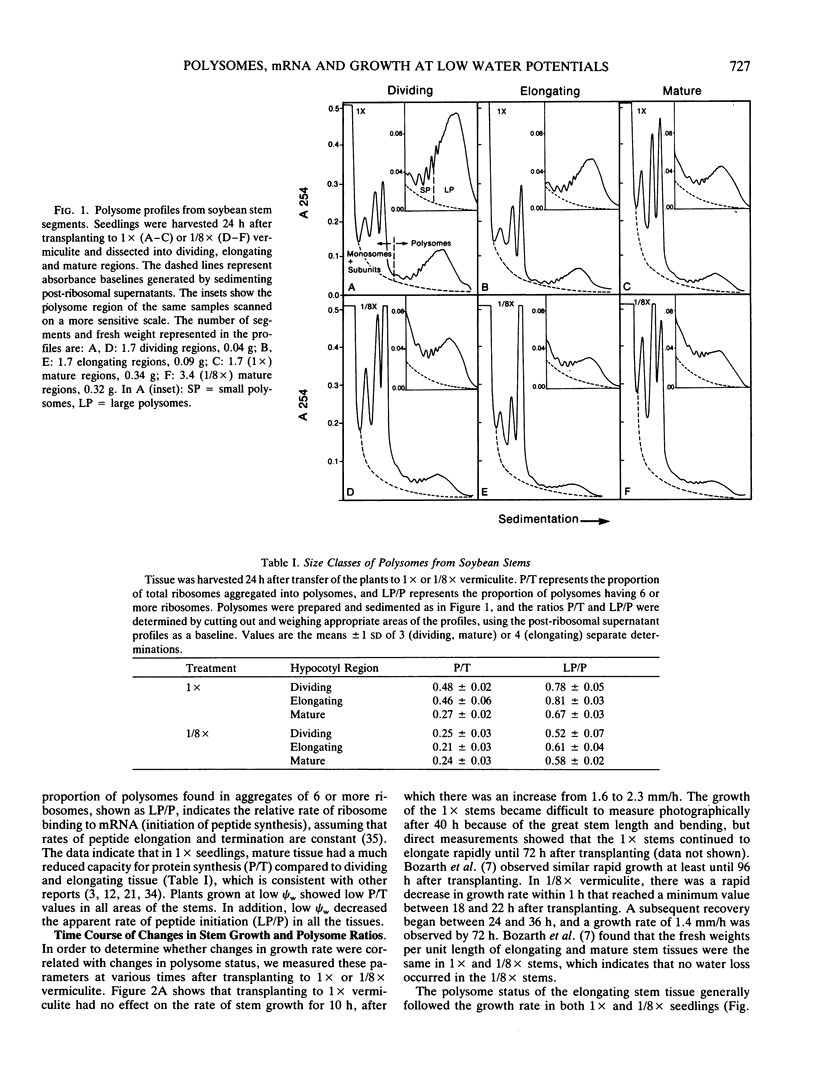
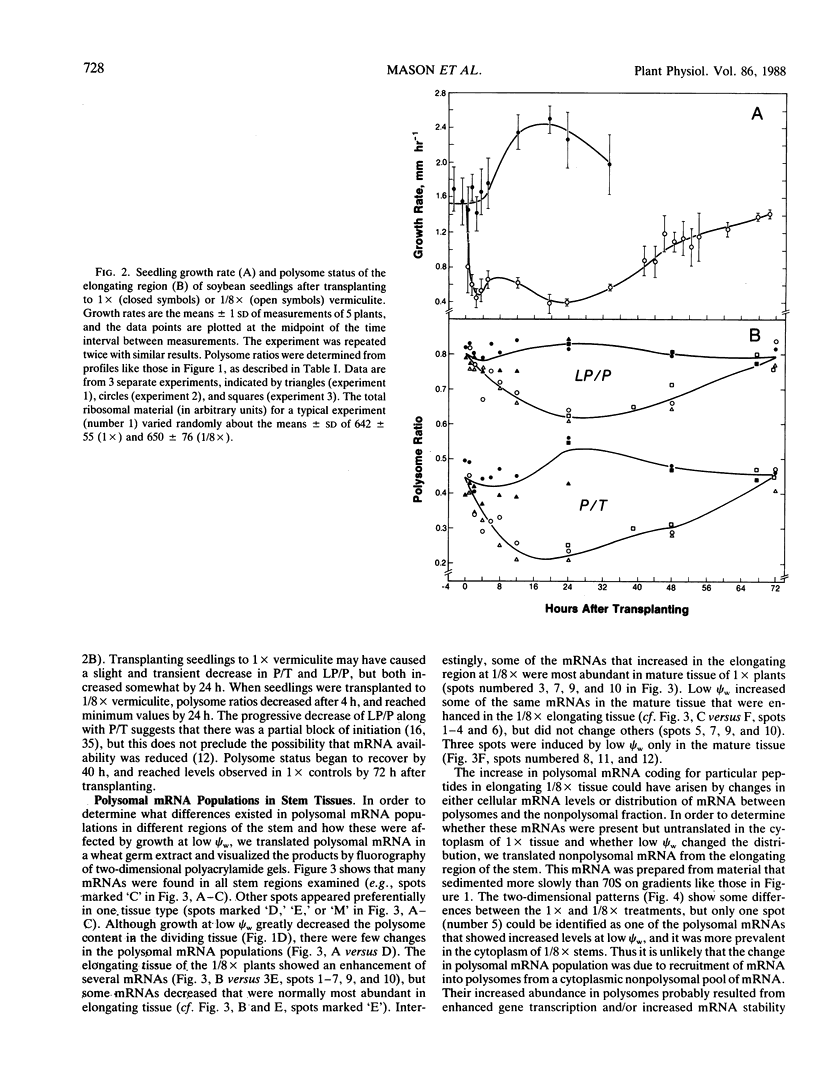
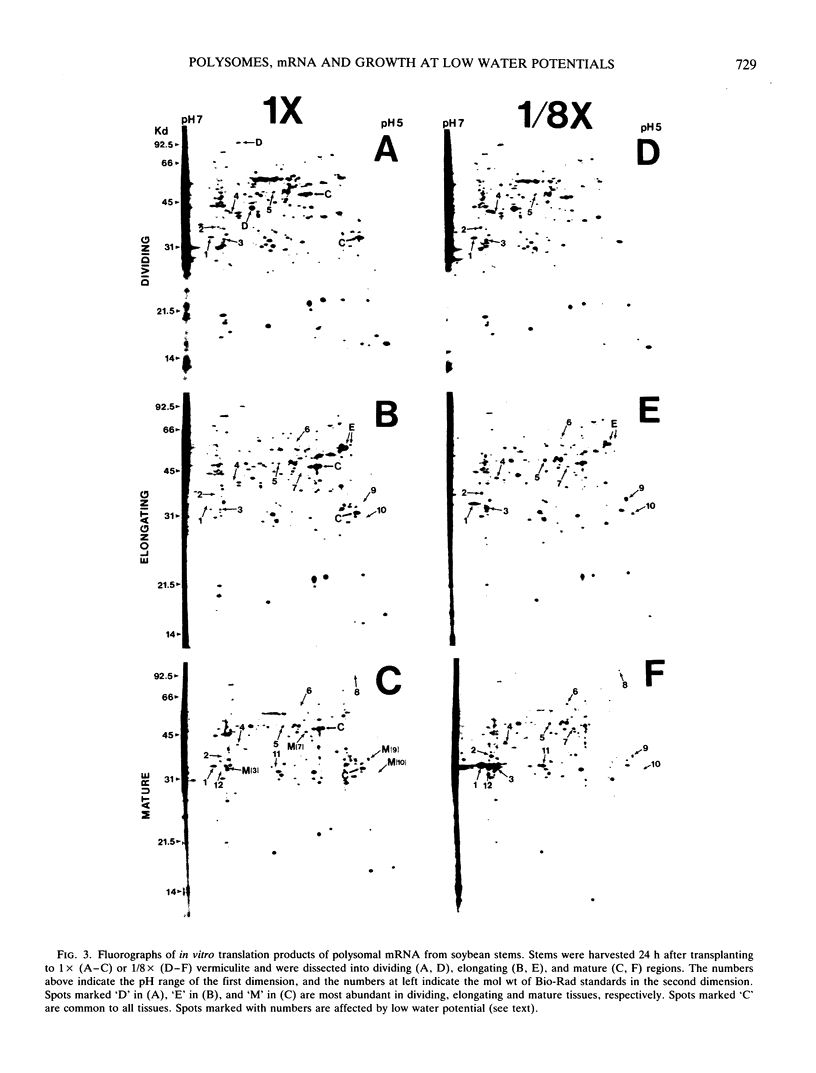
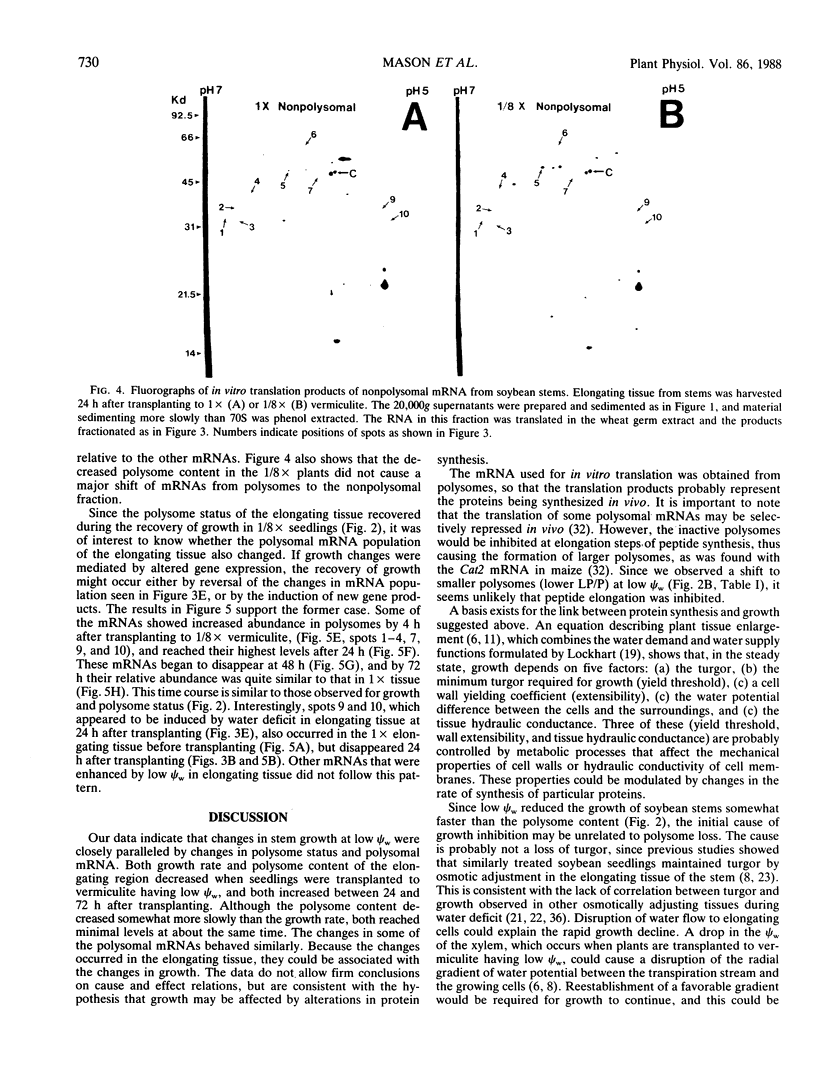
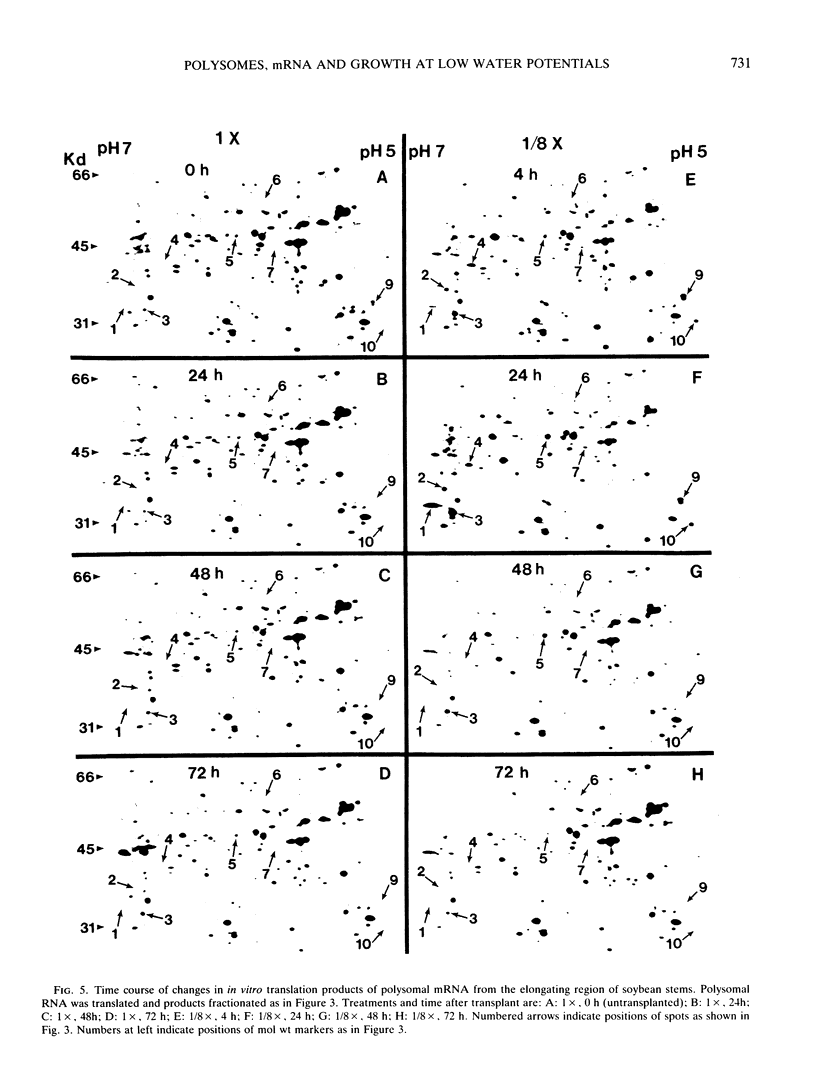
The polysome status and populations of polysomal mRNA were examined in different regions of dark-grown soybean (Glycine max [L.] Merr.) stems that contained either dividing, elongating, or mature (nongrowing) cells. There was a developmental gradient of polysome content in which the dividing tissue had the highest levels and the mature tissue the lowest. A few hours after transplanting the seedlings to vermiculite having low water content (water potential Ψw = −0.29 megapascals), stem growth rate decreased to 30% of well-watered controls and the polysome content decreased most in the dividing and elongating tissues. After 24 to 36 hours, stem growth and polysome content recovered gradually. In vitro translation products of polysomal mRNA from dividing, elongating or mature tissue were examined on two-dimensional gels. In well-watered controls, each of the stem regions was enriched in a small subset of the polysomal mRNA population, probably because of developmentally regulated gene expression. Exposing plants to low Ψw for 24 hours induced a change in the relative abundance of a small number of polysomal mRNAs in the elongating and mature tissues, but not in the dividing tissue. After 24 to 72 hours at low Ψw, the changes in polysomal mRNA population were reversed in the elongating tissue. The data indicate that changes in stem growth at low water potential are associated with changes in polysome status and polysomal mRNA in the elongating tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bozarth C. S., Mullet J. E., Boyer J. S. Cell wall proteins at low water potentials. Plant Physiol. 1987 Sep;85(1):261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri A. J., Boyer J. S. Water potentials induced by growth in soybean hypocotyls. Plant Physiol. 1982 Feb;69(2):492–496. doi: 10.1104/pp.69.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosomes from Peas: II. Polyribosome Metabolism during Normal and Hormone-induced Growth. Plant Physiol. 1973 Oct;52(4):339–345. doi: 10.1104/pp.52.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao T. C. Rapid Changes in Levels of Polyribosomes in Zea mays in Response to Water Stress. Plant Physiol. 1970 Aug;46(2):281–285. doi: 10.1104/pp.46.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Riazi A. Stress-induced osmotic adjustment in growing regions of barley leaves. Plant Physiol. 1981 Sep;68(3):571–576. doi: 10.1104/pp.68.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilla C. A., Boyer J. S., Hageman R. H. Nitrate Reductase Activity and Polyribosomal Content of Corn (Zea mays L.) Having Low Leaf Water Potentials. Plant Physiol. 1973 May;51(5):817–824. doi: 10.1104/pp.51.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S., Steudle E. Pressure probe and isopiestic psychrometer measure similar turgor. Plant Physiol. 1987 Mar;83(3):592–595. doi: 10.1104/pp.83.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rhodes P. R., Matsuda K. Water stress, rapid polyribosome reductions and growth. Plant Physiol. 1976 Nov;58(5):631–635. doi: 10.1104/pp.58.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : II. Response to Wounding in Aged Tissue. Plant Physiol. 1983 Nov;73(3):817–821. doi: 10.1104/pp.73.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Anderson J. M., Key J. L. Influence of auxin and incubation on the relative level of polyribosomes in excised soybean hypocotyl. Plant Physiol. 1973 Dec;52(6):608–612. doi: 10.1104/pp.52.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E., Cantraine F. R. Translational control of protein synthesis: a simulation study. Biochim Biophys Acta. 1971 Oct;247(3):471–485. doi: 10.1016/0005-2787(71)90034-7. [DOI] [PubMed] [Google Scholar]