Abstract
The effects of mutations in host genes on tetracycline resistance mediated by the Tet(O) and Tet(M) ribosomal protection proteins, which originated in Campylobacter spp. and Streptococcus spp., respectively, were investigated by using mutants of Salmonella typhimurium and Escherichia coli. The miaA, miaB, and miaAB double mutants of S. typhimurium specify enzymes for tRNA modification at the adenosine at position 37, adjacent to the anticodon in tRNA. In S. typhimurium, this involves biosynthesis of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A). The miaA mutation reduced the level of tetracycline resistance mediated by both Tet(O) and Tet(M), but the latter showed a greater effect, which was ascribed to the isopentenyl (i6) group or to a combination of the methylthioadenosine (ms2) and i6 groups but not to the ms2 group alone (specified by miaB). In addition, mutations in E. coli rpsL genes, generating both streptomycin-resistant and streptomycin-dependent strains, were also shown to reduce the level of tetracycline resistance mediated by Tet(O) and Tet(M). The single-site amino acid substitutions present in the rpsL mutations were pleiotropic in their effects on tetracycline MICs. These mutants affect translational accuracy and kinetics and suggest that Tet(O) and Tet(M) binding to the ribosome may be reduced or slowed in the E. coli rpsL mutants in which the S12 protein is altered. Data from both the miaA and rpsL mutant studies indicate a possible link between stability of the aminoacyl-tRNA in the ribosomal acceptor site and tetracycline resistance mediated by the ribosomal protection proteins.
Tetracycline is a broad-spectrum bacteriostatic antibiotic. Its usefulness in the early days of antibiotic therapy has subsequently been jeopardized by widespread drug resistance (27). A common mechanism of resistance to tetracycline is referred to as ribosomal protection (for a review, see reference 33). Several related classes of tetracycline resistance (Tcr) determinants have been identified and characterized at the DNA and protein levels (27), and all of these appear to function in a similar fashion. We will confine ourselves to discussing the tet(M) and tet(O) genes, which encode the Tet(M) and Tet(O) proteins, respectively, since most work has been done with these systems. The tet(M) gene was originally cloned from Streptococcus (10) and is found mainly in gram-positive bacteria (27), whereas the tet(O) gene was originally cloned from Campylobacter jejuni (32) and is found in both gram-negative species (Campylobacter species) and several gram-positive species, including Streptococcus species, Enterococcus faecalis (43), and most recently, Streptococcus pneumoniae (41).
Both ribosomal protection proteins are approximately 70 kDa, and their amino-terminal regions show considerable amino acid similarity to those of elongation factors EF-Tu and EF-G, GTPases that participate in protein synthesis (9, 23, 30, 31). Tet(M) and Tet(O) have been shown to bind and hydrolyze GTP (9, 34). In addition, substitution of amino acid Asn-128 within the putative GTP binding region of Tet(O) by several other amino acids resulted in decreased tetracycline resistance, confirming that tetracycline resistance is dependent on GTP binding (18). Recently, purified Tet(M) protein was shown to dramatically reduce the affinity of ribosomes for tetracycline when GTP is present (12). The addition of Tet(M) and Tet(O) proteins in the presence of GTP to ribosome-tetracycline complexes resulted in the displacement of bound drug (12, 37). Both the Tet(M) and Tet(O) proteins confer tetracycline resistance in Escherichia coli (12, 23, 32).
Tet(M), Tet(O), and EF-G have distinct amino acid similarities throughout their lengths and have apparently similar three-dimensional structures (1, 9, 33). EF-G functions to promote translocation, but its precise role in protein synthesis is not clearly understood (21). Protein translocation involves a reassociation of mRNA and tRNA with the functional sites of the ribosome responsible for the respective movement of the acceptor site (A-site) and peptidyl site (P-site) tRNAs into the P site and the exit site (E site) (21, 26). Burdett et al. (10) have demonstrated that Tet(M) cannot substitute for EF-Tu in an in vitro protein synthesis system. It has been suggested that the Tet(M) protein could act in conjunction with EF-G as part of a translocation complex at a step just prior to A-site binding of aminoacyl-tRNA to release the tetracycline bound near this site (12).
Previous work on Tet(M)-mediated Tcr in E. coli has also demonstrated that specific tRNA modification at the adenosine at position 37 (A37) is necessary for resistance because strains with mutations in the miaA gene were more tetracycline sensitive (11). Position A37 is adjacent to the anticodon in tRNA (6).
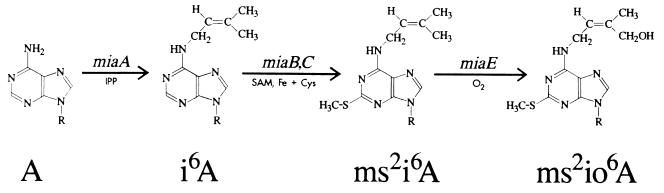
In this study, we examined the effects of the miaA and miaB mutations in Salmonella typhimurium (official designation, Salmonella enterica serovar typhimurium (5, 6, 17) on Tet(O)-mediated tetracycline resistance. The biosynthesis pathway of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) is shown in Fig. 1 (17).
FIG. 1.
Biosynthesis of ms2io6A in S. typhimurium. Adapted with permission from Esberg and Björk (17).
E. coli tRNAs that read codons starting with uridine (except tRNASer, species I and V) contain N6-(isopentenyl)-2-methylthioadenosine (ms2i6A) at position 37 (5). In contrast, S. typhimurium tRNAs contain the 4-hydroxy derivative (ms2io6A) at position 37 (8, 24). The availability of isogenic strains of S. typhimurium LT2 and its miaA and miaB derivatives (17) allowed us to test the effect of the hydroxyisopentenyl (io6) tRNA modification described above and to test the effect of the modification in combination with methylthioadenosine (ms2) as the ms2io6 modification on Tet(O) and Tet(M) Tcr. We also demonstrate that mutations in the rpsL gene encoding the S12 ribosomal protein (21) reduce the level of Tcr mediated by Tet(O) and Tet(M).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli or S. typhimurium strains were used since the tet(M) and tet(O) genes confer resistance in these species and appropriate mutants of these species are available. These are listed in Table 1 and were grown on Luria-Bertani medium (Difco, Detroit, Mich.) or Mueller-Hinton medium (Oxoid, Nepean, Ontario, Canada) at 37°C. Solid medium contained 1.5% agar (Gibco-BRL, Gaithersburg, Md.). Antibiotic concentrations were as follows: kanamycin (Sigma Chemical, St. Louis, Mo.), 100 μg/ml; carbenicillin (Sigma), 100 μg/ml; and tetracycline (Sigma) and streptomycin (Sigma), various concentrations, as indicated.
TABLE 1.
S. typhimurium and E. coli strains used in this study
| Strain | Species | Genotype | Reference | Source |
|---|---|---|---|---|
| GT522 | S. typhimurium LT2 | Prototroph | 17 | G. R. Björk |
| GT523 | S. typhimurium LT2 | miaA1 | 17 | G. R. Björk |
| GT2734 | S. typhimurium LT2 | miaB2508::Tn10dCm | 17 | G. R. Björk |
| GT3635 | S. typhimurium LT2 | miaA1 miaB2508::Tn10dCm | 6a | G. R. Björk |
| Tx2568 | E. coli K-12 | Prototroph | 13 | M. Winkler |
| Tx2571 | E. coli K-12 | miaA::Km | 13 | M. Winkler |
| Tx2569 | E. coli K-12 | mutL::Km | 13 | M. Winkler |
| Tx3346 | E. coli K-12 | miaB::Tn10dCm | 13 | M. Winkler |
| JM83 | E. coli K-12 | strAΔlac-pro thi φ80 lacZ ΔM15 | 42 | J. Messing |
| JM101 | E. coli K-12 | traD36 proA+proB+lacI9 lacZ ΔM15 supE thi Δ(lac-proAB) | 42 | J. Messing |
| JM101-S | E. coli K-12 | Strr derivative of JM101 | This study | M. Bekkering |
| WP2 | E. coli B/R | trpE65 lon-11 sulA1 | 36 | A. Timms |
| CM1234 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strd) | 36 | A. Timms |
| CM1243 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strd) | 36 | A. Timms |
| CM1245 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strd) | 36 | A. Timms |
| CM1235 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strr) | 36 | A. Timms |
| CM1236 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strr) | 36 | A. Timms |
| CM1237 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strr) | 36 | A. Timms |
| CM1238 | E. coli B/R | trpE65 lon-11 sulA1 rpsL (Strr) | 36 | A. Timms |
DNA preparation and transformation.
Plasmids containing cloned tet(O) or tet(M) genes were isolated by a modification of the method of Birnboim and Doly (4). Plasmids were transformed into recipient strains via electroporation (Gene Pulser; Bio-Rad, Hercules, Calif.) with the settings at 2.4 V, 200 Ω (resistance), and 25 μF (capacitance). Competent cells were prepared by growing 20 ml of the culture to the mid-logarithmic phase with appropriate antibiotics and then centrifuging (3,000 × g) and washing the cells several times with ice-cold distilled water before suspending them in 1 ml of 10% glycerol. Transformants were selected with carbenicillin for tet(O) or kanamycin for tet(M). The presence of plasmids in strains was confirmed by agarose gel electrophoresis.
Antibiotic susceptibility testing.
MICs were determined in the following manner. Cultures were grown to a concentration of 108 cells in liquid medium with an appropriate antibiotic to select for plasmid-containing cells. A volume of 10 μl from a 5-ml culture was diluted in 5 ml of 1× phosphate-buffered saline (Oxoid), and 10 μl of this dilution was spotted onto plates containing increasing twofold concentrations of tetracycline ranging from 1 to 128 μg/ml. The plates were incubated at 37°C overnight. For strains that were streptomycin dependent (Strd), streptomycin was added at a concentration of from 100 to 500 μg/ml, depending on the strain.
Protein gels and immunoblot blots.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the plasmids containing tet(O) or tet(M) was performed by the method of Laemmli (22). The total amount of protein loaded onto each gel was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.), and 7.5 μg of protein was loaded in each lane. Electrophoresis was performed with a Protean II apparatus (Bio-Rad) on a 10% polyacrylamide gel at a constant voltage of 150 V. The gels were stained with Coomassie blue stain. Prestained molecular weight standards (Gibco-BRL or Rainbow Markers; Amersham, Buckinghamshire, United Kingdom) were used throughout.
After SDS-PAGE the protein was electroblotted onto a NitroPlus nitrocellulose membrane (Micron Separations Inc., Westboro, Mass.). The membrane was then rinsed in wash buffer (phosphate-buffered saline plus 5% skim milk powder and 0.05% Tween 20). The membrane was blocked for 1 h with wash buffer containing 10% skim milk powder and was then incubated for 1 h with primary antibody against Tet(O) (34) diluted 1:500 in wash buffer, followed by three 5-min washes. Goat anti-rabbit conjugate (Sigma Chemicals), which was diluted 1:1,000 in wash buffer, was added, and the membrane was then reincubated for 1 h. Detection was performed with an ECL Western Blotting kit (Amersham) by following the manufacturer’s recommended protocols. The membrane was exposed to BioMax (Kodak) film, which was developed by using the manufacturer’s protocols.
RESULTS
MICs specified by tet(M) and tet(O) genes in wild-type S. typhimurium.
Burdett (11) has shown that mutations in E. coli miaA reduce the level of tetracycline resistance of strains containing the tet(M) plasmid pVB11. We examined the effects of mutations in two genes (miaA and miaB) involved in the modification of A37 in the tRNA of S. typhimurium, as shown in Fig. 1. Two plasmids carrying the tet(M) gene and two plasmids carrying the tet(O) gene were examined (Table 2). The pVB11 plasmid (11), which carries the tet(M) gene originally cloned from the conjugative transposon Tn916, specified the lowest MIC of 16 μg/ml for a wild-type S. typhimurium strain, whereas the tet(M) gene, originating from pJI3 (10), specified an MIC similar to that specified by the tet(O) gene encoded by pUOA2E1 (MIC, 64 μg/ml) (40). In plasmid pUOA2E2 (40) the region upstream of the tet(O) gene is deleted and specifies a MIC lower than that specified by the wild-type tet(O), which is similar to that specified by the tet(M) gene encoded by plasmid pVB11 (MIC, 16 μg/ml). A tet(B) gene, which encodes a tetracycline efflux system, pRT11 (pVH51::Tn10 derivative), was also used for comparison (19). This plasmid specified MICs of 256 μg/ml for all S. typhimurium strains tested (Table 2).
TABLE 2.
Effect of miaA and miaB mutations in S. typhimurium on MICs of tetracycline resistance mediated by Tet(O) and Tet(M)a
| Strain no. | Genotype | Tetracycline MIC (μg/ml) for strains with the following plasmid:
|
|||||
|---|---|---|---|---|---|---|---|
| No plasmid | pVB11 [Tet(M)] | pJI3 [Tet(M)] | pUOA2E1 [Tet(O)] | pUOA2E2 [Tet(O)] | pRT11 [Tet(B)]b | ||
| GT522 | Wild type (ms2io6 A37) | 1 | 16 | 64 | 64 | NDc | 256 |
| GT523 | miaA (A37) | 0.5 | 4 | 16 | 32 | ND | 256 |
| GT2734 | miaB (i6 A37) | 1 | 16 | 64 | 64 | 16 | 256 |
| GT3635 | miaAmiaB (A37) | 0.5 | 4 | 8 | 32 | 8 | 256 |
Effect of host mutations (miaA and miaB) which undermodify A37 in tRNA.
We used S. typhimurium mutants in these experiments because of the availability of isogenic mutations in miaA and miaB and the double mutant (miaAB) (Table 1). The miaA mutation in S. typhimurium reduced the tetracycline MIC for both tet(M)- and tet(O)-containing strains (Table 2). The levels of reduction in the MICs for tet(M)- and tet(O)-containing strains were compared and were found to differ, with the MIC for the tet(M)-containing strains, specified by pVB11, being reduced from 16 to 4 μg/ml and those specified by pJI3 being reduced from 64 to 16 μg/ml, but the reduction specified by pUOA2E1 [wild-type tet(O)] was smaller (64 to 32 μg/ml). The difficulties encountered in preparing constructs with pUOA2E2 [upstream deletion of tet(O)] made comparisons more difficult, but a twofold decrease in the MIC (from 16 to 8 μg/ml) for the miaB and miaAB mutants was again found (Table 2). The miaB mutation had no effect on the tetracycline MIC for either tet(M)- or tet(O)-containing strains. The MICs for the strain with the double mutation (miaAB) were approximately identical to those observed for strain with the miaA mutation alone.
E. coli miaA mutants with Kmr transposon insertions into miaA, mutL, and miaB (14) were also used to determine MICs. With pVB11, the MIC for the E. coli miaA strain was 4 μg/ml, a definite reduction in the MIC (from 16 μg/ml), whereas for the miaB and mutL (located upstream of miaA) strains, there was no reduction in the MIC. In the case of pJI3, the MIC was also reduced to 16 μg/ml (from 64 μg/ml). However, for pUOA2E1, the MIC remained at 64 μg/ml for all E. coli miaA, miaB, and mutL derivatives tested. Therefore, the miaA mutation appears to reduce the MIC for tet(M) strains to a greater extent than it does for tet(O) strains. For S. typhimurium, the MIC specified by tet(O) is reduced only by one-half, whereas for E. coli no reduction in the MIC was observed.
Effect of mutations in the rpsL gene encoding ribosomal protein S12 on MICs of tetracycline.
During MIC testing of strains harboring plasmids carrying the tet(M) and tet(O) genes, we observed that the MICs of tetracycline were different for E. coli JM83 and JM101 carrying the same plasmid. For example, for JM83(pVB11) the MIC was 16 μg/ml, but for JM101(pVB11) the MIC was 32 μg/ml. Similar results with other plasmids were observed, with the MIC for the JM83 host always being lower than the MIC for JM101, even though no difference in MICs were found for plasmid-free strains. The major difference between JM83 and JM101 was a strA mutation in the former strain. To test the effect of resistance to streptomycin on the tetracycline MIC, JM101(pVB11) was made resistant to streptomycin by selection on 100 μg of streptomycin per ml. The MIC for streptomycin-resistant (Strr) strain JM101(pVB11) on retesting was reduced from 32 to 8 μg/ml. We therefore began to examine the effect of Strr and Strd mutations on the MICs specified by the tet(M) and tet(O) genes.
The strongest evidence that Strr and Strd mutations reduce the MICs specified by tet(M) and tet(O) is shown in Table 3. We used strains with known mutations in ribosomal protein S12; these mutations have been sequenced by Timms and colleagues (35, 36). Almost all rpsL mutations reduce the MICs specified by both tet(M) and tet(O) genes. In general, the level of reduction for each particular mutation for tet(M) and tet(O) is similar; e.g., for strain CM1245 (Strd) the MIC is reduced by half for both determinants, whereas for strain CM1236 (Strr) the MIC is reduced to one-fourth. Nevertheless, the reduction in the MIC for strains with mutations in rpsL is pleiotropic, with the reduction in the MIC for some strains with tet(M) being greater than that for strains with tet(O), as with CM1243 (Strd) and CM1238 (Strr). No reduction in the MIC for strain CM1235(pVB11) was found. In contrast to the effect on tetracycline resistance mediated by Tet(M) and Tet(O), which act by protecting the ribosome, the Tet(B) determinant, which specifies tetracycline efflux, was not affected by the rpsL mutation. The MICs specified by Tet(B) were 256 μg/ml for both parental strains and strains with an rpsL backgrounds (Table 3).
TABLE 3.
Defined mutations in E. coli rpsL gene reduce MICs specified by tet(M) and tet(O) genes
| Strain no. | Genotype | Amino acid changea | Tetracycline MIC (μg/ml) for strains with following plasmid:
|
|||
|---|---|---|---|---|---|---|
| No plasmid | pVB11 [Tet(M)] | pUOA2E1 [Tet(O)] | pRT11 [Tet(B)]b | |||
| WP2 | Wild type | None | 1 | 8 | 64 | 256 |
| CM1234 | rpsL (Strd) | Lys 42→Gln | 0.5 | NDc | 1 | 256 |
| CM1243 | rpsL (Strd) | Pro 90→Arg | 1 | 1 | 16 | 256 |
| CM1245 | rpsL (Strd) | Gly 91→Asp | 1 | 4 | 32 | 256 |
| CM1235 | rpsL (Strr) | Lys 42→Thr | 1 | 8 | 32 | 256 |
| CM1236 | rpsL (Strr) | Lys 42→Ile | ND | 2 | 16 | ND |
| CM1237 | rpsL (Strr) | Lys 42→Arg | 1 | 4–8 | 32 | 256 |
| CM1238 | rpsL (Strr) | Lys 42→Asn | 1 | <1 | 16 | 256 |
We used the CM strains in preference to other rpsL mutants because the rpsL genes have been sequenced and the exact amino acid substitutions in the mutants are known (35, 36). However, other Strr mutants in which the exact mutations involved were not known were also tested. Some Strr mutations in strains with pVB11, pJI3, pUOA2E1, or pUOA2E2 had no effect on tetracycline MICs. In contrast, for two Strd strains known as strM (29), the MICs specified by Tet(M) and Tet(O) plasmids were reduced; e.g., the MIC for CGSC6983(pVB11) was 2 μg/ml, that for CGSC6983(pJI3) was 4 μg/ml, and that for CGSC6983(pUOA2E1) was 2 to 4 μg/ml (data not shown in Table 3). In contrast to the rpsL mutants, no parental strains were available for the strM strains.
Effect of addition of streptomycin to growth medium for MIC testing.
Since streptomycin must be added to Strd strains for growth to occur, it occurred to us that this might be affecting the MICs of tetracycline. Therefore, streptomycin at 100 μg/ml was added to the tetracycline plates used to determine the MICs (Table 3). The presence or absence of streptomycin had no effect on the tetracycline MICs for the Strr strains listed in Table 3. It was not possible to test Strd strains without streptomycin, since they would not be able to grow. We infer from the results of this experiment that streptomycin itself does not reduce the tetracycline MIC.
Examination of Tet(M) and Tet(O) protein production.
Immunoblotting studies were performed with antibody to Tet(O) [which also reacts with Tet(M)] by using the strains listed in Tables 2 and 3. There was no significant difference in the amount of Tet(M) or Tet(O) protein produced by any of the strains tested (data not shown). Consequently, differences in the MICs for particular strains could not be explained by the differences in the levels of ribosomal protection proteins.
DISCUSSION
A major goal of this study was to examine the effect of undermodification of A37 within tRNA on Tcr mediated by Tet(O). This was precipitated by the report that modification of tRNA at A37, which was lacking in an E. coli miaA mutant, was necessary for Tet(M)-mediated Tcr (11). We found that Tet(O)-mediated Tcr was slightly reduced in an S. typhimurium miaA mutant, but the effect was less than that observed with Tet(M)-mediated Tcr. Also, our data on the effect of miaA on Tet(M) are not as compelling as those presented in an earlier study (11). The availability of miaA, miaB, and miaAB isogenic mutations enabled us to test different modifications within ms2io6A37 on both Tet(O)- and Tet(M)-mediated resistance. The miaA mutants should produce primarily unmodified A37, and miaB mutants accumulate mostly isopentenyl (i6) A37, with only small amounts of io6 A37 accumulating in S. typhimurium (17). The effect on both Tcr determinants can be ascribed to the i6 group alone or to the combination of the ms2 and i6 groups but not to the ms2 group alone (Fig. 1). Identical results were found by Esberg and Björk (17) in studying the decoding efficiency of tRNA. Their results indicate that although ms2 and i6 groups contribute to the decoding efficiency of tRNA, the major impact originates from the i6 group or the combination of the i6 and the ms2 groups. The ms2i6 A37 has been shown to stabilize the anticodon-codon interaction by improving the stacking of the hypermodified nucleoside (39).
The effects of the miaA mutation on E. coli are pleiotropic. Mutants grow more slowly, the translation elongation rate is decreased, and the regulation of many operons is affected (16). The ms2i6 modification also influences translational accuracy; fidelity is increased in an miaA mutant, possibly because the absence of the modification which usually stabilizes the codon-anticodon interaction affects the binding of near cognate tRNA more than it affects the binding of cognate tRNA (5).
The effect of undermodification of A37 on Tet(O)- and Tet(M)-mediated Tcr is consistent with the interaction of these proteins near the ribosomal A site, the site of tetracycline action. Tetracycline binds near the ribosomal A site and blocks entry of the aminoacyl-tRNA · EF-Tu · GTP ternary complex. Tet(O) and Tet(M) cause tetracycline to be displaced from the ribosome (12, 37). A decrease in aminoacyl-tRNA stability caused by the loss of the ms2i6 modification may decrease the ability of aminoacyl-tRNA to successfully bind to the A site before the rebinding of tetracycline. The difference in the level of resistance noted between the Tet(O) and Tet(M) derivatives of the miaA strains may reflect differences in the kinetic rates of these ribosomal protection proteins which alter the amount of time that the aminoacyl-tRNA has to bind before the rebinding of tetracycline.
In this study, we also discovered that the levels of resistance mediated by Tet(O) and Tet(M) were reduced in E. coli strains with particular mutations in the rpsL gene encoding ribosomal protein S12. For both Strr and Strd strains with either Tet(O) or Tet(M) some reduction in tetracycline MICs was observed. Although the reductions were quite variable, the MICs specified by Tet(M) (pVB11) and Tet(O) (pUOA2E1) are comparable, in that mutations which reduce tetracycline resistance for strains with Tet(M) reduce it by about the same level as that observed for strains with Tet(O). The effect of rpsL mutants on tetracycline resistance could not be explained by the hypersensitivity of these mutants to tetracycline or to poor growth since the presence of a Tet(B) determinant, which acts via an efflux mechanism, resulted in the maintenance of an MIC of 256 μg/ml for both parental and rpsL mutant derivatives.
Streptomycin induces misreading in the translation of the genetic code (21). Mutations in the rpsL gene, which generate Strr or Strd ribosomes, lead to an error-restrictive (or hyperaccurate) phenotype, i.e., the accuracy of translation is increased, as is the case with the miaA mutation. This phenomenon was once thought to relate to increased proofreading in rpsL mutants (28), but this is no longer thought to be the complete explanation (20). It is now established that error-restrictive S12 mutations reduce the kinetic efficiency of the interaction between the ternary complex (aa-tRNA · EF-Tu · GTP) and the ribosome (3, 7, 38). The restrictive S12 mutations are thought to reduce the saturation level of the ribosome (21). The effects of mutations in Strr and Strd mutants on translational accuracy and kinetics are presumably related to ternary complex binding at the A site, perhaps indirectly, because the S12 protein affects the 16S RNA which in turn affects ternary complex interactions at the A site (2). Mutant ribosomes with decreased translational accuracy (ribosomal ambiguity mutants) have a higher nonspecific affinity for tRNA at the A site, while the mutants with hyperaccurate mutations, such as the ribosomes of rpsL mutants, have a lower general affinity for tRNA, which makes the codon-anticodon interaction of increased importance (20). In addition, some mutants with mutations in S12 were shown to be abnormal with respect to their capacity to stimulate the GTPase activity of EF-Tu (3). S12 has also been cross-linked to EF-G, and EF-Tu is believed to bind in the vicinity of S12. Both elongation factors bind to the same site in a mutually exclusive fashion (14).
The interaction of Tet(O) and Tet(M) with the ribosome appear to be affected by the S12 mutations, as shown in this work. As with the miaA mutation, the rpsL mutation increases translational accuracy, and this is believed to be due to decreases in the affinity of the ribosomes for the common features of the tRNA molecules. It is likely that this effect plays the same role in the reduction of the tetracycline MICs for the rpsL mutants as it does for the miaA mutants. We also looked at Tcr in error-prone ribosomal ambiguity mutants (strains with mutations in either the rpsD and rplL genes) (21). None of the mutations in ribosomal ambiguity mutants had much of an effect on Tet(O)- or Tet(M)-mediated resistance (data not shown). Ribosomal ambiguity mutants are believed to have an increased affinity for tRNA (20).
Another possibility for the strong effect of rpsL mutations on tetracycline resistance involves the S12 interactions with elongation factors. We know that Tet(O) and Tet(M) interact with the ribosome in the GTP conformer (12, 37), and the results obtained for S12 indicate that they may bind to the same site as the elongation factor. If Tet(O) and Tet(M) binding is altered by S12 mutations, it could interfere with the actions of these proteins, so that bound tetracycline is not displaced as well as it is in wild-type ribosomes. Our data indicate that Tet(O) may bind more tightly than Tet(M), because mutations in S12 reduce the MICs for strains with Tet(M)-mediated Tcr more than they do the MICs for strains with Tet(O)-mediated Tcr. Alternatively, if the kinetics of Tet(O) and Tet(M) are altered, tetracycline may be able to rebind to the ribosome faster than the ternary complex, thus decreasing the effects of Tet(O) and Tet(M) on protein synthesis. At present, we favor the latter hypothesis.
In fact, the S12 mutants have a range of kinetic rates (21) and some mutants differ in their ability to stimulate the GTPase activity of EF-Tu (3), which may explain the difference in MICs for the different mutants listed in Table 1. We used S12 mutants with single amino acid changes in our study, although Timms and Bridges (36) have shown that double independent mutational events occur in the rpsL gene (36). Only a single mutation, Lys 42→Arg, is known to have a nonrestrictive Strr phenotype. When this E. coli mutant was used as the host strain, we observed the smallest effect on the MIC for strains with Tet(O) (pUOA2E1; from 64 to 32 μg/ml) and strains with Tet(M) (pVB11; from 8 to 4 to 8 μg/ml). This supports the hypothesis that the accuracy of translation is a factor in strains with this type of Tcr.
Our study demonstrates that mutations in the ribosomal protein S12 and the modification of tRNA at position A37, as shown previously for Tet(M) (11), can both reduce the ribosomal protection mechanism of Tcr. It is of interest that a double mutant with an miaA mutation combined with a mutation resulting in Strr had a streptomycin-dependent phenotype (15, 25). The explanation accounting for this was based on the increase in “proofreading flows to excessive levels” that could be suppressed by streptomycin (15). Although the explanation for the interaction between miaA and rpsL is not clear, it is intriguing in light of our findings.
The effects of these host mutations shed additional light on the mechanism of Tet(O)- and Tet(M)-mediated resistance; however, there is still much to be learned. The rpsL mutations point to an interaction of the ribosomal protection proteins with the elongation factor binding site on the ribosome. Data for both the miaA mutants and the rpsL mutants indicate a possible link between stability of the aminoacyl-tRNA in the A site and tetracycline resistance. The overall accuracy of translation is important for ribosomal protection protein-mediated tetracycline resistance.
ACKNOWLEDGMENTS
We thank G. R. Björk, A. Timms, and M. Winkler for strains and for useful comments, V. Burdett for plasmid pVB11, and C. Spahn for helpful discussion.
Support for this work was provided by the Natural Sciences and Engineering Research Council of Canada (to D.E.T.). D.E.T. is an Alberta Heritage Foundation for Medical Research Scientist. C.A.T. was supported by a fellowship from the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Ævarsson A, Brazhnikov E, Garber M, Zheltonosova J, Chirgadze Y, Al-Karadaghi S, Svensson L A, Liljas A. Three-dimensional structure of the ribosomal translocase: elongation factor G from Thermus thermophilus. EMBO J. 1994;13:3669–3677. doi: 10.1002/j.1460-2075.1994.tb06676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen P N, Noller H F. Mutations in ribosomal proteins S4 and S12 influence the higher order structure of 16 S ribosomal RNA. J Mol Biol. 1989;208:457–468. doi: 10.1016/0022-2836(89)90509-3. [DOI] [PubMed] [Google Scholar]
- 3.Bilgin N, Claesens F, Pahverk H, Ehrenberg M. Kinetic properties of Escherichia coli ribosomes with altered forms of S12. J Mol Biol. 1992;224:1011–1027. doi: 10.1016/0022-2836(92)90466-w. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björk G R. Genetic dissection of synthesis and function of modified nucleosides in bacterial transfer RNA. Prog Nucleic Acid Res Mol Biol. 1995;50:263–338. doi: 10.1016/s0079-6603(08)60817-x. [DOI] [PubMed] [Google Scholar]
- 6.Björk G R. Stable RNA modification. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: molecular and cellular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 861–886. [Google Scholar]
- 6a.Björk, G. R. Unpublished data.
- 7.Bohman K T, Ruusala T, Jelenc P C, Kurland C G. Kinetic impairment of restrictive streptomycin-resistant ribosomes. Mol Gen Genet. 1994;198:90–99. doi: 10.1007/BF00328706. [DOI] [PubMed] [Google Scholar]
- 8.Buck M, McCloskey J A, Basile B, Ames B N. cis-2-Methylthioribosylzeatin (ms2io6A) is present in the transfer RNA of Salmonella typhimurium, but not Escherichia coli. Nucleic Acids Res. 1982;10:5649–5662. doi: 10.1093/nar/10.18.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdett V. Purification and characterization of Tet(M), a protein that renders ribosomes resistant to tetracycline. J Biol Chem. 1991;266:2872–2877. [PubMed] [Google Scholar]
- 10.Burdett V, Inamine J, Rajogapalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982;149:995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdett V. tRNA modification activity is necessary for Tet(M)-mediated tetracycline resistance. J Bacteriol. 1993;175:7209–7215. doi: 10.1128/jb.175.22.7209-7215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdett V. Tet(M)-promoted release of tetracycline from ribosomes is GTP dependent. J Bacteriol. 1996;178:3246–3251. doi: 10.1128/jb.178.11.3246-3251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly D M, Winkler M E. Structure of Escherichia coli K-12 miaA and characterization of the mutator phenotype caused by miaA insertion mutations. J Bacteriol. 1991;173:1711–1721. doi: 10.1128/jb.173.5.1711-1721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czworkowski J, Moore P B. The elongation phase of protein synthesis. Prog Nucleic Acid Res Mol Biol. 1996;54:293–332. doi: 10.1016/s0079-6603(08)60366-9. [DOI] [PubMed] [Google Scholar]
- 15.Diaz I, Ehrenberg M, Kurland C G. How do combinations of rpsL− and miaA− generate streptomycin dependence? Mol Gen Genet. 1986;202:207–211. doi: 10.1007/BF00331638. [DOI] [PubMed] [Google Scholar]
- 16.Ericson J U, Björk G R. Pleiotropic effects induced by modification deficiency next to the anticodon of tRNA from Salmonella typhimurium LT2. J Bacteriol. 1986;166:1013–1021. doi: 10.1128/jb.166.3.1013-1021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esberg B, Björk G R. The methylthio group (ms2) of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) present next to the anticodon contributes to the decoding efficiency of the tRNA. J Bacteriol. 1995;177:1967–1975. doi: 10.1128/jb.177.8.1967-1975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grewal J, Manavathu E K, Taylor D E. Effect of mutational alteration of Asn-128 in the putative GTP-binding domain of tetracycline resistance determinant Tet(O) from Campylobacter jejuni. Antimicrob Agents Chemother. 1993;37:2645–2649. doi: 10.1128/aac.37.12.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen R A, Reznikoff S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979;138:705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimi R, Ehrenberg M. Dissociation rate of cognate peptidyl-tRNA from the A-site of hyper-accurate and error-prone ribosomes. Eur J Biochem. 1994;226:355–360. doi: 10.1111/j.1432-1033.1994.tb20059.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurland C G, Hughes D, Ehrenberg M. Limitations of translational accuracy. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: molecular and cellular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 979–1004. [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Manavathu E K, Hiratsuka K, Taylor D E. Nucleotide sequence analysis and expression of a tetracycline resistance gene from Campylobacter jejuni. Gene. 1988;62:17–26. doi: 10.1016/0378-1119(88)90576-8. [DOI] [PubMed] [Google Scholar]
- 24.Persson B C, Esberg B, Olafsson O, Björk G R. Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie. 1994;76:1152–1160. doi: 10.1016/0300-9084(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 25.Petrullo L A, Gallagher P J, Elseviers D. The role of 2-methyl-thio-N6-isopentenyladenosine in readthrough and suppression of nonsense codons in Escherichia coli. Mol Gen Genet. 1983;190:289–294. doi: 10.1007/BF00330653. [DOI] [PubMed] [Google Scholar]
- 26.Rheinberger H J, Nierhaus K H. Allosteric interactions between the transfer RNA-binding sites A and E. J Biol Chem. 1986;26:9133–9139. [PubMed] [Google Scholar]
- 27.Roberts M C. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol Rev. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 28.Ruusala T, Ehrenberg M, Kurland C G. Is there proofreading during polypeptide synthesis? EMBO J. 1982;1:741–745. doi: 10.1002/j.1460-2075.1982.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Anzaldo F J, Bastarrachea F. Genetic characterization of streptomycin-resistant and -dependent mutants of Escherichia coli K12. Mol Gen Genet. 1974;130:47–64. doi: 10.1007/BF00270518. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Pescador R, Brown J T, Roberts M, Urdea M S. Homology of the TetM with translational elongation factors: implication for potential modes of tetM conferred tetracycline resistance. Nucleic Acids Res. 1988;16:1218. doi: 10.1093/nar/16.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Pescador R, Brown J T, Roberts M, Urdea M S. The nucleotide sequence of the tetracycline resistance determinant tetM from Ureaplasma urealyticum. Nucleic Acids Res. 1988;16:1216–1217. doi: 10.1093/nar/16.3.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D E. Plasmid-mediated tetracycline resistance in Campylobacter jejuni: expression in Escherichia coli and identification of homology with streptococcal class M determinant. J Bacteriol. 1986;165:1037–1039. doi: 10.1128/jb.165.3.1037-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor D E, Chau A. Tetracycline resistance mediated by ribosomal protection. Antimicrob Agents Chemother. 1996;40:1–5. doi: 10.1128/aac.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor D E, Jerome L J, Grewal J, Chang N. Tet(O), a protein that mediates ribosomal protection to tetracycline, binds, and hydrolyses GTP. Can J Microbiol. 1995;41:965–970. [Google Scholar]
- 35.Timms A R, Steingrimsdottir H, Lehmann A R, Bridges B A. Mutant sequences in the rpsL gene of Escherichia coli B/r: mechanistic implications for spontaneous and ultraviolet light mutagenesis. Mol Gen Genet. 1992;232:89–96. doi: 10.1007/BF00299141. [DOI] [PubMed] [Google Scholar]
- 36.Timms A R, Bridges B A. Double, independent mutational events in the rpsL gene of Escherichia coli: an example of hypermutability? Mol Microbiol. 1993;9:335–342. doi: 10.1111/j.1365-2958.1993.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 37.Trieber, C. A., D. E. Taylor, and H. K. Nierhaus. Unpublished data.
- 38.Tubulekas I, Buckingham R H, Hughes D. Mutant ribosomes can generate dominant kirromycin resistance. J Bacteriol. 1991;173:3635–3643. doi: 10.1128/jb.173.12.3635-3643.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vacher J, Grosjean H, Houssier C, Buckingham R H. The effect of point mutations affecting Escherichia coli tryptophan tRNA on anticodon-anticodon interactions and on UGA suppression. J Mol Biol. 1984;177:329–342. doi: 10.1016/0022-2836(84)90460-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Taylor D E. A DNA sequence upstream of the tet(O) gene is required for full expression of tetracycline resistance. Antimicrob Agents Chemother. 1991;35:2020–2025. doi: 10.1128/aac.35.10.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widdowson C A, Klugman K P, Hanslo D. Identification of the tetracycline resistance gene, tet(O), in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2891–2893. doi: 10.1128/aac.40.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 43.Zilhao R, Papadopoulou B, Courvalin P. Occurrence of the Campylobacter resistance gene tet(O) in Enterococcus and Streptococcus spp. Antimicrob Agents Chemother. 1988;32:1793–1796. doi: 10.1128/aac.32.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]