Abstract
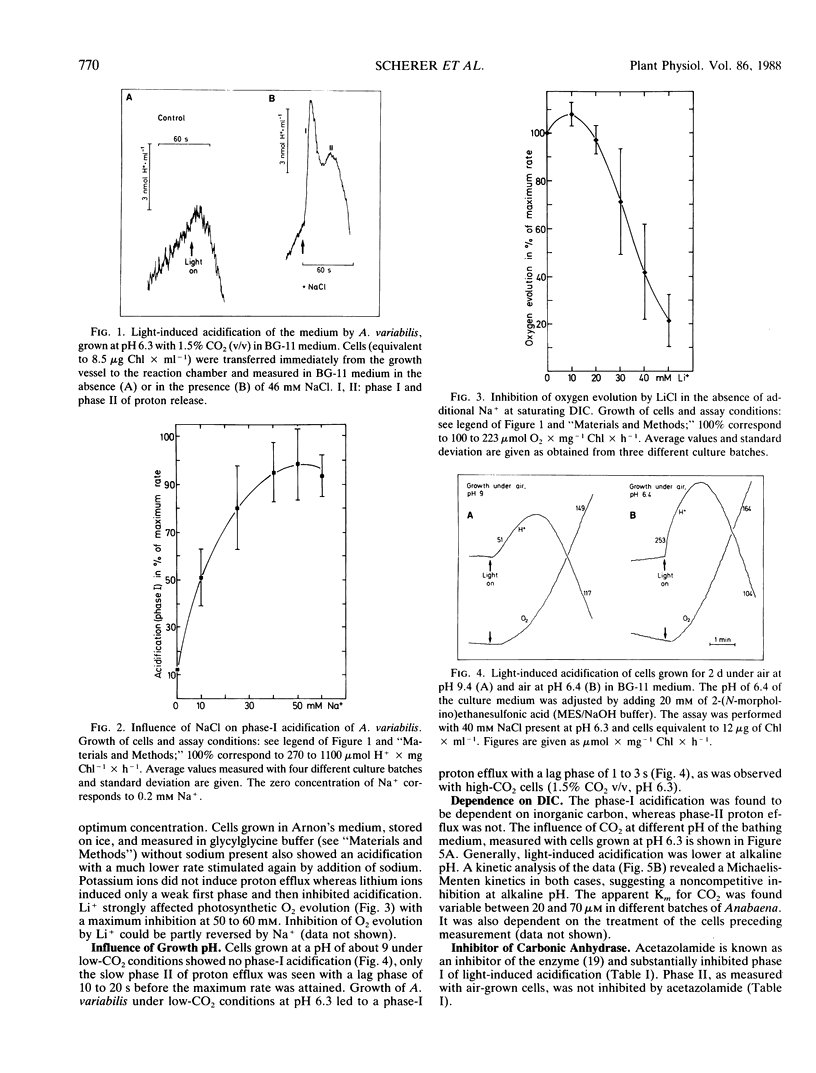
Light-induced acidification by the cyanobacterium Anabaena variabilis is biphasic (a fast phase I and slow phase II) and shown to be sodium-dependent with an optimum concentration of 40 to 60 millimolar Na+. Cells grown under low CO2 concentrations at pH 9 (i.e. mainly HCO3− present in the medium) exhibited the slow phase II of proton efflux only, while cells grown under low CO2 concentrations at pH 6.3 (i.e. CO2 and HCO3− present) exhibited both phases. Light-induced proton release of phase I was dependent on inorganic carbon available in the bathing medium with an apparent Km for CO2 of 20 to 70 micromolar. As was concluded from the CO2 dependence of acidification measured at different pH of the bathing medium, bicarbonate inhibited phase-I acidification noncompetetively. Acidification was inhibited by acetazolamide, an inhibitor of carbonic anhydrase. Apparently, acidification of phase I is due to a light-dependent uptake of CO2 being converted to HCO3− by a carbonic anhydrase-like function of the HCO3−-transport system (M Volokita, D Zenvirth, A Kaplan, L Reinhold 1984 Plant Physiol 76: 599-602) before or during entering the cell, thus releasing one proton per CO2 converted to HCO3−.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Wolosin J. M., Packer L. Na+/H+ exchange in the cyanobacterium Synechococcus 6311. Biochem Biophys Res Commun. 1984 Jul 18;122(1):452–459. doi: 10.1016/0006-291x(84)90497-2. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Zenvirth D., Reinhold L., Berry J. A. Involvement of a Primary Electrogenic Pump in the Mechanism for HCO(3) Uptake by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1982 Apr;69(4):978–982. doi: 10.1104/pp.69.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Hinrichs I., Böger P. Effect of Monochromatic Light on Proton Efflux of the Blue-Green Alga Anabaena variabilis. Plant Physiol. 1986 Jul;81(3):939–941. doi: 10.1104/pp.81.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]


