Abstract
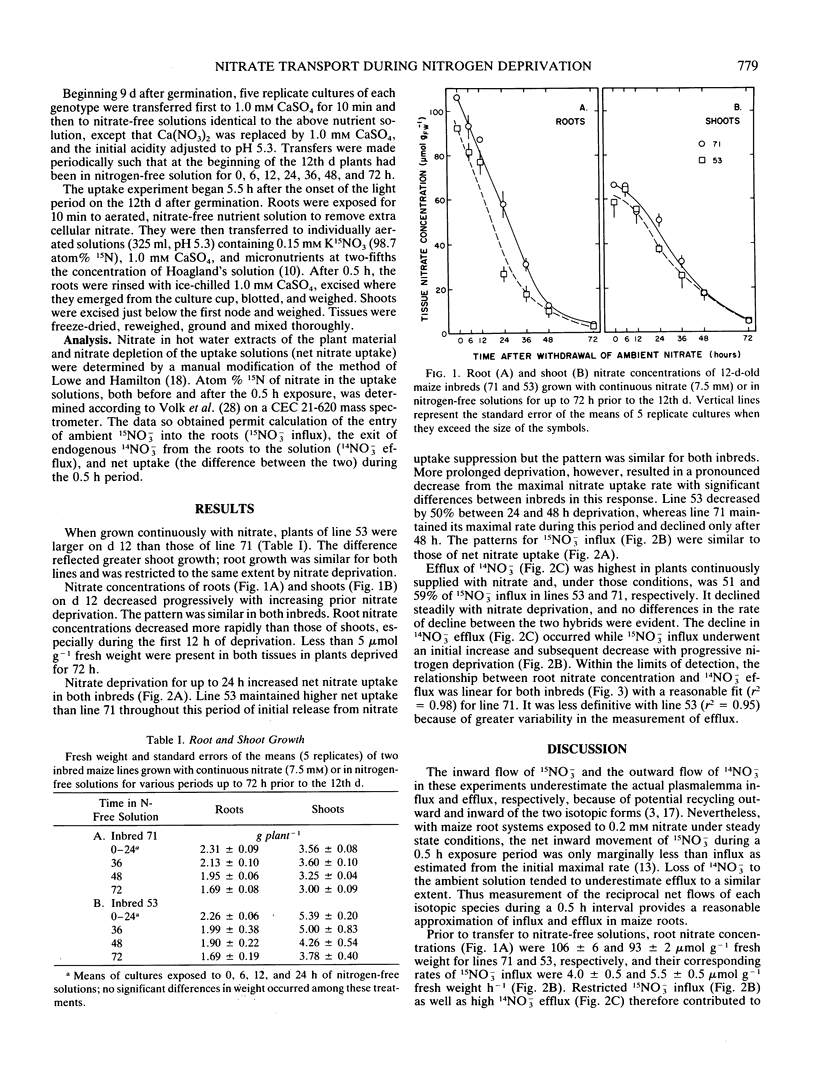
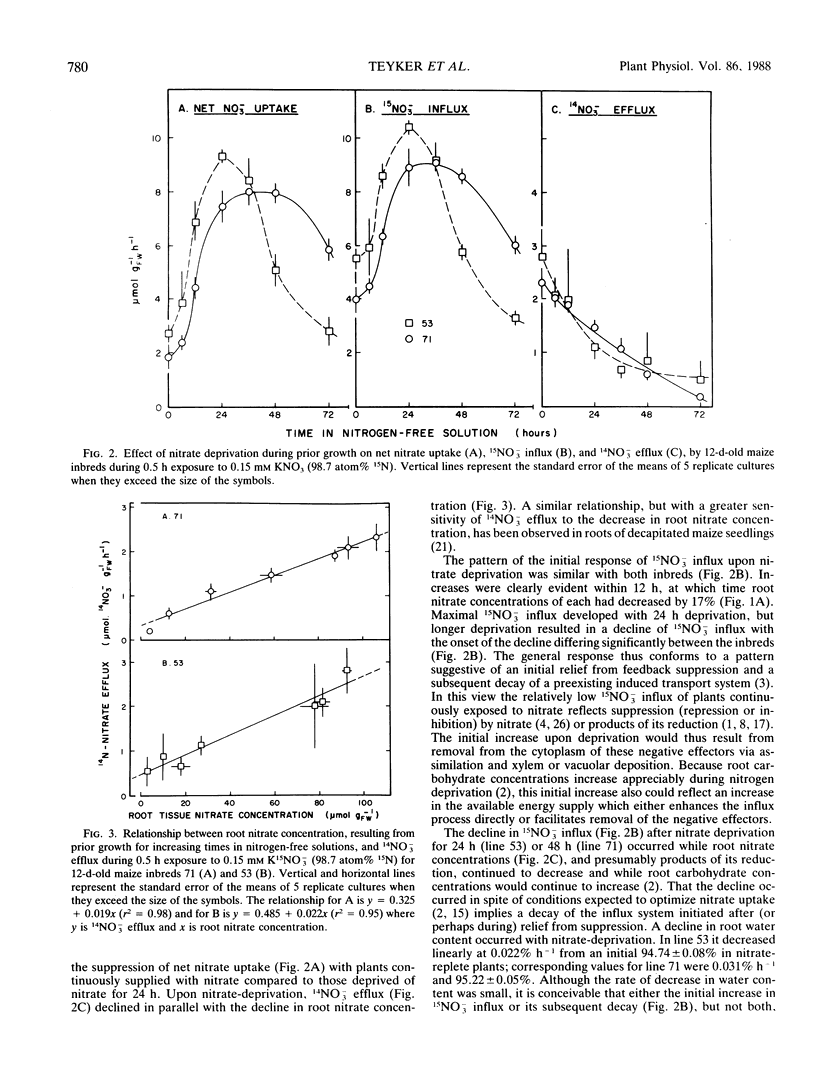
The influence of nitrogen stress on net nitrate uptake resulting from concomitant 15NO3− influx and 14NO3− efflux was examined in two 12-day-old inbred lines of maize. Plants grown on 14NO3− were deprived of nitrogen for up to 72 hours prior to the 12th day and then exposed for 0.5 hour to 0.15 millimolar nitrate containing 98.7 atom% 15N. The nitrate concentration of the roots declined from approximately 100 to 5 micromolar per gram fresh weight during deprivation, and 14NO3− efflux was linearly related to root nitrate concentration. Influx of 15NO3− was suppressed in nitrogen-replete plants and increased with nitrogen deprivation up to 24 hours, indicating a dissipation of factors suppressing influx. Longer periods of nitrogen-deprivation resulted in a decline in 15NO3− influx from its maximal rate. The two inbreds differed significantly in the onset and extent of this decline, although their patterns during initial release from influx suppression were similar. Except for plants of high endogenous nitrogen status, net nitrate uptake was largely attributable to influx, and genetic variation in the regulation of this process is implied.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deane-Drummond C. E., Glass A. D. Short Term Studies of Nitrate Uptake into Barley Plants Using Ion-Specific Electrodes and ClO(3): I. Control of Net Uptake by NO(3) Efflux. Plant Physiol. 1983 Sep;73(1):100–104. doi: 10.1104/pp.73.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D., Thompson R. G., Bordeleau L. Regulation of NO(3) Influx in Barley : Studies Using NO(3). Plant Physiol. 1985 Feb;77(2):379–381. doi: 10.1104/pp.77.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. A., Flesher D., Hageman R. H. Nitrate Uptake by Dark-grown Corn Seedlings: Some Characteristics of Apparent Induction. Plant Physiol. 1973 Jan;51(1):120–127. doi: 10.1104/pp.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackown C. T., Volk R. J., Jackson W. A. Nitrate Accumulation, Assimilation, and Transport by Decapitated Corn Roots : EFFECTS OF PRIOR NITRATE NUTRITION. Plant Physiol. 1981 Jul;68(1):133–138. doi: 10.1104/pp.68.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Volk R. J., Jackson W. A. Simultaneous Influx and Efflux of Nitrate during Uptake by Perennial Ryegrass. Plant Physiol. 1973 Feb;51(2):267–272. doi: 10.1104/pp.51.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Volk R. J., Jackson W. A. p-Fluorophenylalanine-Induced Restriction of Ion Uptake and Assimilation by Maize Roots. Plant Physiol. 1985 Mar;77(3):718–721. doi: 10.1104/pp.77.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomino Y., Sakai H., Woodroffe A. J., Clarkson A. R. Studies on glomerular immune solubilization by complement in patients with IgA nephropathy. Acta Pathol Jpn. 1987 Nov;37(11):1763–1767. doi: 10.1111/j.1440-1827.1987.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Volk R. J., Pearson C. J., Jackson W. A. Reduction of plant tissue nitrate to nitric oxide for mass spectrometric 15N analysis. Anal Biochem. 1979 Aug;97(1):131–135. doi: 10.1016/0003-2697(79)90336-1. [DOI] [PubMed] [Google Scholar]


