Abstract
Renal fibrosis relies on multiple proteins and cofactors in its gradual development. Copper is a cofactor of many enzymes involved in renal microenvironment homeostasis. We previously reported that intracellular copper imbalance occurred during renal fibrosis development and was correlated with fibrosis intensity. In this study, we investigated the molecular mechanisms of how copper affected renal fibrosis development. Unilateral ureteral obstruction (UUO) mice were used for in vivo study; rat renal tubular epithelial cells (NRK-52E) treated with TGF-β1 were adapted as an in vitro fibrotic model. We revealed that the accumulation of copper in mitochondria, rather than cytosol, was responsible for mitochondrial dysfunction, cell apoptosis and renal fibrosis in both in vivo and in vitro fibrotic models. Furthermore, we showed that mitochondrial copper overload directly disrupted the activity of respiratory chain complex IV (cytochrome c oxidase), but not complex I, II and III, which hampered respiratory chain and disrupted mitochondrial functions, eventually leading to fibrosis development. Meanwhile, we showed that COX17, the copper chaperone protein, was significantly upregulated in the mitochondria of fibrotic kidneys and NRK-52E cells. Knockdown of COX17 aggravated mitochondrial copper accumulation, inhibited complex IV activity, augmented mitochondrial dysfunction and led to cell apoptosis and renal fibrosis, whereas overexpression of COX17 could discharge copper from mitochondria and protect mitochondrial function, alleviating renal fibrosis. In conclusion, copper accumulation in mitochondria blocks complex IV activity and induces mitochondrial dysfunction. COX17 plays a pivotal role in maintaining mitochondrial copper homeostasis, restoring complex IV activity, and ameliorating renal fibrosis.
Keywords: renal fibrosis, mitochondria, copper, COX17, cytochrome c oxidase, NRK-52E cells
Introduction
Renal fibrosis is a common pathway associated with progressing chronic kidney disease (CKD) to end-stage kidney disease [1], and many pathogens have been correlated with renal fibrosis development. The extracellular matrix or microenvironment of renal fibrotic cells may play a critical role in fibrosis development. We previously reported that an intracellular copper overload activated lysyl oxidase (LOX), which enhanced cross-linking of collagen and elastin and promoted renal fibrosis.
Copper is a cofactor for many extracellular enzymes, such as LOX in cells [2, 3]. In recent years, studies have shown that copper accumulation is associated with fibrosis [4–6]. Tadakamadla et al. demonstrated that copper levels in the serum of oral submucous fibrosis patients increase and correlate with the severity of fibrosis [7]. Brewer et al. revealed that an anti-copper drug therapy effectively ameliorates bleomycin-induced pulmonary inflammation and fibrosis [8]. Our previous study indicated that intracellular copper accumulation is implicated in renal fibrosis and using a copper chelator ameliorates renal fibrosis [9].
Mitochondria produce ATP via oxidative phosphorylation [10]. This organelle is the vital copper reservoir and the main intracellular copper utiliser for copper-dependent enzymes in mitochondria, such as cytochrome c oxidase (COX) and superoxide dismutase 1 (SOD1) [11]. Renal tubule cells are enriched with mitochondria because of their heavy demands for ATP to maintain body fluid and pH balance [12]. Studies have shown that mitochondrial dysfunction is related to cell apoptosis, which can stimulate fibrotic outcomes and lead to renal fibrosis [13–15].
In this study, we report the accumulation of copper in mitochondria as a leading cause of disruption of complex IV activity, mitochondrial dysfunction, and cellular apoptosis. More importantly, the expression of copper chaperone protein COX17 increased significantly in the mitochondria of fibrotic kidneys. Downregulation of COX17 increased mitochondria copper accumulation, decreased respiratory chain complex IV activity and aggravated cell apoptosis and renal fibrosis, whereas overexpression of COX17 reversed these conditions. Thus, COX17 plays a pivotal role in maintaining mitochondrial copper homeostasis, which restores complex IV activity and ameliorates renal fibrosis.
Materials and methods
Human kidney tissues
Human kidney tissues used in this study were residual biopsy samples from the Department of Nephrology, Tongji Hospital, Shanghai, China. Control samples were kidney tissues obtained from patients with minimal change disease with no fibrosis. Fibrotic kidney tissues were obtained from CKD patients with IgA nephropathy or diabetic nephropathy. The use of all patient biopsy samples was approved by the Human Subjects Committee of Tongji Hospital (No. K-W-2021-012). Informed consent was approved by all participants.
Animals
C57BL/6 mice aged 6 to 8 weeks were used for the in vivo analysis. All animals were kept in the animal room of Tongji Hospital, affiliated with Tongji University, and group-housed under a constant temperature (23 ± 2 °C) and humidity (40%–60%), with free access to water ad libitum. All animal procedures and protocols were approved by the Ethics Committee of Tongji University School of Medicine (No. 2020-DW-003).
Mice were subjected to UUO surgery as described previously [16]. Briefly, after anaesthesia, the left ureter of mice were exposed and ligated by surgical 4-0 silk sutures. Sham-operated mice underwent the same surgery without ligation of the left ureter. Mice (n = 5 per group) were killed on d 14 after UUO for biochemical and histological analyses. Mice were treated with TM (0.7 mg/d, Sigma-Aldrich) [17, 18] via gavage once a day for 14 d after the UUO operation to chelate copper. In addition, mice were subjected to UUO surgery and killed at d 3, 7 or 14 for histological analysis.
Adeno-associated virus-treated mice
The adeno-associated viruses harbouring shRNA targeting the COX17 gene (AAV9-shCOX17) were constructed by Hanheng (Shanghai, China). An empty AAV9 vector (AAV9-Con) was used as the control. All mice were randomly divided into four groups (n = 5 per group): sham-operated mice treated with AAV9-Con (Sham + AAV9-Con), sham-operated mice treated with AAV9-shCOX17 (Sham + AAV9-shCOX17), UUO mice (14 d) treated with AAV9-Con (UUO14d + AAV9-Con) and UUO mice (14 d) treated with AAV9-shCOX17 (UUO14d + AAV9-shCOX17). COX17 overexpression adeno-associated viruses (AAV-COX17) were also constructed by Hanheng. The viruses were delivered by tail injection, and the animals were sacrificed 14 d after surgery for sample harvesting [19].
Cell culture and treatment
Rat renal tubular epithelial cells (NRK-52E) were purchased from the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM containing 4% fetal bovine serum (FBS) at 37 °C in a 5% CO2 air incubator. NRK-52E cells were treated with 5 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN) with or without TM treatment. Overexpression of COX17 was performed by infection with LV-COX17. A COX17 shRNA plasmid was used to generate a lentivirus, and the virus was transferred into NRK-52E cells to knockdown COX17. Puromycin was used to screen out cells successfully transfected with the lentivirus.
Western blotting
NRK-52E cells and kidney tissues were lysed in cold protein lysis buffer, and their protein concentrations were measured using the Bradford assay. Protein samples were analysed via SDS-PAGE, transferred to a polyvinylidene fluoride (PVDF) membrane and blocked in 5% skim milk for 1 h. The membranes were then incubated with primary antibodies overnight at 4 °C and a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The immune complexes were visualised using ECL detection reagents according to the manufacturer’s protocol. The following primary antibodies were used: anti-GAPDH (Santa Cruz, #sc-137179), anti-COX IV (Proteintech, #11242-1-AP), anti-Collagen I (Abcam, #ab-21286), anti-alpha smooth muscle actin (α-SMA; Abcam, #124964), anti-COX17 (Santa Cruz, #sc-100521), anti-cleaved caspase 3 (Cell Signalling Technology, #9664), anti-cleaved caspase 9 (Bioss, #bs-20773R), anti-caspase 3 (Abcam, #ab-184787) and anti-caspase 9 (Abcam, #ab-184786).
Reverse transcription and quantitative real-time PCR
Total RNA was extracted from cells and kidney tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions [20]. RNA was reverse transcribed to cDNA using the cDNA Archival Kit (Applied Biosystems). Subsequently, cDNA was used as a template and real-time PCR was performed using SYBR Green PCR Master Mix (Roche, Mannheim, Germany). Results were calculated using the 2–ΔΔCt method. The primers used in this study targeted mouse GAPDH, collagen I and α-SMA (Table 1) and rat COX17 and GAPDH (Table 2).
Table 1.
Primer sequences of mouse for real-time RT-PCR.
| Name(mouse) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | GTCTTCACTACCATGGAGAAGG | TCATGGATGACCTTGGCCAG |
| α-SMA | CAGCGGGCATCCACGAA | GCCACCGATCCAGACAGA |
| Collagen I | TGGACTTCCTGGTCCTCCTG | AGGCACGGAAACTCCAGC |
Table 2.
Primer sequences of rat for real-time RT-PCR.
| Name (rat) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | GGCAAGTTCAACGGCACA | CCATTTGATGTTAGCGGGAT |
| COX17 | GAGGCTCAGGAGAAGAAGCC | TTGTGGGCCTCGATGAGATG |
Histology and immunohistochemistry
Paraffin-embedded kidney sections (4-mm thickness) were prepared according to a standard procedure. Masson trichrome and HE staining were performed using a standard protocol [21]. For immunohistochemical staining, the tissue sections were deparaffinized and hydrated in ethyl alcohol. Antigen retrieval was carried out in sodium citrate buffer (pH 6.0) for 10 min by microwaving the sections. Subsequently, endogenous peroxidase activity was blocked using 3% hydrogen peroxidase for 10 min. The sections were then blocked in 5% goat serum for 60 min at room temperature and incubated with the primary antibody against COX17 (Proteintech, #11464-1-AP). After washing, the sections were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Staining was visualised under a light microscope using a 3,3′-diaminobenzidine (DAB) kit (Vector, #SK4100).
Kidney cryosections were fixed with 4% paraformalin for 30 min at room temperature and blocked with 5% goat serum for immunofluorescence staining. The primary antibodies used targeted TOM20 (mitochondrial marker; Proteintech, #11802-1-AP) and COX17 (Santa Cruz, #sc-100521). The sections were then incubated with Alexa Fluor-conjugated secondary antibody (Invitrogen, #Alexa Fluor 568-A11011, Alexa Fluor 488-A11029). The sections were visualised with a Zeiss confocal microscope (LSM510, Germany).
TUNEL assay
Kidney tissues were fixed in formalin and embedded in paraffin. TUNEL staining was carried out using the One Step TUNEL Apoptosis Assay Kit (Beyotime, Jiangsu, China), and the nuclei were stained with DAPI. Staining was visualised under a fluorescence microscope (DMI400B, Leica Company, Germany).
Transmission electron microscopy
We used transmission electron microscopy (TEM) to assess the mitochondrial structure. Kidney cortexes and NRK-52E cells were fixed with 2.5% glutaraldehyde/0.1 M phosphate buffer, dehydrated and embedded in Epon according to routine procedures. Mitochondria were examined using an electron microscope (JEOL JEM-1010, Tokyo, Japan).
mRNA sequencing and analysis
Total RNA was extracted from three control and three severe fibrotic kidney biopsy specimens using TRIzol reagent, according to a routine protocol. The mRNA from total RNA was further purified using an mRNA Purification Kit (Invitrogen, #61006). cDNA libraries were generated for each sample using the Collibri Stranded RNA Library Prep Kit (Invitrogen, #a38994024) following the manufacturer’s procedures. The cDNA libraries were sequenced using an Illumina platform. Library construction and RNA sequencing were performed at GENEWIZ Technology Corporation (SuZhou, China). Another 53 CKD kidney biopsy specimens were selected from the GSE66494 dataset.
Raw sequencing data were examined for quality issues using FastQC, which can provide GC content biases, adapter content and overrepresented sequences for quality profiling. Trimmomatic was used to trim and filter low-quality reads [22]. A STAR spliced read aligner was used to map the clean reads to the Homo sapiens reference genome GRCh38.p13 [23]. The fold-change in gene expression between the control and fibrotic kidney tissues was estimated using the GFOLD algorithm [24].
Metal content determination
Copper levels in subcellular fractions were analysed via inductively coupled plasma–mass spectrometry (ICP–MS) (NexION 300D, PerkinElmer, USA). The samples were wet-washed with 65% nitric acid and diluted with ultrapure water. We also used standard samples for quantification [25, 26].
Isolation of mitochondria
Mitochondria from the kidney cortex were isolated using a commercial Mitochondria Isolation Kit for Tissue (Abcam, #ab-110168) according to the manufacturer’s protocol. Mitochondria from NRK-52E cells were isolated using a Mitochondria Isolation Kit for Cultured Cells (Abcam, #ab-110170) following the manufacturer’s instructions. Isolated mitochondria were resuspended in mitochondrial storage buffer (included in the product kit), and the protein concentration in the isolated mitochondria was assessed via the BCA method.
Mitochondrial superoxide ROS (mtROS) detection
mtROS production was assessed by incubating freshly prepared snap-frozen sections and cells with 5 µM MitoSOX Red fluorescent probe (M36008, Invitrogen, USA) for 10 min at 37 °C in darkness [27, 28]. Images were examined under a Zeiss confocal microscope.
Detection of ATP
ATP levels in the cells and kidney tissues were determined using a luciferase-based luminescence ATP assay kit (Beyotime Institute of Biotechnology) according to the manufacturer’s protocol. Cells and kidney tissues were homogenised in ATP-releasing buffer and centrifuged at 12,000 × g and 4 °C for 5 min. Then, 20 μL of supernatant was mixed with 100 μL of the detection working solution in 96-well plates. The luminance was measured and normalised to the protein concentration.
Respiratory chain complex enzymatic activity
Mitochondrial respiratory chain complex activity was assessed, as described previously (40). Briefly, the respiratory chain complex I-II activities were measured using a commercial assay kit (Solarbio, # BC0515 and # BC3235, respectively) according to the manufacturer’s instructions. The activity of complex I (NADH dehydrogenase) was measured by monitoring the absorbance at 340 nm. Complex II (succinate dehydrogenase) produced ubiquinol, which was coupled to reduce the dye DCPIP, and the activity of complex II was measured by monitoring the absorbance of DCPIP at 605 nm. The activity of complex III (cytochrome c reductase) was determined using a commercial assay kit (Solarbio, # BC3240) according to the manufacturer’s protocol. The activity of complex III was measured by monitoring the absorbance at 550 nm. The activity of complex IV (cytochrome c oxidase) was determined using a commercial assay kit (CYTOCOX1, Sigma-Aldrich, St Louis, MO, USA) following the manufacturer’s protocol. The activity of complex IV was measured by monitoring the absorbance at 550 nm. Enzyme activities are presented as the fold change of reaction rates compared to the control group.
Protein structure
The protein structures of COX1 and COX2 were downloaded from the AlphaFold2 modelling results of the UniProt website [29], where the UniProt number of COX1 is P05503 and the UniProt number of COX2 is P00406. The PDB file (PDB ID: 1OCC) was selected as a reference for positioning copper ions in the COX crystal structure. COX1-Cu1+(1) and COX2-Cu1+(2) protein structures were then established for MD simulations.
Molecular dynamics simulations
The CuB metal active centre in the subunit I of COX comprises a single copper ion, whereas the CuA centre in the subunit II of COX contains two copper ions [30]. The COX1-Cu1+(1) and COX2-Cu1+(2) protein structures were established as the initial conformation for MD simulations. We used Gromacs2019.6 as the dynamics simulation software [31] and GROMOS96 54a7 as the dynamic force field [32]. The TIP3P water model was used along with NaCl. An extra one or two copper ions were separately added to COX1 and COX2 systems to construct two experimental simulation systems, namely COX1-Cu1+(2) and COX2-Cu1+(4). COX1-Cu1+(1) and COX2-Cu1+(2) were the control groups. The simulated copper ion content was increased two-fold in the experimental group. The van der Waals interactions were smoothly switched off over 14 Å by a force-based switching function. Long-range electrostatic interactions were calculated by the particle-mesh Ewald method with a mesh size of 12 Å. NVT dynamics was applied during the equilibration run with a 1 fs time step for 50,000 ps. Thereafter, 2 fs time steps for 100 ns were used for production simulations. Root mean square deviations (RMSDs) were used to observe the allosteric situation of local sites. The radius of gyration (Rg) was used to evaluate the closeness of an architecture. The solvent-accessible surface area (SASA) was calculated to observe the solvent accessible surface area. Root mean square fluctuations (RMSFs) were determined to examine the allosteric situation of local sites.
Determination of blood urea nitrogen and serum creatinine
Blood urea nitrogen (BUN) and serum creatinine were examined by an automatic chemistry analyser. The serum creatinine and BUN levels are expressed as μmol/L and mmol/L, respectively.
Statistical methods
All data examined are presented as the mean ± SEM. Student’s t-test was used to analyse differences between two groups. One-way analysis of variance (ANOVA) was performed to determine differences among multiple groups. Differences at P < 0.05 were considered statistically significant. SPSS 21.0 statistical software (Chicago, IL, USA) was used for statistical analyses.
Results
Mitochondrial copper chaperone COX17 is upregulated in renal interstitial fibrosis
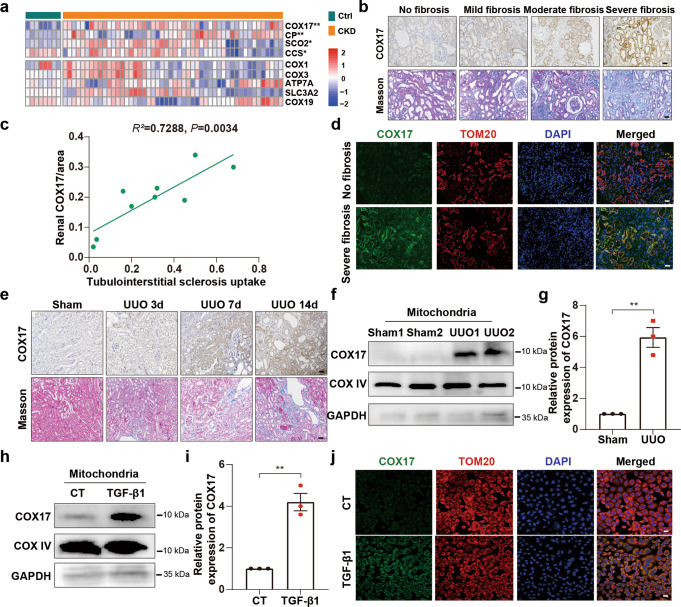
We screened for copper transporter proteins and analysed the transcriptome RNA sequence of human CKD kidney biopsy specimens to identify the gene responsible for intracellular copper homeostasis in renal fibrosis. Notably, expression of the copper chaperone COX17 increased significantly in severely fibrotic tissue (Fig. 1a). COX17 expression positively correlated with tubulointerstitial fibrosis (Fig. 1b). Quantitative analysis of COX17 staining positively correlated with quantification of the Masson’s trichrome-positive areas in patients (R2 = 0.7288, P = 0.0034) (Fig. 1c). COX17 co-stained with mitochondrial marker TOM20, indicating that this protein is primarily a protein located in the mitochondria (Fig. 1d). Moreover, COX17 expression was increased at d 3 of unilateral ureteral obstruction (UUO) and was continuously upregulated in a time-dependent manner, which correlated with collagen deposition (Fig. 1e). Mitochondrial COX17 was upregulated significantly in both the UUO 14d model and NRK-52E cells treated with TGF-β1 (Fig. 1f–j).
Fig. 1. COX17 is upregulated in renal interstitial fibrosis.
a Heatmap comparing copper-related gene expression in the kidneys of patients with chronic kidney disease (CKD) with that in control subjects (n = 53). b Immunohistochemical staining of COX17 and Masson staining in kidney sections with different fibrotic degrees. Original magnification, ×200. Bar = 50 μm. c Scatter plots with linear regression show the correlation between COX17 expression levels and the extent of renal fibrosis (R2 = 0.7288, P = 0.0034). d Immunofluorescence co-staining of TOM20 (mitochondrial marker, red) and COX17 (green). Original magnification, ×200. Bar = 20 μm. e Immunohistochemical staining of COX17 and Masson staining in kidney sections. Original magnification, ×200. Bar = 50 μm. f Western immunoblots and g densitometric analysis of COX17 expression in the mitochondria from sham and UUO kidney tissues (n = 5). h Western immunoblots and i densitometric analysis showing COX17 expression in the mitochondria of NRK-52E cells treated with TGF-β1. j Immunofluorescence for COX17 and TOM20, showing COX17 expression in the mitochondria of NRK-52E cells stimulated with TGF-β1. Original magnification, ×200. Bar = 20 μm. Each bar represents the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01.
Downregulation of COX17 aggravates apoptosis and renal fibrosis
Differentially expressed genes in CKD patients with severe fibrosis were identified by RNA sequencing (Fig. S1a). Our results showed that signalling pathways associated with energy reserve metabolic processes and regulating the generation of precursor metabolites and energy were downregulated in severely fibrotic kidneys (Fig. S1b). We also found dramatic mitochondrial swelling with a loss of cristae in fibrotic kidney tissues of human specimens (Fig. S1c).
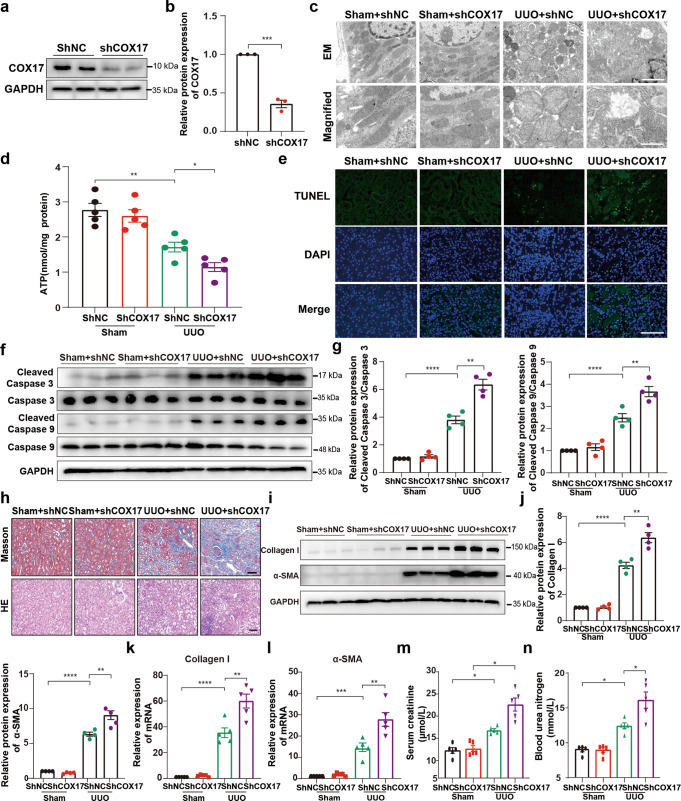
Mitochondria play a pivotal role in apoptosis, and mitochondrial dysfunction contributes to cell apoptosis [33], which can stimulate fibrotic outcomes directly or indirectly [15]. To explore the role of COX17 in mitochondrial dysfunction, induction of cell apoptosis and renal fibrosis development, we depleted COX17 by delivering AAV9-shCOX17 into UUO mice. AAV9-shCOX17 depleted COX17 efficiently (Fig. 2a, b). Renal tubular cell mitochondrial swelling with disorganised, fragmented cristae deteriorated in COX17-shRNA-treated kidneys (Fig. 2c). ATP content was lower in the UUO model treated with AAV9-shCOX17 (Fig. 2d). Furthermore, downregulation of COX17 augmented renal apoptosis, as shown by higher TUNEL-positive cells and increased expression of cleaved caspase 3 and cleaved caspase 9 (Fig. 2e–g). Consistently, the downregulation of COX17 markedly exacerbated collagen deposition in the fibrotic mouse model (Fig. 2h–l). Moreover, serum creatinine and BUN levels increased markedly upon COX17 depletion (Fig. 2m, n). We also constructed an adeno-associated virus (AAV) to overexpress COX17 (Fig. S2a, b) and found that collagen deposition in fibrotic kidneys was alleviated after COX17 overexpression (Fig. S2c–e). Taken together, these results confirmed the critical role of COX17 in restricting renal fibrosis.
Fig. 2. Downregulation of COX17 in vivo aggravates renal fibrosis.
a Western blot and b densitometric analysis showing COX17 expression in kidney tissues transfected with AAV9-shCOX17 or AAV9-Con. c Representative TEM images of mitochondria in tubular cells at low magnification (upper panel: magnified ×7000; bar = 2 μm) and high magnification (bottom panel: magnified ×15,000; bar = 1 μm). d Quantification of ATP levels (n = 5). e Representative images for TUNEL staining of the kidney tissues. Original magnification, ×400. Bar = 100 μm. f Western blot and g densitometric analysis of the expression of caspase 3, caspase 9, cleaved caspase 3 and cleaved caspase 9 in different groups. h Masson and HE staining of renal sections from the indicated groups. Original magnification, ×200. Bar = 50 μm. i Western blot and j densitometric analysis of the expression of collagen I and α-SMA in different groups. k RT–PCR analysis of collagen I and l α-SMA in different groups. m Serum creatinine and n urea nitrogen levels in different groups (n = 5). Data are the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Downregulation of COX17 exacerbates TGF-β1 induced-mitochondrial dysfunction and cell apoptosis
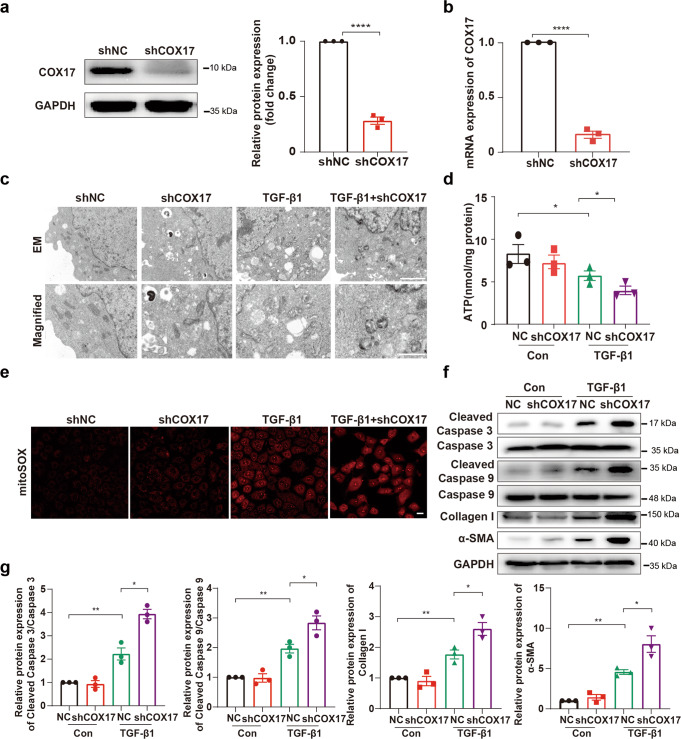
To further confirm the role of COX17 in mitochondrial function and cell apoptosis, we generated stable COX17 knockdown cell lines and treated them with TGF-β1. As shown in Fig. 3a, b, COX17 levels drastically decreased in the cell lines with stable COX17 downregulation. We then found that mitochondrial shrinkage and disorganised cristae induced by TGF-β1 were aggravated in COX17 knockdown cells (Fig. 3c). Furthermore, downregulation of COX17 decreased intracellular ATP content and increased mtROS generation (Fig. 3d, e). In addition, TGF-β1-induced expression of cleaved caspase 3, cleaved caspase 9, collagen I and α-SMA increased significantly in COX17 knockdown cells (Fig. 3f, g). Together, these data indicated that deficiency of COX17 augments TGF-β1 induced-mitochondrial dysfunction, cell apoptosis and cellular fibrotic changes.
Fig. 3. Downregulation of COX17 aggravates TGF-β1 induced-mitochondrial dysfunction and cell apoptosis.
a Western blot and b RT-PCR showing COX17 expression in COX17 knockdown and control short hairpin RNA (shRNA) cell lines (n = 3). c Representative electron microscopy images of mitochondria in tubular cells among groups, as indicated at low magnification (upper panel: magnified ×7000; bar = 2 μm) and high magnification (bottom panel: magnified ×15,000; bar = 1 μm). d Quantification of intracellular ATP levels (n = 3). e Representative MitoSOX staining micrographs in different groups, as indicated. Original magnification, ×200. Bar = 20 μm. f Immunoblots and g densitometric analysis of caspase 3, caspase 9, cleaved caspase 3, cleaved caspase 9, collagen I and α-SMA expression in tubular cells among different groups. Data are the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01 and ****P < 0.0001.
Overexpression of COX17 ameliorates TGF-β1 induced-mitochondrial dysfunction and cell apoptosis
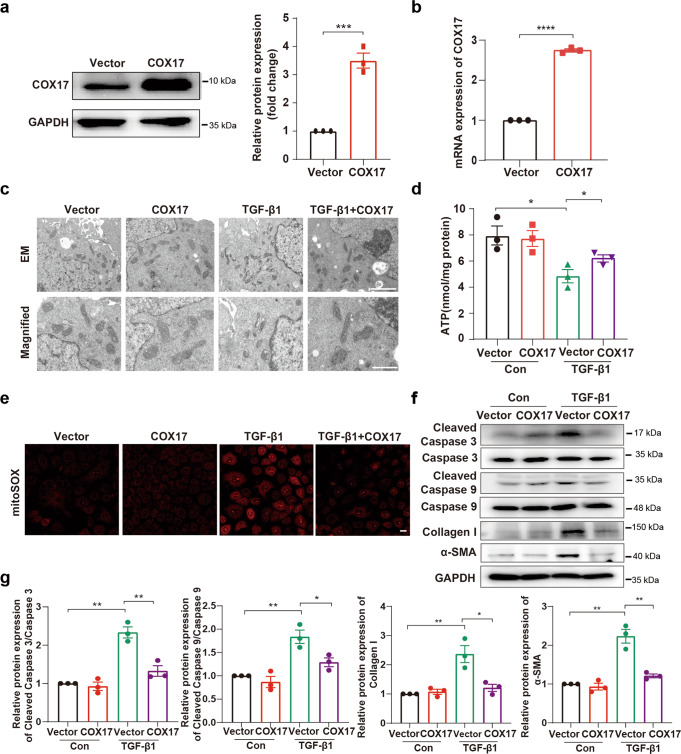
We next studied whether overexpression of COX17 in tubular epithelial cells alleviates mitochondrial dysfunction and cell apoptosis. We generated stable COX17 overexpressing cell lines with an approximately three-fold increase in COX17 expression compared to the control group (Fig. 4a, b). The results showed mitochondrial shrinkage and disorganised cristae induced by TGF-β1 were ameliorated in cells overexpressing COX17 (Fig. 4c). Upregulation of COX17 expression increased intracellular ATP content and decreased mtROS generation (Fig. 4d, e). We also found overexpression of COX17 mitigated cleaved caspase 3, cleaved caspase 9, collagen I and α-SMA expression (Fig. 4f, g). Thus, these results demonstrated that overexpression of COX17 alleviates TGF-β1 induced-mitochondrial dysfunction, cell apoptosis and cellular fibrotic changes.
Fig. 4. Overexpression of COX17 mitigates mitochondrial dysfunction and cell apoptosis.
a, b The efficiency of COX17 overexpression in NRK-52E cells was detected by Western blot and RT-PCR (n = 3). c EM images showing mitochondria in tubular cells of the indicated groups at low magnification (upper panel: magnified ×7000; bar = 2 μm) and high magnification (bottom panel: magnified ×15,000; bar = 1 μm). d Quantification of intracellular ATP levels (n = 3). e Representative MitoSOX staining micrographs in different groups as indicated. f Immunoblots and g densitometric analysis of caspase 3, caspase 9, cleaved caspase 3, cleaved caspase 9, collagen I and α-SMA expression in tubular cells among different groups. Original magnification, ×200. Bar = 20 μm. Data are the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
COX17 maintains mitochondrial copper homeostasis and restores respiratory chain complex IV activity
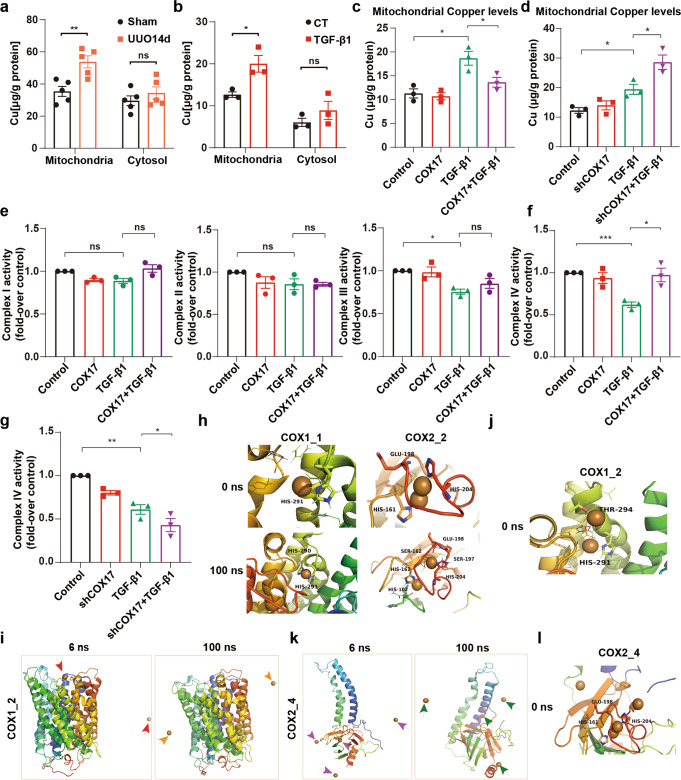
Elevated copper levels in mitochondria of the UUO fibrotic model and tubule cells treated with TGF-β1 (Fig. 5a, b) were consistent with our previous finding that the intracellular copper level increases in renal fibrosis [9]. Further, overexpression of COX17 reversed mitochondrial copper accumulation, whereas downregulation of COX17 augmented mitochondrial copper accumulation (Fig. 5c, d). Because COX17 is essential for the transport of copper into respiratory chain complex IV, also known as COX, we verified that the decline of complex IV activity induced by TGF-β1 was markedly ameliorated by overexpression of COX17. In contrast, the decrease in complex III activity induced by TGF-β1 cannot be restored by COX17 (Fig. 5e, f). Downregulation of COX17 was found to exacerbate the inhibition of complex IV activity (Fig. 5g). Because respiratory chain complex IV is copper sensitive, we further evaluated the effect of mitochondrial copper overload on COX.
Fig. 5. COX17 reduces mitochondrial copper content and restores respiratory chain complex IV activity.
a The copper content was detected in the cytosol and mitochondria in the kidneys of the UUO model (n = 5). b Copper content was detected in the mitochondria of NRK-52E cells after 48 h of TGF-β1 treatment (n = 3). c, d Copper content was detected in the mitochondria of control, COX17-overexpressing and knockdown cell lines treated with TGF-β1 (n = 3). e, f The relative activities of respiratory chain complexes I, II, III and IV in control and COX17-overexpressing cell lines treated with TGF-β1 (n = 3). g The relative activity of respiratory chain complex IV in control and COX17-knockdown cell lines treated with TGF-β1 (n = 3). h MD simulations of COX1 and COX2. i MD simulation of COX1_2. j MD simulation of COX1_2 at 0 ns. k MD simulation of COX2_4. l MD simulation of COX2_4 at 0 ns. Data are the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001. COX1_1, COX1-Cu1+(1); COX1_2, COX1-Cu1+(2); COX2_2, COX2-Cu1+(2); COX2_4, COX2-Cu1+(4). ns indicates no significance.
Firstly, we used molecular dynamics (MD) simulations that had been verified stable and viable (Fig. S3a–g) to determine the combing site of copper ions with COX subunits (COX1, COX2) (Fig. 5h), which were consistent with previous studies [34, 35]. We then simulated the COX copper overload system. We found that copper ions escaped during the initial (6 ns) simulation of the COX1 copper-overload system (Fig. 5i). According to the trajectory, the increase in copper ions strengthened the local positive charge, thus forcing the opening of the pocket of residues 289–305 and therefore decreasing the steric hindrance for the copper ion escape path (Fig. 5j). Similar to the COX1 system, the COX2 copper overload system also experienced ion escape (Fig. 5k). An extra copper ion was found to attract the His204 side chain to break the balance of the pocket, thereby enabling the escape of the native active copper in the pocket (Fig. 5l). These results suggest COX17 may restore respiratory chain complex IV activity by attenuating mitochondrial copper overload.
Confirming that reducing mitochondrial copper accumulation improves mitochondrial dysfunction, cell apoptosis and renal fibrosis
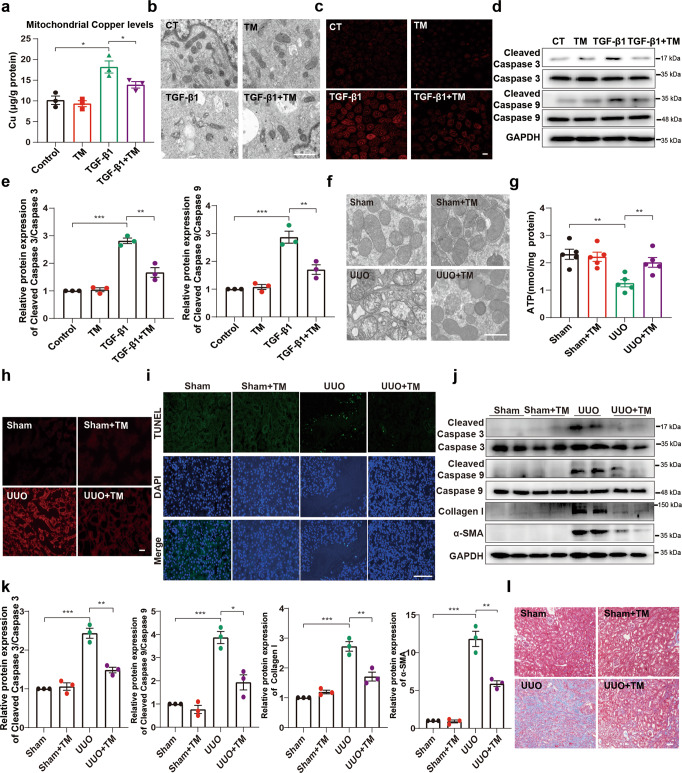
Given that ameliorating mitochondrial copper overload improved mitochondrial respiratory chain complex IV activity, we further verified whether reducing mitochondrial copper accumulation improved mitochondrial dysfunction, cell apoptosis and renal fibrosis. We used the copper chelator tetrathiomolybdate (TM) to reduce mitochondrial copper accumulation in response to TGF-β1 (Fig. 6a). TM markedly restored the deformation of mitochondria and the disappearance of mitochondrial cristae induced by TGF-β1 (Fig. 6b). TM also attenuated mtROS generation and cleaved caspase 3 and cleaved caspase 9 expression (Fig. 6c–e). Consistently, we found that renal tubular cell mitochondrial swelling with disorganised, fragmented cristae, observed in the UUO model, was blocked after treatment with TM (Fig. 6f). TM treatment also improved ATP content and reduced mtROS generation in the UUO model (Fig. 6g, h). In addition, the TUNEL-positive cell number decreased following copper chelation by TM (Fig. 6i). The expression levels of cleaved caspase 3, cleaved caspase 9, collagen I and α-SMA and collagen deposition were markedly attenuated by using copper chelator TM (Fig. 6j–l). Our results indicated that the overload of copper in mitochondria is implicated in mitochondrial dysfunction and cell apoptosis.
Fig. 6. Alleviating mitochondrial copper overload improves mitochondrial dysfunction, cell apoptosis and renal fibrosis.
NRK-52E cells were treated with TGF-β1 (5 ng/mL) or TM (7.5 µmol/L) as indicated. a The copper content was detected in the mitochondria of NRK-52E cells after 48 h of TGF-β1 treatment (n = 3). b Representative electron micrographs of mitochondria in NRK-52E cells (magnified ×15,000; bar = 1 μm). c Representative MitoSOX staining micrographs in different groups as indicated. d Immunoblots and e densitometric analysis of caspase 3, caspase 9, cleaved caspase 3 and cleaved caspase 9 expression in tubular cells among different groups. f Representative TEM images of mitochondria in different groups (magnified ×15,000; bar = 1 μm). g Quantification of ATP levels (n = 5). h Representative MitoSOX staining micrographs in different groups as indicated. i Representative sections of TUNEL-positive cells. Original magnification, ×400. Bar = 100 μm. j Immunoblot and k densitometric analyses of caspase 3, caspase 9, cleaved caspase 3, cleaved caspase 9, collagen I and α-SMA among different groups. l Masson staining of renal sections from the indicated groups. Original magnification, ×200. Bar = 50 μm. Each bar represents the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001.
Discussion
In this study, there are two main highlights. (1) Copper accumulation in mitochondria leads to renal fibrosis. (2) COX17 alleviates renal fibrosis by attenuating copper accumulation in mitochondria.
The native activity of complex IV in the respiratory chain reaction largely depends on its cofactor copper in mitochondria. A slight change in the copper level disrupts complex IV activity in the electron transport chain. Therefore, this disruption may explain why an elevated intracellular copper level promotes renal fibrosis. Moreover, COX17 was found to ameliorate renal fibrosis by improving mitochondrial function via maintaining mitochondrial copper homeostasis to restore complex IV activity.
As a copper chaperone, the transport of copper by COX17 mainly consists of two parts: transferring copper afflux or efflux from mitochondria and transporting copper to the respiratory chain inside mitochondria. Controversy remains over transferring copper afflux or efflux from mitochondria by COX17. COX17 was initially hypothesised to ferry cytosolic copper to mitochondria because COX17 is localised in both the cytosol and mitochondrial intermembrane space [36]. However, Andrew et al. demonstrated that the function of COX17 was confined to the mitochondrial intermembrane space [37]. Wang et al. found that siRNA targeting COX17 increased the mitochondrial copper concentration in human umbilical vein endothelial cells significantly [38]. Recently, Singh et al. also reported that siRNA targeting COX17 increased copper accumulation in mitochondria, suggesting COX17 participates in copper efflux from mitochondria [39]. Consistent with these observations, our results showed downregulation of COX17 aggravated an increase in mitochondrial copper concentration induced by TGF-β1 in renal tubular epithelial cells, whereas overexpression of COX17 attenuated an increase in mitochondrial copper content. We revealed that COX17 might be involved in copper release from mitochondria and play a vital role in maintaining mitochondrial copper homeostasis under pathological situations.
In mitochondria, COX17 accepts copper from the copper ligand (CuL) and is required for loading copper onto the mitochondrial respiratory chain complex IV, which is the only complex activated by copper in the respiratory chain, leading to ATP production [40, 41]. Previous studies showed that copper overload impairs complex IV activity [42–44]. Excessive copper ions may affect the stability of the copper ion binding pocket of complex IV, resulting in the escape of copper ions from the binding site and, thus, the eventual inactivation of the complex. COX17 attenuates mitochondrial copper overload to protect mitochondrial function. Complex IV activity was inhibited by downregulation of COX17 and restored by overexpression of COX17 in renal tubular epithelial cells (RTECs) treated with TGF-β1. Therefore, COX17 mitigates mitochondrial injury by attenuating mitochondrial copper overload to restore complex IV activity.
Cuproptosis, a challenging traditional cell death dogma proposed by Tsvetkov et al. recently, is caused by excessive copper sedimentation, which impairs the mitochondrial TCA cycle [45]. In this previous report, cuproptosis was induced by pulse treatment with the Cu2+ ionophore elesclomol, resulting in a 15- to 60-fold increase in intracellular copper levels and the triggering of cell death [45]. However, in our study, we found that only a 1.5- to 2-fold increase in intracellular copper levels caused renal fibrosis [9]. In addition, Tsvetkov et al. reported that excess copper promoted lipoylated protein aggregation and iron-sulfur cluster protein loss in the TCA cycle [45]. In this study, we showed that copper overload directly affects the activity of respiratory chain complex IV. Our results suggest that some degree of copper accumulation in RTECs is sufficient to impair complex IV activity and induce mitochondria impairment.
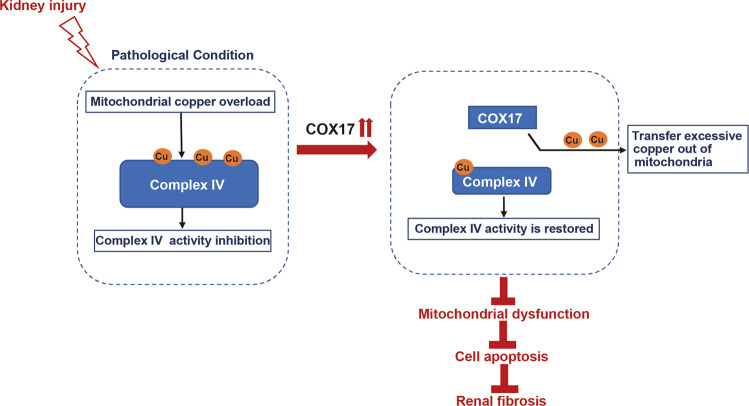
In conclusion, this study highlights that mitochondrial copper is overloaded in fibrotic kidneys and involved in disrupting respiratory chain complex IV activity, mitochondrial dysfunction, and cell apoptosis. COX17 functions in an antifibrotic manner in kidneys by attenuating mitochondrial copper overload and restoring complex IV activity (Fig. 7).
Fig. 7. Diagram depicts the role of COX17 in renal fibrosis.
This study highlights that mitochondrial copper is overloaded in fibrotic kidneys and involved in disrupting respiratory chain complex IV activity, mitochondrial dysfunction, and cell apoptosis. The antifibrotic activity of COX17 in kidneys attenuates mitochondrial copper overload and restores complex IV activity.
Supplementary information
Author contributions
CY, SYZ and YYN designed the study; SYZ performed the experiments; SYZ, WQZ and XL analysed and interpreted the results; SYZ wrote and edited the manuscript. WQZ and CZ assisted with the main experiments. YYZ provided essential reagents and techniques for this study and reviewed the manuscript. CY, SYZ and YYN approved the final version of the manuscript. CY conceived and supervised the study.
Funding
This study was supported by the National Natural Science Foundation of China (No 81873609, 82170696, 82000673).
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01098-3.
References
- 1.Panizo S, Martinez-Arias L, Alonso-Montes C, Cannata P, Martin-Carro B, Fernandez-Martin JL, et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci. 2021;22:408–26. [DOI] [PMC free article] [PubMed]
- 2.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr Opin Chem Biol. 2010;14:211–7. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 4.Janssen R, de Brouwer B, von der Thusen JH, Wouters EFM. Copper as the most likely pathogenic divergence factor between lung fibrosis and emphysema. Med Hypotheses. 2018;120:49–54. doi: 10.1016/j.mehy.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Yadav A, Kumar L, Misra N, Deepak U, Shiv GC. Kumar, Estimation of serum zinc, copper, and iron in the patients of oral submucous fibrosis. Natl J Maxillofac Surg. 2015;6:190–3. doi: 10.4103/0975-5950.183851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirksen K, Fieten H. Canine copper-associated hepatitis. Vet Clin North Am Small Anim Pract. 2017;47:631–44. doi: 10.1016/j.cvsm.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Tadakamadla J, Kumar S, Gp M. Evaluation of serum copper and iron levels among oral submucous fibrosis patients. Med Oral Patol Oral Cir Bucal. 2011;16:e870–3. doi: 10.4317/medoral.17083. [DOI] [PubMed] [Google Scholar]
- 8.Brewer GJ, Dick R, Ullenbruch MR, Jin H, Phan SH. Inhibition of key cytokines by tetrathiomolybdate in the bleomycin model of pulmonary fibrosis. J Inorg Biochem. 2004;98:2160–7. doi: 10.1016/j.jinorgbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Niu YY, Zhang YY, Zhu Z, Zhang XQ, Liu X, Zhu SY, et al. Elevated intracellular copper contributes a unique role to kidney fibrosis by lysyl oxidase mediated matrix crosslinking. Cell Death Dis. 2020;11:211. doi: 10.1038/s41419-020-2404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Pracheil T, Thornton J, Liu Z. Adenosine Triphosphate (ATP) is a candidate signaling molecule in the mitochondria-to-nucleus retrograde response pathway. Genes (Basel) 2013;4:86–100. doi: 10.3390/genes4010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zischka H, Einer C. Mitochondrial copper homeostasis and its derailment in Wilson disease. Int J Biochem Cell Biol. 2018;102:71–5. doi: 10.1016/j.biocel.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–77. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–12. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson A, DiPietro LA. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J. 2013;27:3893–901. doi: 10.1096/fj.12-214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy. 2016;12:976–98. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu PY, Yen HH, Yang TH, Su CC. Tetrathiomolybdate, a copper chelator inhibited imiquimod-induced skin inflammation in mice. J Dermatol Sci. 2018;92:30–7. doi: 10.1016/j.jdermsci.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Brewer GJ, Ullenbruch MR, Dick R, Olivarez L, Phan SH. Tetrathiomolybdate therapy protects against bleomycin-induced pulmonary fibrosis in mice. J Lab Clin Med. 2003;141:210–6. doi: 10.1067/mlc.2003.20. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Jiang W, Zhao H, He C, Tang X, Xu S, et al. PSTPIP2 inhibits cisplatin-induced acute kidney injury by suppressing apoptosis of renal tubular epithelial cells. Cell Death Dis. 2020;11:1057. doi: 10.1038/s41419-020-03267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Wang Y, Niu Y, Fu L, Chin YE, Yu C. Leukemia inhibitory factor attenuates renal fibrosis through Stat3-miR-29c. Am J Physiol Ren Physiol. 2015;309:F595–603. doi: 10.1152/ajprenal.00634.2014. [DOI] [PubMed] [Google Scholar]
- 21.Han SH, Wu MY, Nam BY, Park JT, Yoo TH, Kang SW, et al. PGC-1alpha protects from notch-induced kidney fibrosis development. J Am Soc Nephrol. 2017;28:3312–22. doi: 10.1681/ASN.2017020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng J, Meyer CA, Wang Q, Liu JS, Shirley Liu X, Zhang Y. GFOLD: a generalized fold change for ranking differentially expressed genes from RNA-seq data. Bioinformatics. 2012;28:2782–8. doi: 10.1093/bioinformatics/bts515. [DOI] [PubMed] [Google Scholar]
- 25.Feng L, Wang J, Li H, Luo X, Li J. A novel absolute quantitative imaging strategy of iron, copper and zinc in brain tissues by Isotope Dilution Laser Ablation ICP-MS. Anal Chim Acta. 2017;984:66–75. doi: 10.1016/j.aca.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Li SW, Shao YZ, Zhao HJ, Wang Y, Li JL, Xing MW. Analysis of 28 trace elements in the blood and serum antioxidant status in chickens under arsenic and/or copper exposure. Environ Sci Pollut Res Int. 2017;24:27303–13. doi: 10.1007/s11356-017-0291-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Lu M, Xiong L, Fan J, Zhou Y, Li H, et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020;11:29. doi: 10.1038/s41419-019-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Kang H, Zhang Q, D’Agati VD, Al-Awqati Q, Lin F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest. 2019;129:2374–89. doi: 10.1172/JCI122256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadenbach B, Huttemann M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion. 2015;24:64–76. doi: 10.1016/j.mito.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26:1701–18. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 32.Schmid N, Eichenberger AP, Choutko A, Riniker S, Winger M, Mark AE, et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J. 2011;40:843–56. doi: 10.1007/s00249-011-0700-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Wang K, Lei Y, Li Q, Nice EC, Huang C. Redox signaling: potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med. 2015;89:452–65. doi: 10.1016/j.freeradbiomed.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Wikstrom M, Krab K, Sharma V. Oxygen activation and energy conservation by cytochrome c oxidase. Chem Rev. 2018;118:2469–90. doi: 10.1021/acs.chemrev.7b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timon-Gomez A, Nyvltova E, Abriata LA, Vila AJ, Hosler J, Barrientos A. Mitochondrial cytochrome c oxidase biogenesis: recent developments. Semin Cell Dev Biol. 2018;76:163–78. doi: 10.1016/j.semcdb.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevitt T, Ohrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim Biophys Acta. 2012;1823:1580–93. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxfield AB, Heaton DN, Winge DR. Cox17 is functional when tethered to the mitochondrial inner membrane. J Biol Chem. 2004;279:5072–80. doi: 10.1074/jbc.M311772200. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Dong D, Kang YJ. Copper chaperone for superoxide dismutase-1 transfers copper to mitochondria but does not affect cytochrome c oxidase activity. Exp Biol Med (Maywood) 2013;238:1017–23. doi: 10.1177/1535370213497327. [DOI] [PubMed] [Google Scholar]
- 39.Singh RP, Jeyaraju DV, Voisin V, Hurren R, Xu C, Hawley JR, et al. Disrupting mitochondrial copper distribution inhibits leukemic stem cell self-renewal. Cell Stem Cell. 2020;26:926–37. doi: 10.1016/j.stem.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22:102–13. doi: 10.1038/s41568-021-00417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leary SC. Redox regulation of SCO protein function: controlling copper at a mitochondrial crossroad. Antioxid Redox Signal. 2010;13:1403–16. doi: 10.1089/ars.2010.3116. [DOI] [PubMed] [Google Scholar]
- 42.Sokol RJ, Devereaux MW, O’Brien K, Khandwala RA, Loehr JP. Abnormal hepatic mitochondrial respiration and cytochrome C oxidase activity in rats with long-term copper overload. Gastroenterology. 1993;105:178–87. doi: 10.1016/0016-5085(93)90024-7. [DOI] [PubMed] [Google Scholar]
- 43.Roberts EA, Robinson BH, Yang S. Mitochondrial structure and function in the untreated Jackson toxic milk (tx-j) mouse, a model for Wilson disease. Mol Genet Metab. 2008;93:54–65. doi: 10.1016/j.ymgme.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 44.Zischka H, Lichtmannegger J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann N Y Acad Sci. 2014;1315:6–15. doi: 10.1111/nyas.12347. [DOI] [PubMed] [Google Scholar]
- 45.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.