Abstract
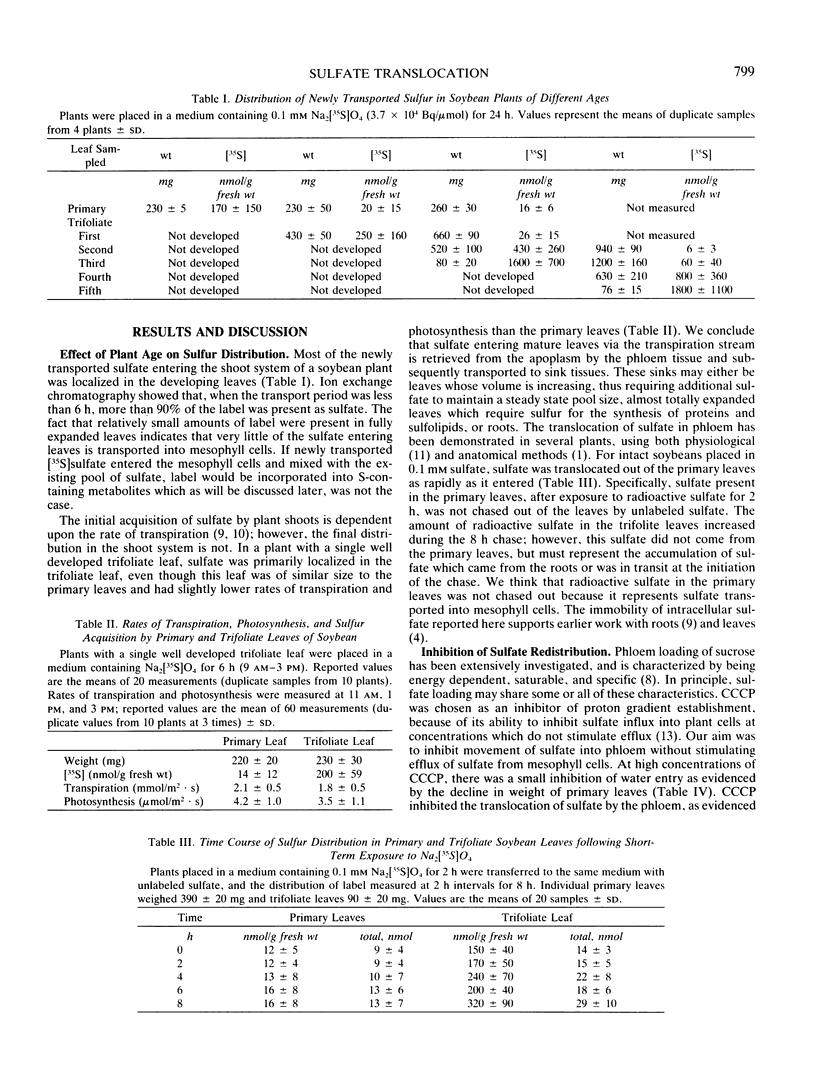
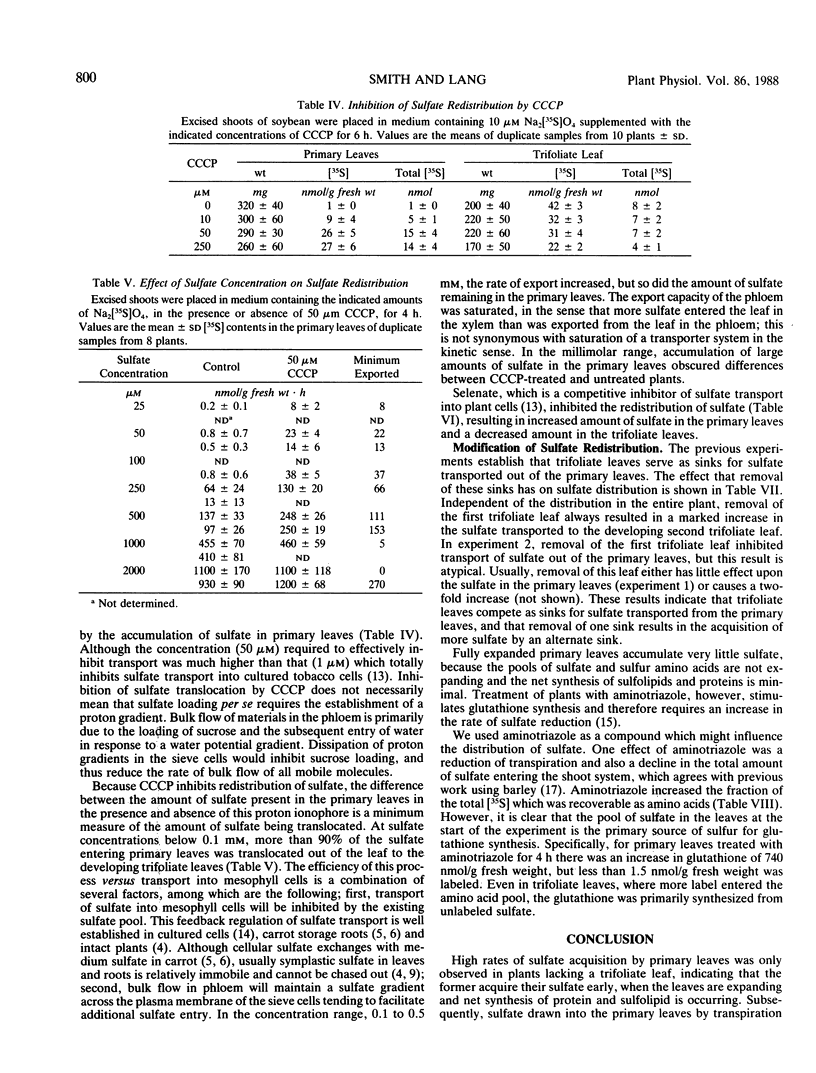
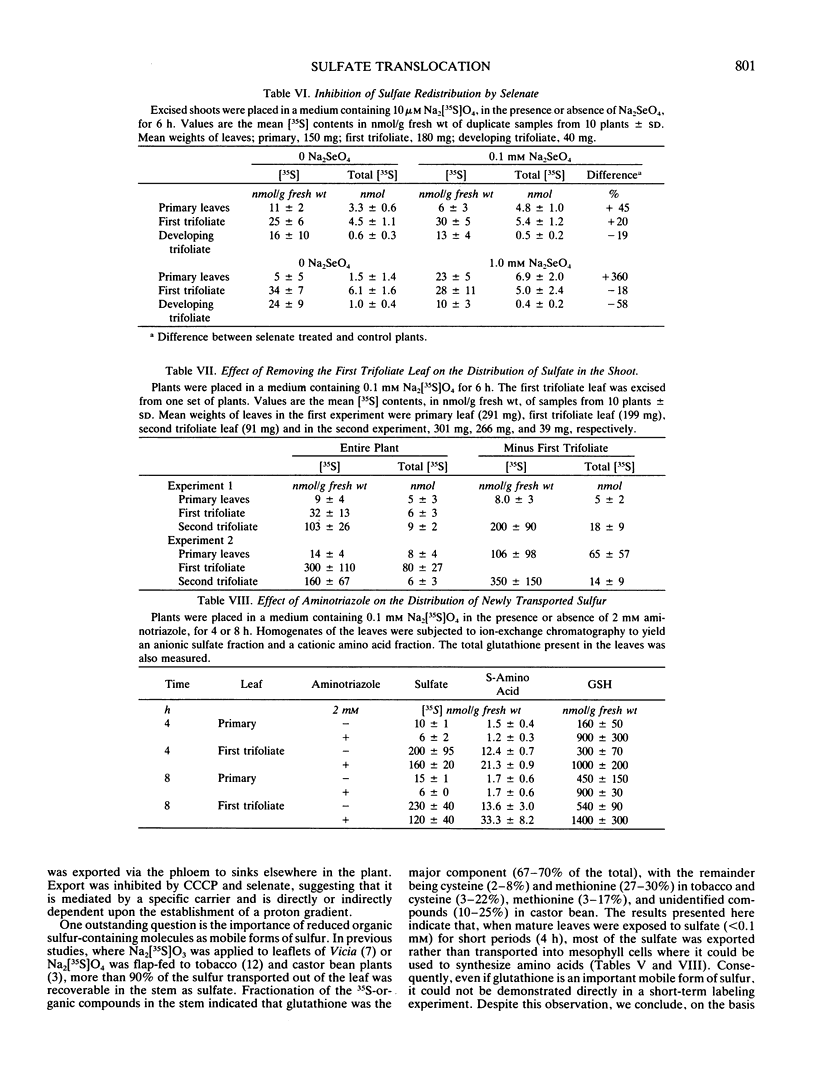
Sulfate translocation in soybean (Glycine max L. Merr) was investigated. More than 90% of the sulfate entering the shoot system was recoverable in one or two developing trifoliate leaves. In young plants, the first trifoliate leaf contained between 10 to 20 times as much sulfate as the primary leaves, even though both types of leaf had similar rates of transpiration and photosynthesis. We conclude that most of the sulfate entering mature leaves is rapidly loaded into the phloem and translocated to sinks elsewhere in the plant. This loading was inhibited by carbonylcyanide m-chlorophenylhydrazone and selenate. At sulfate concentrations below 0.1 millimolar, more than 95% of the sulfate entering primary leaves was exported. At higher concentrations the rate of export increased but so did the amount of sulfate remaining in the leaves. Removal of the first trifoliate leaf increased two-fold the transport of sulfate to the apex, indicating that these are competing sinks for sulfate translocated from the primary leaves. The small amount of sulfate transported into the mesophyll cells of primary leaves is a result of feedback regulation by the intracellular sulfate pool, not a consequence of their metabolic inactivity. For example, treatment of plants with 2 millimolar aminotriazole caused a 700 nanomoles per gram fresh weight increase in the glutathione content of primary leaves, but had no effect on sulfate aquisition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddulph O., Biddulph S., Cory R., Koontz H. Circulation Patterns for Phosphorus, Sulfur and Calcium in the Bean Plant. Plant Physiol. 1958 Jul;33(4):293–300. doi: 10.1104/pp.33.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram J. Characteristics of sulfate transport across plasmalemma and tonoplast of carrot root cells. Plant Physiol. 1983 May;72(1):204–211. doi: 10.1104/pp.72.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Characterization of sulfate transport in cultured tobacco cells. Plant Physiol. 1976 Sep;58(3):358–362. doi: 10.1104/pp.58.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K., Lang A. L. Decoloration and solubilization of plant tissue prior to determination of 3H, 14C, and 35S by liquid scintillation. Anal Biochem. 1987 Aug 1;164(2):531–536. doi: 10.1016/0003-2697(87)90529-x. [DOI] [PubMed] [Google Scholar]
- Smith I. K. Regulation of Sulfate Assimilation in Tobacco Cells: EFFECT OF NITROGEN AND SULFUR NUTRITION ON SULFATE PERMEASE AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1980 Nov;66(5):877–883. doi: 10.1104/pp.66.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 1985 Dec;79(4):1044–1047. doi: 10.1104/pp.79.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


