Abstract
Antibiotic-resistant enterococci are being increasingly identified as causal agents of infection. Trovafloxacin is a new fluoronaphthyridone with enhanced activity against gram-positive cocci and variable activity reported against Enterococcus spp. Twenty-one strains of vancomycin-resistant Enterococcus faecium and two strains of Enterococcus faecalis (one vancomycin resistant) were studied at an initial inoculum of 106 CFU/ml in time-kill assays with trovafloxacin (3 mg/liter), ampicillin-sulbactam (100/50 mg/liter), and the combination. Six strains of E. faecium (five vancomycin resistant) also were studied in an in vitro two-compartment dynamic model that mimics human pharmacokinetics with trovafloxacin simulated at 300 mg every 12 h (q12h), ampicillin-sulbactam at 2/1 g q6h, and the combination. Peripheral compartments were sampled q2h for 30 h for bacterial counts. Trovafloxacin MICs ranged from 0.5 to 32 mg/liter, and the nine strains of vancomycin-resistant E. faecium for which MICs were ≤2 mg/liter were more likely to show a reduction of 2 log units or more in viable counts in time-kill assays than were strains for which MICs were higher. Synergism with ampicillin-sulbactam was found for only one strain (trovafloxacin MIC, 16 mg/liter). Similar results were obtained in the pharmacokinetic model, with 2- to 4-log-unit reductions in viable bacteria for trovafloxacin-susceptible strains. Although no convincing evidence of synergism was found, ampicillin-sulbactam in combination minimized late bacterial regrowth of two trovafloxacin-susceptible strains. These data suggest that this high dose of trovafloxacin (with or without ampicillin-sulbactam) might be useful against strains of vancomycin-resistant E. faecium for which MICs were ≤2 mg/liter.
With increasing antimicrobial resistance reported from all over the world, it is important to find antibiotics with enhanced activity against resistant organisms (1). Antibiotic resistance is a particular problem in Enterococcus spp., with some strains showing high-level resistance to aminoglycosides, beta-lactams, and vancomycin. Vancomycin-resistant Enterococcus faecium is being isolated with increasing frequency around the world, particularly from hospitalized patients (5, 12). This organism is quite difficult to eradicate from infected heart valves and from other infected sites (12, 19). Although some success in early trials has been obtained with the new antibiotic combination quinupristin-dalfopristin (Synercid), the search for antibiotics with activity against these organisms continues.
Trovafloxacin is a new fluoronaphthyridone with a long elimination half-life and enhanced activity against gram-positive cocci, including penicillin-susceptible and -resistant strains of Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, etc. Its activity against Enterococcus faecalis and E. faecium is more variable but still greater than those of older fluoroquinolones (6).
The in vitro bactericidal activity of trovafloxacin against Enterococcus spp. was studied with time-kill assays and in an in vitro pharmacokinetic-pharmacodynamic model of infection that allows for the exposure of bacteria to changing concentrations of antibiotic that mimic human pharmacokinetics. Some strains also were studied with ampicillin-sulbactam alone and in combination with trovafloxacin.
MATERIALS AND METHODS
Twenty-one strains of vancomycin-resistant E. faecium and two strains of E. faecalis (one of which was vancomycin resistant) were studied (kindly supplied by John Boyce, Antone Medeiros, Leonard Mermel, Lance Peterson, and Steven Opal). Time-kill studies were performed in 10 ml of brain heart infusion broth, and each strain was studied with trovafloxacin alone, with ampicillin-sulbactam alone, and with the combination. An initial inoculum of 106 CFU/ml was used, and antibiotic concentrations approximated levels in serum following a single 300-mg dose of trovafloxacin (3 mg/liter) and a single 2/1-g dose of ampicillin-sulbactam (100/50 mg/liter). These mixtures were incubated at 37°C and were sampled at 0, 4, 8, and 24 h for bacterial counts, which were performed with serial 1:10 dilutions of 20-μl volumes plated in triplicate on antibiotic-free plates (Mueller-Hinton agar supplemented with 5% sheep blood). Bacterial counts are expressed as change of log CFU at 24 h.
Six strains of E. faecium (five of six were vancomycin resistant) also were studied in an in vitro two-compartment dynamic model that mimics human pharmacokinetics (2, 3). This model includes a central compartment that mimics concentrations in serum and a peripheral compartment consisting of hollow-fiber artificial-capillary units placed in series (Bioreactor chambers). Although the probable oral dose of trovafloxacin is likely to be 200 mg per day, in this model trovafloxacin was simulated as 300-mg doses q12h and ampicillin-sulbactam was simulated at 2/1 g q6h intravenously. The same doses were simulated when the drugs were studied in combination. In these studies, log-phase bacteria were used at initial inocula that ranged from 5.7 × 105 to 1.7 × 106 CFU/ml. The peripheral compartments were sampled for cell counts q2h for 30 h (except hour 16). The central compartment was sampled seven to eight times following the first dose and five to seven times following subsequent doses for bioassay determinations of drug concentrations.
Trovafloxacin concentrations in the central and peripheral compartments were determined by bioassay. Bacillus subtilis (ATCC 6633)-seeded plates containing Antibiotic Medium no. 1 (Difco) were used. Wells were made in the agar and filled with 25 ml of sample. Before the wells were filled, Penase (Difco Penase concentrate [penicillinase, 10,000,000 IU/ml]) was added to all samples that contained both trovafloxacin and ampicillin-sulbactam in order to inactivate the ampicillin. Plates were incubated overnight at 37°C, and zone sizes were measured with calipers the following day.
Trovafloxacin concentrations were calculated against a four-point standard curve by linear regression. The calibration plots were linear within the range of 0.25 to 1.2 mg/ml. The r2 for standard curves ranged from 0.98 to 0.99 (median, 0.99). The median percent error in the standard curves was 1.9% (range, −9.8 to 12.2). The median coefficient of variation for standards and unknown drug concentrations was 8.52% (range, 0 to 24.6) when three to four replicates per sample were studied.
Ampicillin concentrations in the central and peripheral compartments were determined by a well-plate bioassay (14) using the same B. subtilis-seeded plates as for trovafloxacin determinations. The median coefficient of variation for standards and unknown drug concentrations was 16.2, with a range of 0 to 28.7 when three or four replicates per sample were studied. Sulbactam did not interfere with ampicillin determinations at concentrations between 4.7 and 75 mg/ml.
RESULTS
MICs and kill curve results.
The MICs of trovafloxacin and ampicillin-sulbactam for the 21 strains of E. faecium are shown in Table 1. Most of the organisms were resistant to ampicillin and to ampicillin-sulbactam. Trovafloxacin MICs ranged from 0.5 to 32 mg/liter. When E. faecium strains were divided by their susceptibilities to trovafloxacin, those strains for which MICs were ≤2 mg/liter were more likely to exhibit a 2-log-unit or greater reduction in CFU per milliliter after exposure to 3 mg of trovafloxacin/liter than were strains for which MICs were higher (Table 2).
TABLE 1.
MICs of trovafloxacin and ampicillin-sulbactam for 21 strains of E. faecium
| Strain | MIC (mg/liter) of:
|
|
|---|---|---|
| Trovafloxacin | Ampicillin-sulbactam | |
| 46 | 16.0 | 32/16 |
| 67 | 16.0 | 64/32 |
| 211 | 8.0 | 64/32 |
| 221 | 1.0 | 64/32 |
| 229 | 16.0 | 64/32 |
| 359 | 2.0 | 64/32 |
| 392 | 4.0 | 64/32 |
| 7924 | 0.5 | 32/16 |
| 1777 | 1.0 | 64/32 |
| 2951 | 16.0 | 32/16 |
| 2952 | 32.0 | 32/16 |
| 2953 | 8.0 | 64/32 |
| 2954 | 1.0 | 32/16 |
| 2955 | 8.0 | 64/32 |
| 2956 | 8.0 | 64/32 |
| 2957 | 8.0 | 64/32 |
| 3608 | 1.0 | 32/16 |
| 4162 | 16.0 | 128/64 |
| 5924 | 1.0 | 32/16 |
| 5558 | 1.0 | 16/8 |
| 6947 | 0.5 | 32/16 |
TABLE 2.
Decrease in log CFU per milliliter by trovafloxacin MIC for 21 strains of vancomycin-resistant E. faecium exposed to 3 mg of trovafloxacin/liter
| Strain | Change in log CFU/ml at 24 h |
|---|---|
| MIC ≤ 2 mg/litera | |
| 5294 (1) | −3.37 |
| 359 (2) | −1.82 |
| 221 (1) | −1.39 |
| 1777 (1) | −2.7 |
| 3608 (1) | −1.0 |
| 5558 (1) | −2.15 |
| 6947 (1) | −2.23 |
| 792 (0.5) | −2.18 |
| 2954 (0.5) | −3.15 |
| MIC > 2 mg/liter | |
| 4162 | +2.96 |
| 392 | +2.39 |
| 46 | +2.51 |
| 67 | +2.26 |
| 211 | +1.46 |
| 229 | +1.52 |
| 2951 | +2.57 |
| 2952 | +2.7 |
| 2953 | +2.8 |
| 2955 | +1.73 |
| 2956 | +2.02 |
| 2957 | +1.96 |
Numbers in parentheses represent trovafloxacin MICs in milligrams per liter.
Ampicillin-sulbactam alone produced a 2-log-unit or greater drop in CFU in 10 of 21 (48%) strains, and for 9 of these (90%), trovafloxacin MICs were ≥4 mg/liter. When trovafloxacin and ampicillin-sulbactam were administered simultaneously, a drop of ≥2 log CFU/ml was found with 16 of 21 strains (76%). Trovafloxacin alone produced a 3.2-log-CFU/ml decrease in the one strain of vancomycin-sensitive E. faecium tested (trovafloxacin MIC, 1 mg/liter) but did not have any bactericidal effect on the two strains of E. faecalis tested (MIC = 8 mg/liter). Both of the latter strains were susceptible to ampicillin-sulbactam, with MICs of ≤2/2 mg/liter, and this drug produced a 2- to 3-log-unit decrease in CFU per milliliter.
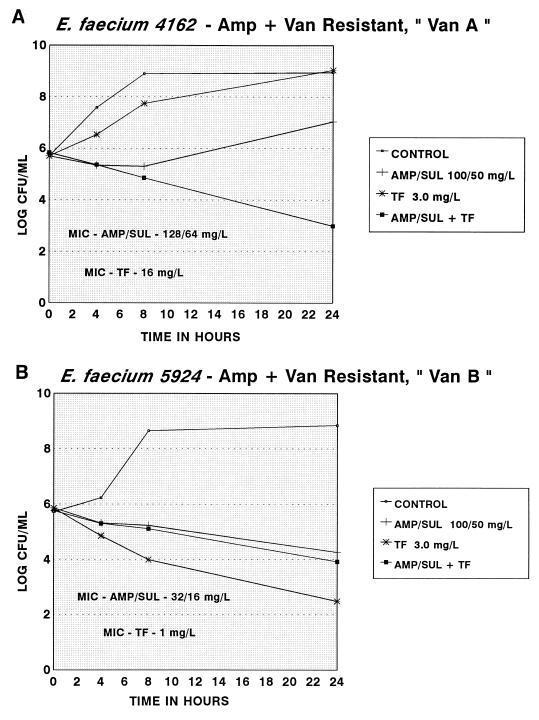
Convincing evidence of antibiotic synergism was found with only one strain, E. faecium 4162, for which the MIC of trovafloxacin was 16 mg/liter and the MIC of ampicillin-sulbactam was 128/64 mg/liter. Some inhibition was observed with ampicillin-sulbactam alone and a 3-log-CFU/ml increase was seen with trovafloxacin alone, although the combination produced a 2.66-log-unit reduction in CFU per milliliter at 24 h (Fig. 1A). One trovafloxacin-susceptible strain (5924) was antagonized slightly when exposed to the combination (Fig. 1B).
FIG. 1.
Time-kill curves of trovafloxacin (TF), ampicillin-sulbactam (AMP/SUL), and the combination (AMP/SUL + TF) for a trovafloxacin- and ampicillin-sulbactam-resistant strain (A) and for a trovafloxacin-susceptible strain (B) of vancomycin-resistant E. faecium.
Results in the in vitro model.
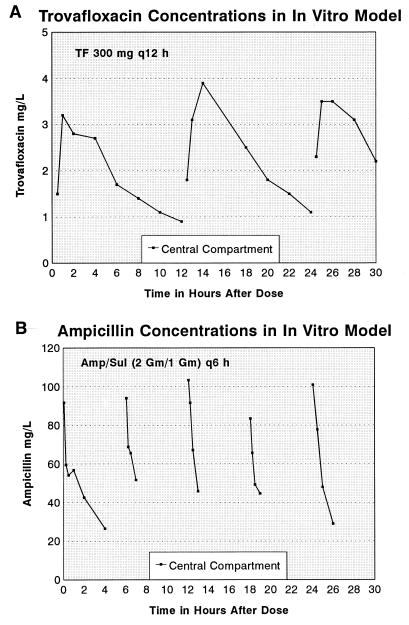
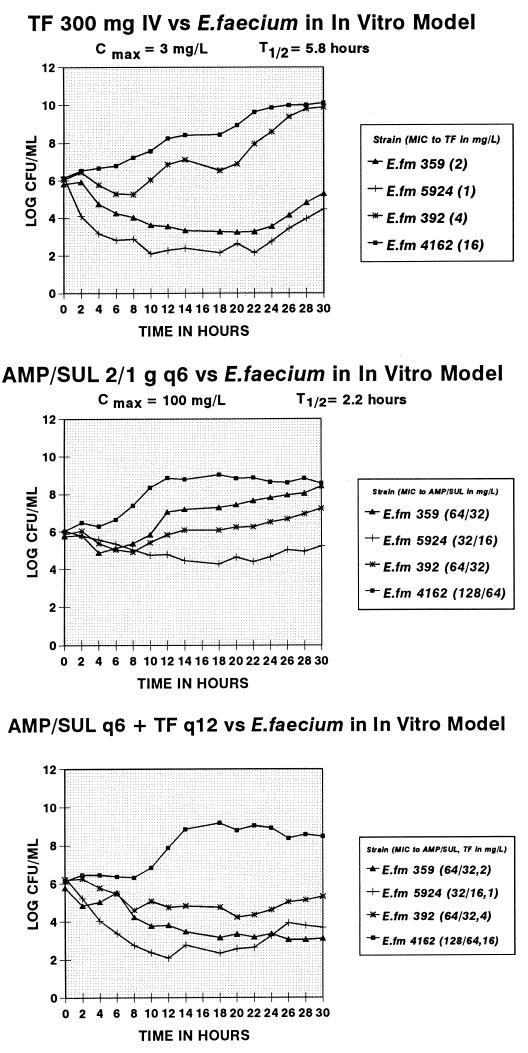
The concentrations of each antibiotic in the central compartment as measured by bioassay are shown in Fig. 2. Reasonable approximations of human concentration-time curves were achieved in the model (17). Data for four strains of vancomycin-resistant E. faecium tested in the in vitro dynamic model are shown in Fig. 3. Two of these strains, 359 and 5924, were susceptible to trovafloxacin (MICs, ≤2 mg/liter), and the MICs for the other two, 391 and 4162, were higher (≥4 mg/liter). Both of the sensitive strains showed a 2- to 4-log-unit reduction in bacterial counts, with a maximal effect at 10 to 18 h (Fig. 3). Ampicillin-sulbactam alone produced inhibition with one strain, but the other three strains were neither inhibited nor killed.
FIG. 2.
Concentrations of trovafloxacin (A) and ampicillin (B) in the central compartment in in vitro model experiments.
FIG. 3.
Effects of trovafloxacin (TF) (300 mg q12h), ampicillin-sulbactam (AMP/SUL) (2/1 g q6h), and the combination against four strains of E. faecium studied in an in vitro pharmacokinetic-pharmacodynamic model.
With the combination, results similar to those achieved with trovafloxacin alone were obtained for three of the four strains (4162, 5924, and 359). Some enhanced killing of strain 392 was found, with high MICs of both drugs. When results for the combination obtained in time-kill experiments at constant antibiotic concentrations were compared, results similar to those in the model were found with strains 359 and 5924 (both of which were trovafloxacin susceptible) and discrepant results were seen with strains 392 and 4162 (both of which were trovafloxacin resistant). Although no clear-cut evidence for synergism could be demonstrated, the combination of trovafloxacin and ampicillin-sulbactam appeared to suppress late regrowth (beginning at 22 h) of strain 359 and, to a lesser extent, of strain 5924, for both of which the MIC of trovafloxacin was ≤2 mg/liter (Fig. 3).
DISCUSSION
This paper shows that trovafloxacin simulating a dose of 300 mg q12h (which is higher than the proposed daily oral dose of 200 mg) has modest bactericidal activity against strains of vancomycin-resistant E. faecium if the MICs for these strains are ≤2 mg/liter. While it is unlikely that trovafloxacin or any of the new fluoroquinolone antibiotics will have major roles in the treatment of infections caused by vancomycin-resistant E. faecium, 9 of 21 (43%) strains studied here were inhibited by ≤2 mg of trovafloxacin/liter. All nine of these strains showed 1- to 3-log-unit reductions in viable counts at 24 h in time-kill assays. This study also shows the utility of in vitro dynamic models that allow for multiple dosing. As seen in Fig. 3, simulated trovafloxacin dosing (albeit at the higher, 300-mg dose) did result in 2- to 4-log-unit killing of susceptible strains, although some regrowth was apparent beginning at 22 h.
Other investigators have reported that 30 to 81% of vancomycin-resistant E. faecium strains are susceptible in vitro to trovafloxacin at concentrations of ≤2 mg/liter (9, 10). Several recent studies also suggest that trovafloxacin might have some useful activity against vancomycin-resistant E. faecium in a variety of models. Using agar dilution, Coque and colleagues reported trovafloxacin MICs for 16 strains of vancomycin-resistant E. faecium ranging from 0.12 to 8 mg/liter, and 81% of these strains were susceptible to 2 mg/liter (10). Using 3 mg of trovafloxacin/liter in time-kill assays, they reported reductions of 2 or more log units at 24 h with five of six strains of vancomycin-resistant E. faecium (10). Also, Cormican and Jones (9) used broth microdilution and disk diffusion methods to study trovafloxacin activity against 131 vancomycin-resistant enterococci and reported potentially useful activity against 30 to 37% of tested strains.
The MIC at which 90% of isolates are inhibited (MIC90) of trovafloxacin was reported to be 0.5 mg/liter for 29 enterococci unidentified at the species level in a recent report (7), and Bonilla et al. reported a trovafloxacin MIC50 of 2 mg/liter (4). Another group reported trovafloxacin MIC90s of 2 mg/liter against 10 strains of E. faecium, 16 mg/liter against 15 ciprofloxacin-resistant strains of E. faecium, and 0.25 mg/liter against 20 strains of E. faecalis (8). Endtz and colleagues reported that trovafloxacin was active against 20 strains of vancomycin-resistant E. faecalis, with MICs ranging from 0.125 to 2 mg/liter (13). Also, trovafloxacin was found to be active in media against three vancomycin-susceptible and three vancomycin-resistant E. faecium strains, but less activity was found intracellularly against vancomycin-resistant E. faecium (15).
Consistent demonstration of synergism or antagonism with ampicillin-sulbactam was not obtained in these studies. However, careful examination of Fig. 3 suggests that for two of the four tested strains, the addition of ampicillin-sulbactam might inhibit the late bacterial regrowth seen with trovafloxacin, despite rather unimpressive activity of the beta-lactam alone. Coque et al. (11) also showed that combinations of trovafloxacin with ampicillin-sulbactam did not show enhanced activity except against one strain of E. faecium. Another report suggested that trovafloxacin and ampicillin-sulbactam were synergistic or partially synergistic against 10 strains of enterococci unidentified at the species level when tested with an agar dilution checkerboard method (16).
Although some anti-vancomycin-resistant E. faecium activity has been demonstrated in vitro and in some experimental models of infection, it is unlikely that trovafloxacin will be uniformly effective in eradicating these organisms from patients with serious infections. In addition, the reported 70% binding to serum protein (18) might further reduce its activity. Nonetheless, for some select patients infected with E. faecium strains for which MICs are low (≤2 mg/liter), trovafloxacin in high doses with or without other antibiotics might have a beneficial effect. Also, the possible suppression of late bacterial regrowth by the combination of trovafloxacin plus ampicillin-sulbactam seen with some strains in the in vitro kinetic model presented here is of interest. The demonstrated potential activity of trovafloxacin against some vancomycin-resistant E. faecium strains suggests that further study with clinically infected patients may be of benefit.
ACKNOWLEDGMENT
This work was supported by a grant from Pfizer, Inc.
REFERENCES
- 1.Bacquero F the Task Force of the General Direction for Health Planning of the Spanish Ministry of Health. Antibiotic resistance in Spain: what can be done? Clin Infect Dis. 1996;23:819–823. doi: 10.1093/clinids/23.4.819. [DOI] [PubMed] [Google Scholar]
- 2.Blaser J, Stone B B, Zinner S H. Efficacy of intermittent versus continuous administration of netilmicin in a two-compartment in vitro model. Antimicrob Agents Chemother. 1985;27:343–349. doi: 10.1128/aac.27.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser, J., B. B. Stone, and S. H. Zinner. 1985. Two-compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl. A):131–137. [DOI] [PubMed]
- 4.Bonilla H F, Zarins L T, Bradley S F, Kaufmann C A. Susceptibility of ciprofloxacin-resistant staphylococci and enterococci to trovafloxacin. Diagn Microbiol Infect Dis. 1996;26:17–21. doi: 10.1016/s0732-8893(96)00146-0. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 6.Child J, Boswell F, Brenwald N, Andrews J M, Wise R. The in vitro activity of CP-99,219, a new naphthyridone antimicrobial: a comparison with fluoroquinolone agents. J Antimicrob Chemother. 1995;35:869–876. doi: 10.1093/jac/35.6.869. [DOI] [PubMed] [Google Scholar]
- 7.Citron D M, Fiorentino N, Jiminez M, Leal R, Appleman M D. Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Comparative in vitro activity of trovafloxacin (TV) with ten other agents against 275 strains of aerobic and anaerobic organisms isolated from patients with intraabdominal infection, abstr. E80; p. 95. [Google Scholar]
- 8.Cohen M A, Huband M D, Gage J W, Yoder S L. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of clinafloxacin, trovafloxacin, and ciprofloxacin, abstr. E85; p. 96. [Google Scholar]
- 9.Cormican M G, Jones R N. In-vitro activity of trovafloxacin (CP-99,219) tested by two methods against 150 vancomycin resistant enterococcal isolates. J Antimicrob Chemother. 1996;37:847–849. doi: 10.1093/jac/37.4.847. [DOI] [PubMed] [Google Scholar]
- 10.Coque T M, Singh K V, Murray B E. Comparative in-vitro activity of the new fluoroquinolone trovafloxacin (CP-99,219) against Gram-positive cocci. J Antimicrob Chemother. 1996;37:1011–1016. doi: 10.1093/jac/37.5.1011. [DOI] [PubMed] [Google Scholar]
- 11.Coque T M, Singh K V, Murray B E. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Assessment of the bactericidal activity of trovafloxacin (CP 99,129) against multiresistant strains of enterococci, abstr. E77; p. 95. [Google Scholar]
- 12.Edmond M B, Ober J F, Weinbaum D L, Pfaller M L, Hwang T, Sanford M D, Wenzel R P. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis. 1995;20:1126–1133. doi: 10.1093/clinids/20.5.1126. [DOI] [PubMed] [Google Scholar]
- 13.Endtz H P, Mouton J W, den Hollander J G, van den Braak N, Verbrugh H A. Comparative in vitro activities of trovafloxacin (CP-99,219) against 445 gram-positive isolates from patients with endocarditis and those with other bloodstream infections. Antimicrob Agents Chemother. 1997;41:1146–1149. doi: 10.1128/aac.41.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firsov A A, Savarino D, Ruble M, Gilbert D, Manzano B, Medeiros A A, Zinner S H. Predictors of effect of ampicillin-sulbactam against TEM-1 β-lactamase-producing Escherichia coli in an in vitro dynamic model: enzyme activity versus MIC. Antimicrob Agents Chemother. 1996;40:734–738. doi: 10.1128/aac.40.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera-Insua I, Jacques-Palaz K, Murray B E, Rakita R M. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Intracellular activity of trovafloxacin (CP-99,219) against vancomycin-susceptible (VSEF) and vancomycin-resistant (VREF) Enterococcus faecium, abstr. E78; p. 95. [Google Scholar]
- 16.Jenkins S G. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro synergy between trovafloxacin and ampicillin/sulbactam against bacterial pathogens, abstr. E82; p. 96. [Google Scholar]
- 17.Kinishi T. Pharmacokinetic profile of antimicrobial agents. II-1. Penicillins. In: Kuemmerle H-P, Murakawa T, Nightingale C H, editors. Pharmacokinetics of antimicrobial agents: principles, methods, application. Landsberg, Germany: Ecomed Verlag; 1993. pp. 43–64. [Google Scholar]
- 18.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 19.Wells C L, Juni B A, Cameron S B, Mason K R, Dunn D L, Ferrieri P, Rhame F S. Stool carriage, clinical isolation, and mortality during an outbreak of vancomycin-resistant enterococci in hospitalized medical and/or surgical patients. Clin Infect Dis. 1995;21:45–50. doi: 10.1093/clinids/21.1.45. [DOI] [PubMed] [Google Scholar]