Abstract
Clinical predictors for pacemaker-induced cardiomyopathy (PICM) (e.g., a wide QRS duration and left bundle branch block at baseline) have been reported. However, factors involved in the development of PICM in patients with preserved left ventricular ejection fraction (LVEF) remain unknown. This study aimed to determine the risk factors for PICM in patients with preserved LVEF. The data of 113 patients (average age: 71.3 years; men: 54.9%) who had echocardiography before and after pacemaker implantation (PMI) among 465 patients undergoing dual-chamber PMI were retrospectively analyzed. Thirty-three patients were diagnosed with PICM (18.0/100 person-years; 95% CI 12.8–25.2). A univariate Cox regression analysis showed that an estimated glomerular filtration rate (eGFR) ≤ 30 mL/min/1.73 m2 (HR 3.47; 95% CI 1.48–8.16) and a past medical history of coronary artery disease (CAD) (HR 2.76; 95% CI 1.36–5.60) were significantly associated with the onset of PICM. After adjusting for clinical variables, an eGFR ≤ 30 mL/min/1.73 m2 (HR 2.62; 95% CI 1.09–6.29) and a medical history of CAD (HR 2.32; 95% CI 1.13–4.80) were independent risk factors for developing PICM. A medical history of CAD and low eGFR are independent risk factors for PICM in patients with preserved LVEF at baseline. These results could be helpful in predicting a decreased LVEF by ventricular pacing before PMI. Close follow-up by echocardiography is recommended to avoid a delay in upgrading to physiological pacing, such as cardiac resynchronization therapy or conduction system pacing.
Subject terms: Cardiology, Cardiac device therapy, Kidney diseases
Introduction
Chronic right ventricular (RV) pacing sometimes leads to a deterioration in the left ventricular ejection fraction (LVEF) because RV pacing provokes interventricular dyssynchrony and intraventricular dyssynchronous contraction in the left ventricle (LV)1. A combination of electrical and mechanical dyssynchrony leads to adverse LV remodeling, which promotes heart failure (HF), atrial fibrillation (AF), and mortality2–4.
A deterioration in LV systolic function related to pacemaker implantation (PMI) is termed pacemaker-induced cardiomyopathy (PICM)5,6. Previous studies have used different LVEF thresholds to define PICM. Therefore, there is currently no internationally accepted definition of PICM. The following three definitions of PICM have been used in past clinical studies: (1) an LVEF ≤ 40% if the baseline LVEF is ≥ 50% or an absolute reduction in the LVEF ≥ 5% if the baseline is < 50%; (2) an LVEF ≤ 40% if the baseline LVEF is ≥ 50% or an absolute reduction in the LVEF ≥ 10% if the baseline is < 50%; and (3) an absolute reduction in the LVEF ≥ 10%, regardless of baseline LV function2,7–9.
There is wide variation in the published prevalence of the development of PICM (5–27%)10,11. The highest prevalence was reported to be 39% according to the definition of an absolute reduction in the LVEF ≥ 10% during a follow-up of 3–4 years2. Furthermore, a worldwide survey showed that the number of patients undergoing PMI had increased12. Therefore, the number of patients with PICM should also be increased.
Identifying risk factors for PICM before PMI is beneficial for preventing PICM-associated heart diseases, such as HF. It is reported that an older age6,7,9, male sex7,13, a history of myocardial infarction13, a lower baseline LVEF14,15, a wider intrinsic QRS duration7,13,16, left bundle branch block (LBBB)13, a history of AF6,17, and a chronic higher RV pacing burden8 could be risk factors for PICM. However, the precise mechanism and established risk factors for PICM have not been fully identified18. Therefore, the present study aimed to identify additional risk factors for PICM.
Methods
Definition of PICM
In this study, PICM was defined as follows: (1) exclusion of alternative causes of heart diseases, such as acute myocardial ischemia, uncontrollable tachyarrhythmia, frequent premature contractions, and untreated hypertension; and (2) an absolute reduction in the LVEF ≥ 10% as measured by transthoracic echocardiography (TTE) after PMI compared with before PMI. TTE data were collected within six months before PMI and from three months to three years after PMI. All TTE data were over-read by cardiovascular experts.
Data collection and the study population
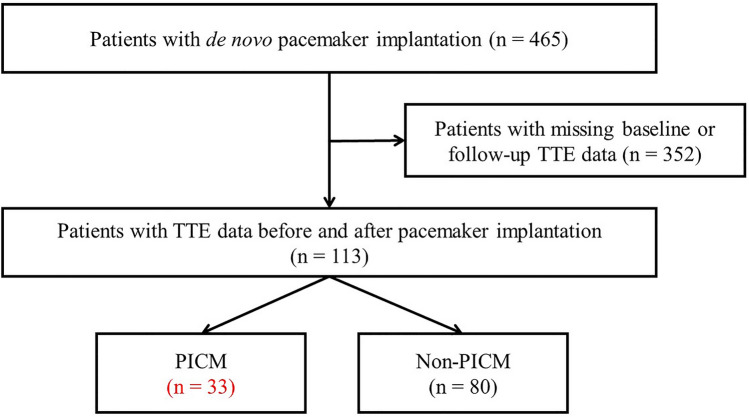
All data were retrospectively acquired at the University of Tokyo Hospital, Tokyo, Japan, from patients who underwent de novo PMI between December 2006 and November 2018. PMI was performed in patients with sick sinus syndrome (SSS) or atrioventricular block (AVB). A total of 465 patients undergoing PMI were included in this study. To identify the risk factors for PICM, the medical history and clinical data were retrospectively collected from all patients. The flow of data collection for the study is shown in Fig. 1.
Figure 1.
Flowchart of data collection for the study population. Patients who underwent de novo pacemaker implantation were studied between December 2006 and November 2018. Among them, 113 patients who matched the criteria were enrolled. TTE transthoracic echocardiogram, PICM pacing-induced cardiomyopathy.
The inclusion criteria were as follows: (1) age of ≥ 20 years; (2) de novo PMI; and (3) available data of TTE before and after PMI. The exclusion criteria were as follows: (1) implantation of a prior cardiac implantable electronic device; (2) lack of TTE before and/or after PMI; (3) congenital heart diseases; (4) heart transplantation; and (5) alternative cause of a reduction in the LVEF, such as acute myocardial ischemia, valvular heart disease, uncontrollable tachyarrhythmia, frequent premature contractions, and untreated hypertension. In addition, all of the patients’ laboratory data were obtained upon admission to our hospital and at a follow-up LVEF examination after discharge.
Clinical outcome and definition of clinical variables
The primary outcome was an incidence of a reduced ejection fraction, which was defined as a ≥ 10% reduction in the LVEF in a follow-up TTE after PMI. The New York Heart Association (NYHA) class was categorized on the basis of symptoms and evaluation of a medical examination on admission by expert cardiologists. AF was diagnosed by an electrocardiogram on admission. Diabetes mellitus was diagnosed as a glycated hemoglobin value ≥ 6.5% or determined by a history of taking oral hypoglycemic agents or insulin injections. Hypertension (HT) was diagnosed as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. A history of antihypertensive agents was also used to determine HT. The estimated glomerular filtration rate (eGFR) was calculated as 194 × (serum creatinine)−1.094 × (age)−0.287 × 0.739 (for female patients)19. Chronic kidney disease (CKD) was defined as an eGFR < 60 mL/min/1.73 m2.
Statistical analysis
The patients were divided into two groups, the PICM group and the non-PICM group, on the basis of the definition of PICM as previously described. The differences in baseline characteristics were compared between the two groups by Student’s t-test or Mann–Whitney U test for continuous variables and the Chi-square test for categorical variables, as appropriate. The incidence rate of PICM and its 95% confidence interval (CI) were calculated in person-years with the assumption of Poisson distribution for the total patients. The cumulative rates of PICM were calculated with the Kaplan–Meier method, and time-to-event data are shown in Kaplan–Meier curves. Cox univariate and multivariate analyses were performed to estimate hazard ratios (HRs) of clinical variables for developing PICM. All statistical analyses were performed using IBM SPSS version 28.0.0.0 (IBM Corp., Armonk, NY), and a two-tailed P value < 0.05 indicated statistical significance.
Ethical approval
This study was approved by the University of Tokyo institutional ethics committee (approval number 2650-[13]). All patient information was deidentified and the requirement for written informed consent was waived by the University of Tokyo institutional ethics committee. The study protocol was conducted in accordance with the Declaration of Helsinki.
Results
Clinical and procedural characteristics
One hundred and thirteen patients who underwent de novo PMI and had TTE data before and after PMI were enrolled. The patients’ characteristics are shown in Table 1. The mean age was 71.3 ± 11.2 years old (range: 25–91 years), the mean LVEF before PMI was 65.0 ± 13.0%, and the mean eGFR before PMI was 59.3 ± 24.8 mL/min/1.73 m2. All patients underwent implantation of the dual-chamber pacemakers, and 77 (68.1%) underwent PMI for SSS and 36 (31.9%) for AVB. Of the 36 patients diagnosed with atrioventricular block (AVB), the following classifications were observed: 26 patients, or 72.2%, exhibited complete AVB; six patients, representing 16.7%, had 2:1 AVB; and the remaining four patients, accounting for 11.1%, were diagnosed with advanced AVB.
Table 1.
The characteristics of patients.
| Characteristics | Total (n = 113) | Non-PICM (n = 80) | PICM (n = 33) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 71.3 ± 11.2 | 71.8 ± 10.4 | 70.0 ± 13.2 | 0.44 |
| Male sex (n, %) | 62 (54.9) | 42 (52.5) | 20 (60.6) | 0.28 |
| BMI (kg/m2) | 22.9 ± 3.8 | 22.6 ± 3.9 | 23.4 ± 3.5 | 0.32 |
| Echocardiography | ||||
| LVEF (%) | 65.0 ± 13.0 | 63.7 ± 11.8 | 68.0 ± 15.2 | 0.11 |
| LVDd (mm) | 47.2 ± 7.1 | 47.1 ± 6.8 | 47.7 ± 7.7 | 0.66 |
| LVDs (mm) | 30.2 ± 7.4 | 30.5 ± 7.4 | 29.5 ± 7.2 | 0.51 |
| LAD (mm) | 42.8 ± 8.7 | 42.9 ± 9.0 | 42.6 ± 8.1 | 0.81 |
| Medical history and clinical findings | ||||
| CAD (n, %) | 44 (38.9) | 26 (32.5) | 18 (54.5) | 0.03* |
| CABG | 8 (7.1) | 5 (6.3) | 3 (9.1) | 0.69 |
| PCI | 33 (29.2) | 14 (42.4) | 19 (23.8) | 0.05* |
| DM (n, %) | 39 (34.5) | 26 (32.5) | 13 (39.4) | 0.31 |
| HT (n, %) | 74 (65.5) | 55 (68.8) | 19 (57.6) | 0.18 |
| HF (n, %) | 33 (29.2) | 23 (28.7) | 10 (30.3) | 0.52 |
| NYHA class ≥ II, n (%) | 52 (46.0) | 36 (45.0) | 16 (48.5) | 0.45 |
| Arrythmia and ECG findings | ||||
| AF (n, %) | 48 (42.5) | 34 (42.5) | 14 (42.4) | 0.58 |
| AVB indicating PMI (n, %) | 36 (31.9) | 23 (28.7) | 13 (39.4) | 0.19 |
| RBBB (n, %) | 23 (20.4) | 16 (20.0) | 7 (21.2) | 0.54 |
| LBBB (n, %) | 10 (8.8) | 10 (12.5) | 0 (0) | 0.03* |
| QRS duration (ms) | 113.5 ± 24.4 | 116.9 ± 25.6 | 105.2 ± 19.1 | 0.02* |
| Kidney function | ||||
| eGFR (mL/min/1.73 m2) | 59.3 ± 24.8 | 62.7 ± 23.9 | 50.9 ± 25.2 | 0.02* |
| eGFR ≥ 90 (n, %) | 7 (6.2) | 5 (6.3) | 2 (6.1) | 0.67 |
| eGFR ≥ 60 and < 90 (n, %) | 52 (46.0) | 40 (50.0) | 12 (36.4) | 0.13 |
| eGFR < 60 (n, %) | 54 (47.8) | 35 (43.8) | 19 (57.6) | 0.13 |
| eGFR ≤ 30 (n, %) | 13 (11.5) | 5 (6.3) | 8 (24.2) | 0.01* |
PICM pacemaker-induced cardiomyopathy, BMI body mass index, LVEF left ventricular ejection fraction, LVDd left ventricular end-diastolic diameter, LVDs left ventricular end-systolic diameter, LAD left atrial diameter, CAD coronary artery disease, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, DM diabetes mellitus, HT hypertension, HF heart failure, NYHA New York Heart Association, AF atrial fibrillation, AVB atrioventricular block, RBBB right bundle branch block, LBBB left bundle branch block, eGFR estimated glomerular filtration rate. *P < 0.05.
Among the enrolled patients, 44 (38.9%) had a medical history of coronary artery disease (CAD), and those of 33 (29.2%) patients had undergone percutaneous coronary intervention (PCI). Patients in the PICM group had a more prevalent medical history of CAD (54.5% vs. 32.5%, P = 0.03) and a history of previous PCI (42.4% vs. 29.2%, P = 0.05), lower eGFR (50.9 ± 25.2 mL/min/1.73 m2 vs. 62.7 ± 23.9 mL/min/1.73 m2, P = 0.02), higher rate of eGFR ≤ 30 mL/min/1.73 m2 (24.2% vs. 6.3%, P = 0.01), lower rate of LBBB (0% vs. 12.5%, P = 0.03), and shorter QRS duration (105.2 ± 19.1 ms vs. 116.9 ± 25.6 ms, P = 0.02) than the non-PICM group. There were no significant differences in other background factors between the two groups.
Supplementary Table 1 outlines the positions of the RV lead tips. The distribution of these lead tips across various regions is as follows: apex with 32 patients (28.3%), septum with 70 patients (61.9%), left bundle area with 3 patients (2.7%), and His bundle area with 8 patients (7.1%). It is noteworthy that there were no significant disparities in the frequency of RV lead placement at the RV apex between patients diagnosed with PICM and those without.
Right atrial (RA) and RV pacing frequencies in patients in the PICM and non-PICM groups after PMI were investigated retrospectively. Pacemaker interrogation data were obtained at the closest date of the TTE examination. There were no significant differences in RA or RV pacing frequencies between the two groups (Table 2).
Table 2.
Right atrial and right ventricular pacing frequencies.
| Characteristics | Total (n = 113) | Non-PICM (n = 80) | PICM (n = 33) | P |
|---|---|---|---|---|
| RAp (%) | 40.2 ± 38.5 | 38.9 ± 38.8 | 43.4 ± 38.3 | 0.58 |
| RVp (%) | 52.9 ± 44.8 | 49.7 ± 44.9 | 60.4 ± 44.5 | 0.25 |
PICM pacemaker-induced cardiomyopathy, RAp right atrial pacing, RVp right ventricular pacing.
Prevalence of PICM
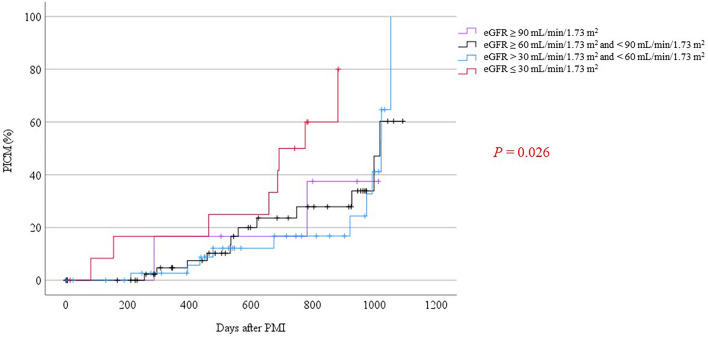
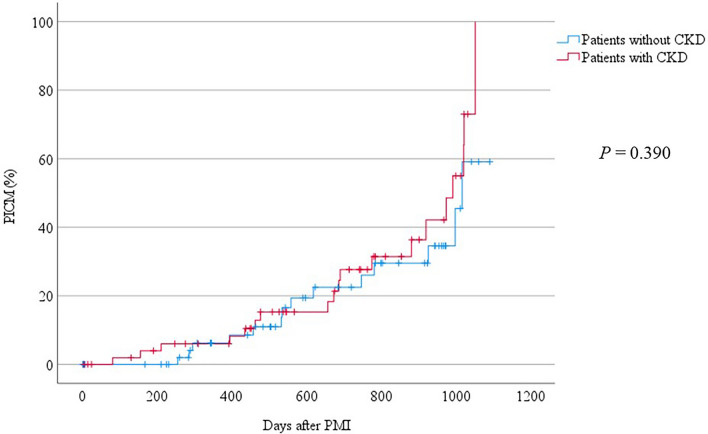
During a mean follow-up of 1.6 ± 0.9 years, 33 (29.2%) patients developed PICM, and the incidence rate was 18.0/100 person-years (95% CI 12.8–25.2) in the total follow-up of 184 person-years. Kaplan–Meier curves for the rates of developing PICM are shown in Figs. 2 and 3. These rates were evaluated with the log-rank test for determining differences between patients with and without CKD. In addition, these patients were divided into four groups by the eGFR as follows: (A) eGFR ≥ 90 mL/min/1.73 m2, (B) eGFR ≥ 60 mL/min/1.73 m2 and < 90 mL/min/1.73 m2, (C) eGFR > 30 mL/min/1.73 m2 and < 60 mL/min/1.73 m2, and (D) eGFR ≤ 30 mL/min/1.73 m2. There was a significant difference in the incidence of PICM between the four groups by the eGFR (log-rank, P = 0.03). Additionally, there were marked differences in the incidence of PICM between groups B and D, as well as between groups C and D, with log-rank values of P = 0.02 and P < 0.01, respectively. These findings are detailed in Table 3. Remarkably, patients with an eGFR < 30 mL/min/1.73 m2 showed a higher incidence of PICM than those with an eGFR ≥ 30 mL/min/1.73 m2.
Figure 2.
Incidence of PICM in patients with chronic kidney disease. Kaplan–Meier curves are shown for the development of PICM over 3 years of follow-up. The eGFR was categorized into four groups: eGFR ≥ 90 mL/min/1.73 m2, eGFR ≥ 60 mL/min/1.73 m2 and < 90 mL/min/1.73 m2, eGFR > 30 mL/min/1.73 m2 and < 60 mL/min/1.73 m2, and eGFR ≤ 30 mL/min/1.73 m2. A significant difference in the incidence of PICM among the four groups was detected. PICM pacing-induced cardiomyopathy, eGFR estimated glomerular filtration rate (mL/min/1.73 m2).
Figure 3.
Incidence of PICM in patients with CKD with an eGFR < 60 mL/min/1.73 m2. Kaplan–Meier curves are shown for the development of PICM. The log-rank test was used for comparing patients with and without CKD. There was no significant difference in the incidence of developing PICM between the two groups. PICM pacing-induced cardiomyopathy, CKD chronic kidney disease.
Table 3.
Log-rank analysis for incidence of pacemaker-induced cardiomyopathy based on renal function status.
| eGFR | P |
|---|---|
| eGFR ≥ 90 mL/min/1.73 m2 (control) | |
| eGFR ≥ 60 mL/min/1.73 m2 and < 90 mL/min/1.73 m2 | 0.90 |
| eGFR > 30 mL/min/1.73 m2 and < 60 mL/min/1.73 m2 | 0.64 |
| eGFR ≤ 30 mL/min/1.73 m2 | 0.21 |
| eGFR ≥ 60 mL/min/1.73 m2 and < 90 mL/min/1.73 m2 (control) | |
| eGFR > 30 mL/min/1.73 m2 and < 60 mL/min/1.73 m2 | 0.90 |
| eGFR ≤ 30 mL/min/1.73 m2 | 0.02* |
| eGFR > 30 mL/min/1.73 m2 and < 60 mL/min/1.73 m2 (control) | |
| eGFR ≤ 30 mL/min/1.73 m2 | < 0.01** |
eGFR estimated glomerular filtration rate. *P < 0.05; **P < 0.01.
Risk factors for PICM
A univariate Cox regression analysis of clinical features that were associated with the prediction of PICM was performed (Table 4). Every 10 mL/min/1.73 m2 decrease in the eGFR (HR 1.19, 95% CI 1.01–1.40), an eGFR ≤ 30 mL/min/1.73 m2 (HR 3.47, 95% CI 1.48–8.16), and a medical history of CAD (HR 2.76, 95% CI 1.36–5.60) were significantly related to the development of PICM. However, neither LBBB nor every 10-ms prolongation of the QRS duration was significantly associated with PICM (HR 0.86, 95% CI 0.73–1.01; HR 1.19, 95% CI 1.01–1.40, respectively).
Table 4.
Univariate Cox model of hazard ratios for pacemaker-induced cardiomyopathy.
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Every 10-year increase in age | 0.97 | 0.72–1.31 | 0.83 |
| Male sex | 1.00 | 0.50–2.01 | 1.00 |
| Every 10% decrease in the baseline LVEF | 0.98 | 0.70–1.36 | 0.90 |
| Every 10-ms prolongation of the QRS duration | 0.86 | 0.73–1.01 | 0.07 |
| LBBB | 0.05 | 0.00–170.00 | 0.46 |
| History of AF | 0.84 | 0.42–1.69 | 0.63 |
| Every 10 mL/min/1.73 m2 decrease in eGFR | 1.19 | 1.01–1.40 | 0.04* |
| eGFR ≤ 30 mL/min/1.73 m2 | 3.47 | 1.48–8.16 | < 0.01** |
| CAD | 2.76 | 1.36–5.60 | < 0.01** |
HR hazard ratio, CI confidence intervals, LVEF left ventricular ejection fraction, LBBB left bundle branch brock, AF atrial fibrillation, eGFR estimated glomerular filtration rate, CAD coronary artery disease. *P < 0.05; **P < 0.01.
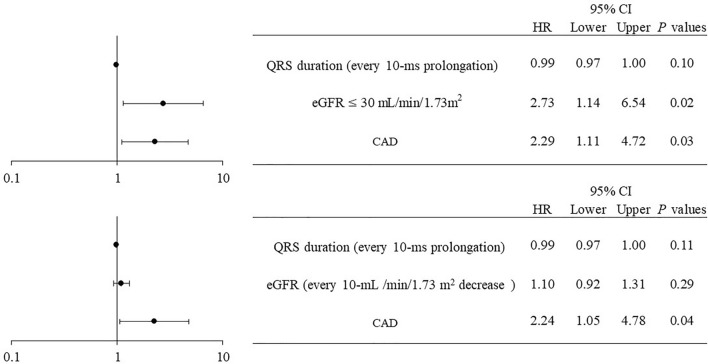
After adjusting for every 10-ms prolongation of the QRS duration, an eGFR ≤ 30 mL/min/1.73 m2, and a medical history of CAD, the multivariate Cox regression analysis showed that an eGFR ≤ 30 mL/min/1.73 m2 and CAD were significant independent risk factors for PICM (HR 2.62, 95% CI 1.09–6.29, and HR 2.32, 95% CI 1.13–4.80, respectively) (Fig. 4).
Figure 4.
Forest plot of hazard ratios for PICM according to a multivariate analysis by the Cox model. After adjusting for the QRS duration, eGFR, and CAD, a multivariate Cox regression analysis showed that an eGFR ≤ 30 mL/min/1.73 m2 and CAD were independent risk factors for developing PICM. CI confidence interval, HR hazard ratio, eGFR estimated glomerular filtration rate, CAD coronary artery disease.
Discussion
Previous studies have reported that an older age6,7,9, male sex7,13, a history of myocardial infarction13, a lower baseline LVEF14,15, a wider intrinsic QRS duration7,13,16, LBBB13, a history of AF6,17, and a chronic higher RV pacing burden8 could be risk factors for PICM. However, these variables, but for a medical history of CAD, were not risk factors for PICM in our analysis.
Although it has been the general opinion that RV pacing burden has detrimental effects on left ventricular function, the present study revealed a higher proportion of patients who underwent PMI because of SSS rather than AVB in the PICM group (Table 1). Additionally, there was no difference in the percentage of RV pacing between patients with PICM and no PICM (Table 2). Recently, it was reported that no significant difference in the development of severe LV dysfunction is observed among patients with SSS and AVB in a large cohort of pacemaker recipients with normal LVEF20. Sanchez et al. showed that developing HF was not associated with pacing mode, %VP, or ventricular lead localization in patients with SSS21. These findings suggested that PICM could be developed in patients with SSS regardless of RV pacing burden.
The patients with PICM had a more prevalent medical history of CAD and a lower eGFR than those without PICM in this study. The multivariate Cox hazard model analysis also showed that a medical history of CAD or stages 4–5 CKD defined as an eGFR < 30 mL/min/1.73 m2 were significant risk factors associated with PICM. The result indicated that the medical history of CAD could be an independent risk factor for PICM as a past study reported. Tayal et al. reported that PMI patients with an antecedent history of MI and CKD had an increased risk of HF22. However, there is no previous study reported an association between renal dysfunction and PICM13. To the best of our knowledge, this study is the first report to show that an eGFR ≤ 30 mL/min/1.73 m2 is an independent risk factor for PICM.
The frequencies of PICM have been reported to be 5.1–26.8% at follow-up, with a mean period of 1–15 years23. The incidence rate of PICM in our study was 29.2% during a mean of 1.6 years of follow-up, which was slightly higher than that in many previous studies. However, a previous study reported a 39% prevalence of PICM according to the same definition as this study2. From the other point of view, this difference in the incidence in current study could be explained by the high prevalence of severe CKD (eGFR ≤ 30 mL/min/1.73 m2) in patients with PICM, in other words, a total of 11.5% of patients had a low GFR < 30 ml/min/1.73 m2.
Renal impairment confers a high risk for poor cardiovascular outcomes24, increasing mortality in patients with HF25. In particular, moderate/severe renal impairment has a high risk of developing HF26. Various mechanistic insights have been proposed for this finding. Renal impairment can upregulate the renin–angiotensin–aldosterone system and enhance basal sympathetic nerve discharge, increasing pro-inflammatory factors and oxidative stress. Additionally, endothelial dysfunction27, exacerbation of underlying anemia, and worsening of LV hypertrophy and myocyte contractility are related to the incidence of HF and impairment of ventricular systolic function.
The high phosphorus status associated with CKD also promotes the calcification of cardiac vessels and valves, further accelerating a reduced LVEF28. Atherosclerosis and CKD affect cardiac function through their interaction that worsens with each other.
Metabolic causality has also been reported as the cause of PICM. Several studies have proposed that RV pacing can induce abnormal myocardial metabolism and altered regional perfusion, increase fibrosis, and cause myofibrillar disarray29–31. However, these possibilities have still not been proven.
Recently, Lin et al. suggested a mechanism of PICM by which intracellular lipid accumulation induced by pacing increases fibrosis in the LV myocardium in some animal models, including pigs and rats32,33. In addition, they showed that the inhibition of the liver X receptor/retinoid X receptor pathway, which regulates lipid metabolism, inflammation, and cholesterol to bile acid catabolism, was associated with pacing32.
Moreover, the lipid-lowering and pleiotropic effects of treatment with statins are attributed to preventing PICM and reducing HF hospitalization, cardiovascular death, and all-cause mortality in patients with AVB33. These results suggest a causative role of lipid accumulation in PICM. Even in non-pacemaker-implanted patients, such as patients with diabetes mellitus and obesity, abnormal intramyocardial lipid accumulation has been frequently observed owing to metabolic changes in the heart. In addition, cardiac lipid accumulation is positively correlated with cardiac dysfunction, which is called lipotoxic cardiomyopathy34. Taking these findings into consideration, we believe that cardiac metabolic modulation due to RV pacing plays an important role in the impairment of cardiac function.
Our results could help to identify patients who develop PICM before PMI. Severe renal dysfunction and a history of CAD could be independent risk factors for PICM. Therefore, patients with these risk factors require closer follow-up by TTE to avoid missing the appropriate timing for upgrading to cardiac resynchronization therapy. Furthermore, conduction system pacing, such as His-bundle pacing and left-bundle pacing, are alternative options in patients with AVB and high-risk factors when they undergo PMI.
Limitations
Our study has some limitations. First, this was a retrospective study from a single center with a comparatively small number of participants, and it did not reflect direct causation. Second, measurement with TTE was performed among not all patients with pacemaker, and the time window for performing follow-up TTE was unclear. Our observational periods for the occurrence of PICM were not long term but medium term instead. Third, PMI procedures in the current study likely included some older materials and methods, and our data lacked information on the lead tip position. Finally, further studies regarding the effects of CAD and CKD on the incidence of PICM after PMI are required to confirm our findings.
Conclusions
A past medical history of CAD and severe stage CKD, stage 4 or 5, at PMI are newly discovered risk factors of PICM. Therefore, intensive follow-up is required to detect a deterioration in the LVEF at an early stage.
Supplementary Information
Author contributions
M.O. contributed to the study design, data collection, data analysis and interpretation, and manuscript writing. E.H. and K.F. contributed to data interpretation and writing of the manuscript. G.K., K.K., T.O., T.M., Y.S., G.O., and T.K. contributed to data collection and management. I.K. was the senior supervisor of the study. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eriko Hasumi, Email: ehasumi-circ@umin.ac.jp.
Katsuhito Fujiu, Email: fujiu-tky@g.ecc.u-tokyo.ac.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-43953-7.
References
- 1.Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: Echocardiographic and clinical outcome. J. Am. Coll. Cardiol. 2003;42:614–623. doi: 10.1016/S0735-1097(03)00757-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaye G, Ng JY, Ahmed S, Valencia D, Harrop D, Ng ACT. The prevalence of pacing-induced cardiomyopathy (PICM) in patients with long term right ventricular pacing—Is it a matter of definition? Heart Lung Circ. 2019;28:1027–1033. doi: 10.1016/j.hlc.2018.05.196. [DOI] [PubMed] [Google Scholar]
- 3.Gebauer RA, Tomek V, Salameh A, Marek J, Chaloupecký V, Gebauer R, Matějka T, Vojtovič P, Janoušek J. Predictors of left ventricular remodelling and failure in right ventricular pacing in the young. Eur. Heart J. 2009;30:1097–1104. doi: 10.1093/eurheartj/ehp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillis AM. Optimal pacing for right ventricular and biventricular devices minimizing, maximizing, and right ventricular/left ventricular site considerations. Circulation. 2014;7:968–977. doi: 10.1161/CIRCEP.114.001360. [DOI] [PubMed] [Google Scholar]
- 5.Bansal R, Parakh N, Gupta A, Juneja R, Naik N, Yadav R, Sharma G, Roy A, Verma SK, Bahl VK. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. J. Interv. Cardiac Electrophysiol. 2019;56:63–70. doi: 10.1007/s10840-019-00602-2. [DOI] [PubMed] [Google Scholar]
- 6.Merchant FM, Mittal S. Pacing-induced cardiomyopathy. Card. Electrophysiol Clin. 2018;10:437–445. doi: 10.1016/j.ccep.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, Frankel DS. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11:1619–1625. doi: 10.1016/j.hrthm.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, Kanj M, Wazni OM, Saliba WI, Varma N, Wilkoff BL, Cantillon DJ. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Lee SA, Cha MJ, Cho Y, Oh IY, Choi EK, Oh S. Paced QRS duration and myocardial scar amount: Predictors of long-term outcome of right ventricular apical pacing. Heart Vessels. 2016;31:1131–1139. doi: 10.1007/s00380-015-0707-8. [DOI] [PubMed] [Google Scholar]
- 10.Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, Fang F, Lam KH, Chan HC, Fung JW. Biventricular pacing in patients with bradycardia and normal ejection fraction. N. Engl. J. Med. 2009;361:2123–2134. doi: 10.1056/NEJMoa0907555. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi Y, Mitsuhashi T, Akashi N, Hayashi T, Umemoto T, Sugawara Y, Fujita H, Momomura SI. Clinical characteristics associated with pacing-induced cardiac dysfunction: A high incidence of undiagnosed cardiac sarcoidosis before permanent pacemaker implantation. Heart Vessels. 2018;33:1505–1514. doi: 10.1007/s00380-018-1206-5. [DOI] [PubMed] [Google Scholar]
- 12.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A World Society of Arrhythmia's project. Pacing Clin. Electrophysiol. 2011;34:1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 13.Cho SW, Gwag HB, Hwang JK, Chun KJ, Park KM, On YK, Kim JS, Park SJ. Clinical features, predictors, and long-term prognosis of pacing-induced cardiomyopathy. Eur. J. Heart Fail. 2019;21:643–651. doi: 10.1002/ejhf.1427. [DOI] [PubMed] [Google Scholar]
- 14.Sharma AD, Rizo-Patron C, Hallstrom AP, O'Neill GP, Rothbart S, Martins JB, Roelke M, Steinberg JS, Greene HL, Investigators D. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm. 2005;2:830–834. doi: 10.1016/j.hrthm.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Curtis AB, Worley SJ, Chung ES, Li P, Christman SA, St John Sutton M. Improvement in clinical outcomes with biventricular versus right ventricular pacing: The BLOCK HF study. J. Am. Coll. Cardiol. 2016;67:2148–2157. doi: 10.1016/j.jacc.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Kang KW, Chin JY, Kim TS, Park JH, Choi YJ. Major determinant of the occurrence of pacing-induced cardiomyopathy in complete atrioventricular block: A multicentre, retrospective analysis over a 15-year period in South Korea. BMJ Open. 2018;8:e019048. doi: 10.1136/bmjopen-2017-019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchant FM, Hoskins MH, Musat DL, Prillinger JB, Roberts GJ, Nabutovsky Y, Mittal S. Incidence and time course for developing heart failure with high-burden right ventricular pacing. Circ. Cardiovasc. Qual. Outcomes. 2017;10:e003564. doi: 10.1161/CIRCOUTCOMES.117.003564. [DOI] [PubMed] [Google Scholar]
- 18.Merchant FM, Mittal S. Pacing induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 2020;31:286–292. doi: 10.1111/jce.14277. [DOI] [PubMed] [Google Scholar]
- 19.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Ebert M, Jander N, Minners J, Blum T, Doering M, Bollmann A, Hindricks G, Arentz T, Kalusche D, Richter S. Long-term impact of right ventricular pacing on left ventricular systolic function in pacemaker recipients with preserved ejection fraction: Results from a large single-center registry. J. Am. Heart Assoc. 2016;5:e003485. doi: 10.1161/JAHA.116.003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riahi S, Nielsen JC, Hjortshøj S, Thomsen PEB, Højberg S, Møller M, Dalsgaard D, Nielsen T, Asklund M, Friis EV, Christensen PD, Simonsen EH, Eriksen UH, Jensen GVH, Svendsen JH, Toff WD, Healey JS, Andersen HR, DANPACE Investigators Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: No association with pacing mode or right ventricular pacing site. EP Europace. 2012;14:1475–1482. doi: 10.1093/europace/eus069. [DOI] [PubMed] [Google Scholar]
- 22.Tayal B, Fruelund P, Sogaard P, Riahi S, Polcwiartek C, Atwater BD, Gislason G, Risum N, Torp-Pedersen C, Kober L, Kragholm KH. Incidence of heart failure after pacemaker implantation: A nationwide Danish Registry-based follow-up study. Eur. Heart J. 2019;40:3641–3648. doi: 10.1093/eurheartj/ehz584. [DOI] [PubMed] [Google Scholar]
- 23.Abbas J, Zulqarnain M, Waqar F, Waqar Z, Malik J, Satti DI, Zaidi SMJ. Incidence and predictors of pacemaker-induced cardiomyopathy with right ventricular pacing: A systematic review. Expert Rev. Cardiovasc. Ther. 2022;20:267–273. doi: 10.1080/14779072.2022.2062323. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 25.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J. Am. Coll. Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 26.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J. Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 27.Zuchi C, Tritto I, Carluccio E, Mattei C, Cattadori G, Ambrosio G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2020;25:21–30. doi: 10.1007/s10741-019-09881-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, Ford V, Raj D, Porter AC, Soliman EZ, Wright JT, Jr, Wolf M, He J. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017;2:635–643. doi: 10.1001/jamacardio.2017.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karpawich PP, Justice CD, Cavitt DL, Chang CH. Developmental sequelae of fixed-rate ventricular pacing in the immature canine heart: An electrophysiologic, hemodynamic, and histopathologic evaluation. Am Heart J. 1990;119:1077–1083. doi: 10.1016/S0002-8703(05)80237-6. [DOI] [PubMed] [Google Scholar]
- 30.Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am. J. Physiol. 1990;259:H300–H308. doi: 10.1152/ajpheart.1990.259.2.H300. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen JC, Bottcher M, Nielsen TT, Pedersen AK, Andersen HR. Regional myocardial blood flow in patients with sick sinus syndrome randomized to long-term single chamber atrial or dual chamber pacing–effect of pacing mode and rate. J. Am. Coll. Cardiol. 2000;35:1453–1461. doi: 10.1016/S0735-1097(00)00593-3. [DOI] [PubMed] [Google Scholar]
- 32.Lin YS, Chang TH, Shi CS, Wang YZ, Ho WC, Huang HD, Chang ST, Pan KL, Chen MC. Liver X receptor/retinoid X receptor pathway plays a regulatory role in pacing‐induced cardiomyopathy. J. Am. Heart Assoc. 2019;8:e009146. doi: 10.1161/JAHA.118.009146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YS, Lip GYH, Ho WC, Shi CS, Lin MH, Kuo TY, Chung CM, Chang ST, Chen YL, Chen HC, Lee WC, Chen MC. Statins to prevent pacing-induced cardiomyopathy: Evidence from the bench applied to clinical studies. Heart Rhythm. 2022;19:960–968. doi: 10.1016/j.hrthm.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Ruberg FL. Myocardial lipid accumulation in the diabetic heart. Circulation. 2007;116:1110–1112. doi: 10.1161/CIRCULATIONAHA.107.721860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.