Abstract
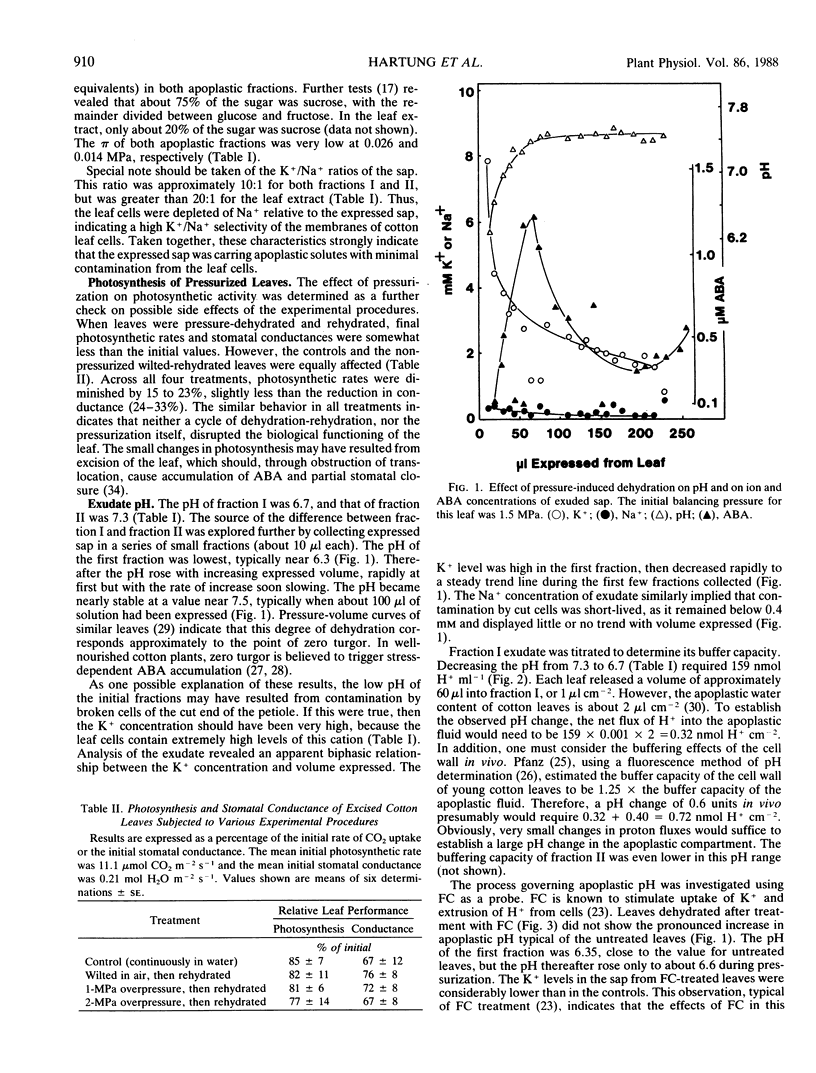
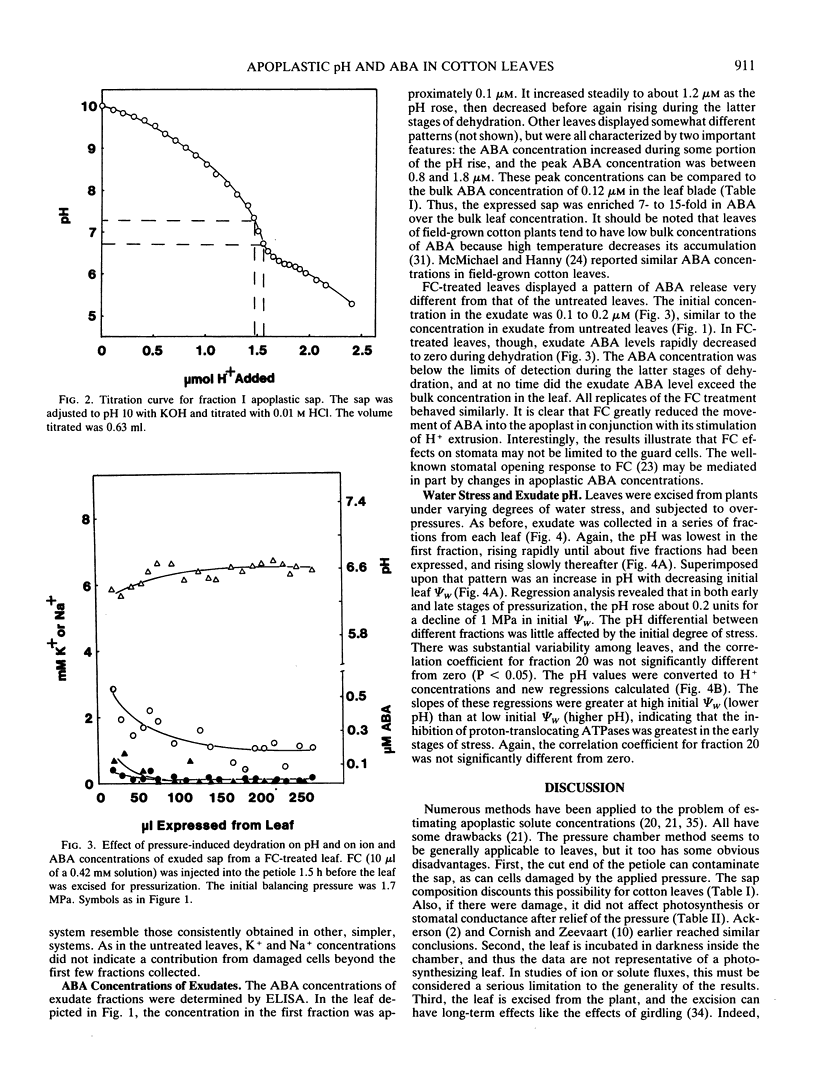
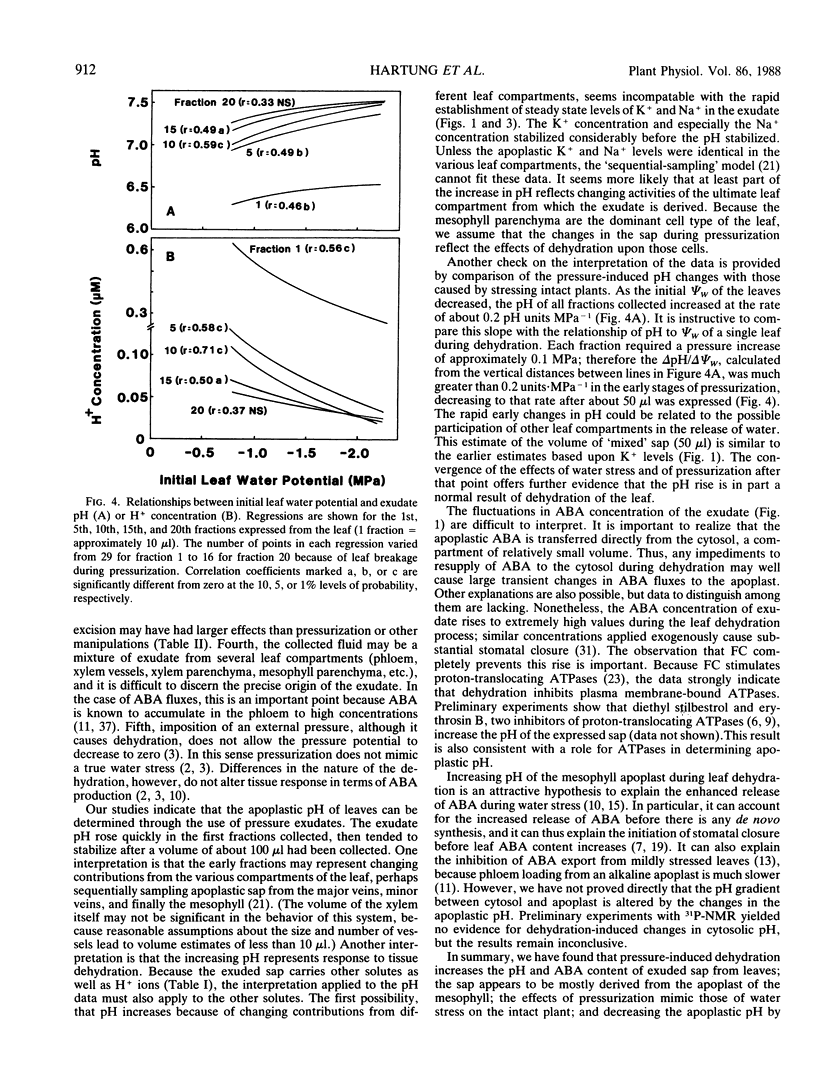
Leaves of cotton (Gossypium hirsutum L.) were subjected to overpressures in a pressure chamber, and the exuded sap was collected and analyzed. The exudate contained low concentrations of solutes that were abundant in total leaf extracts, and photosynthetic rates and stomatal conductance were completely unaffected by a cycle of pressurization and rehydration. These criteria and others indicate that the experimental techniques inflicted no damage upon the leaf cells. The pH and abscisic acid (ABA) content of the apoplastic fluid both increased greatly with pressure-induced dehydration. Although ABA concentrations did not reach a steady state, the peak levels were above 1 micromolar, an order of magnitude greater than bulk ABA concentrations of the leaf blades. Treatment of leaves with fusicoccin decreased the K+ concentration, greatly reduced the pH rise, and completely eliminated the increase in ABA in the apoplast upon dehydration. When water-stressed leaves were pressurized, the pH of the exuded sap was increased by 0.2 units per 1 megapascal decrease in initial leaf water potential. Buffer capacity of the sap was least in the pH range of interest (6.5-7.5), allowing extremely small changes in H+ fluxes to create large changes in apoplastic pH. The data indicate that dehydration causes large changes in apoplastic pH, perhaps by effects on ATPases; the altered pH then enhances the release of ABA from mesophyll cells into the apoplastic fluid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerson R. C., Radin J. W. Abscisic Acid accumulation in cotton leaves in response to dehydration at high pressure. Plant Physiol. 1983 Feb;71(2):432–433. doi: 10.1104/pp.71.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerson R. C. Stomatal response of cotton to water stress and abscisic Acid as affected by water stress history. Plant Physiol. 1980 Mar;65(3):455–459. doi: 10.1104/pp.65.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerson R. C. Synthesis and movement of abscisic Acid in water-stressed cotton leaves. Plant Physiol. 1982 Mar;69(3):609–613. doi: 10.1104/pp.69.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthon G. E., Spanswick R. M. Purification and properties of the h-translocating ATPase from the plasma membrane of tomato roots. Plant Physiol. 1986 Aug;81(4):1080–1085. doi: 10.1104/pp.81.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsell M. F., Cohen D. Relationships between Leaf Water Status, Abscisic Acid Levels, and Stomatal Resistance in Maize and Sorghum. Plant Physiol. 1975 Aug;56(2):207–212. doi: 10.1104/pp.56.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Metabolism in Relation to Water Stress and Leaf Age in Xanthium strumarium. Plant Physiol. 1984 Dec;76(4):1029–1035. doi: 10.1104/pp.76.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn G., Brummett D. L. Concentrations of abscisic Acid and indoleacetic Acid in cotton fruits and their abscission zones in relation to fruit retention. Plant Physiol. 1987 Jan;83(1):199–202. doi: 10.1104/pp.83.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Huber S. C. Diurnal fluctuations in cotton leaf carbon export, carbohydrate content, and sucrose synthesizing enzymes. Plant Physiol. 1986 Jun;81(2):584–586. doi: 10.1104/pp.81.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F. C., Bennett A. B., Spanswick R. M. Concentrations of sucrose and nitrogenous compounds in the apoplast of developing soybean seed coats and embryos. Plant Physiol. 1984 May;75(1):181–186. doi: 10.1104/pp.75.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jachetta J. J., Appleby A. P., Boersma L. Use of the pressure vessel to measure concentrations of solutes in apoplastic and membrane-filtered symplastic sap in sunflower leaves. Plant Physiol. 1986 Dec;82(4):995–999. doi: 10.1104/pp.82.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Radin J. W., Parker L. L., Guinn G. Water Relations of Cotton Plants under Nitrogen Deficiency: V. Environmental Control of Abscisic Acid Accumulation and Stomatal Sensitivity to Abscisic Acid. Plant Physiol. 1982 Oct;70(4):1066–1070. doi: 10.1104/pp.70.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Parker L. L. Water Relations of Cotton Plants under Nitrogen Deficiency: I. Dependence upon Leaf Structure. Plant Physiol. 1979 Sep;64(3):495–498. doi: 10.1104/pp.64.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W. Water Relations of Cotton Plants under Nitrogen Deficiency: III. STOMATAL CONDUCTANCE, PHOTOSYNTHESIS, AND ABSCISIC ACID ACCUMULATION DURING DROUGHT. Plant Physiol. 1981 Jan;67(1):115–119. doi: 10.1104/pp.67.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A., Brenner M. L. Effect of obstructed translocation on leaf abscisic Acid, and associated stomatal closure and photosynthesis decline. Plant Physiol. 1980 Jun;65(6):1111–1115. doi: 10.1104/pp.65.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M. E., Bonner B. A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980 Aug;66(2):321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Sites of Abscisic Acid Synthesis and Metabolism in Ricinus communis L. Plant Physiol. 1977 May;59(5):788–791. doi: 10.1104/pp.59.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]


