Abstract
People with HIV (PWH) often develop HIV-related neurological impairments known as HIV-associated neurocognitive disorder (HAND), but cognitive dysfunction in older PWH may also be due to age-related disorders such as Alzheimer's disease (AD). Discerning these two conditions is challenging since the specific neural characteristics are not well understood and limited studies have probed HAND and AD spectrum (ADS) directly. We examined the neural dynamics underlying motor processing during cognitive interference using magnetoencephalography (MEG) in 22 biomarker-confirmed patients on the ADS, 22 older participants diagnosed with HAND, and 30 healthy aging controls. MEG data were transformed into the time-frequency domain to examine movement-related oscillatory activity and the impact of cognitive interference on distinct stages of motor programming. Both cognitively impaired groups (ADS/HAND) performed significantly worse on the task (e.g., less accurate and slower reaction time) and exhibited reductions in frontal and cerebellar beta and parietal gamma activity relative to controls. Disease-specific aberrations were also detected such that those with HAND exhibited weaker gamma interference effects than those on the ADS in frontoparietal and motor areas. Additionally, temporally distinct beta interference effects were identified, with ADS participants exhibiting stronger beta interference activity in the temporal cortex during motor planning, along with weaker beta interference oscillations dispersed across frontoparietal and cerebellar cortices during movement execution relative to those with HAND. These results indicate both overlapping and distinct neurophysiological aberrations in those with ADS disorders or HAND in key motor and top-down cognitive processing regions during cognitive interference and provide new evidence for distinct neuropathology.
Keywords: neuroHIV, Magnetoencephalography, MEG, Cognitive interference, Top-down
1. Introduction
Emergence of combination antiretroviral therapies (cARTs) have transformed a diagnosis of HIV from a death sentence to a manageable, chronic condition. However, many HIV-related comorbidities remain prevalent despite these recent advances in medicine, with cognitive and related neurological conditions such as HIV-related neurocognitive disorder (HAND) affecting between 30 and 70% of the entire HIV-infected population (Heaton et al., 2004; Namagga et al., 2019). Onset of impairments in PWH who develop HAND typically occurs in mid adulthood (e.g., thirties and forties), although the use of cART has also drastically increased life expectancy of PWH, and now the underlying cause of cognitive decline has become more ambiguous in PWH. For example, age-related neurological conditions such as Alzheimer's disease (AD) arise in late adulthood (Milanini and Valcour, 2017), and thus, may contribute to cognitive impairment, especially in PWH who first experience deficits in later adulthood. Therefore, identifying the pathological origin of cognitive dysfunction in older PWH and how this differs relative to those with other forms of dementia (e.g., AD) is critical.
Challenges arise when disentangling the root of cognitive impairment in older PWH because HAND and AD spectrum (ADS) disorders have similar phenotypes. For example, ADS and HAND are often characterized by deficits in functioning across multiple cognitive domains (e.g., visuospatial processing, attention, cognitive control) and these common deficits extend to motor control. Such dysfunction would likely be exacerbated by deficits in cognitive control and conflict resolution, although studies in this area are sparse. Cognitive testing and amyloid imaging using positron emission tomography (PET) have formulated a standard diagnostic criterion for ADS disorders. Meanwhile, PET studies examining beta-amyloid deposition in PWH have shown amyloid levels similar to those found in cognitively unimpaired controls (Fulop et al., 2019; Howdle et al., 2020; Mohamed et al., 2020). These findings suggest ADS and HAND are at least, in part, pathologically distinct. However, interestingly, several case studies have reported on older PWH who have hallmark ADS biomarkers (e.g., amyloid deposits and neurofibrillary tangles) and presented with cognitive impairment (Calcagno et al., 2021; Turner et al., 2016). Thus, it is likely that at least some cognitively impaired older PWH have ADS-like pathology and not HAND, which is intuitive since the prevalence of AD would not be expected to differ in older PWH. Identifying the unique neuropathological signatures of HAND and ADS is critical and may help inform new therapeutic methods aimed at the disease-specific features of each condition while simultaneously filling an expansive gap in the literature. Identifying such features using noninvasive measures would have an even broader impact.
Previous neuroimaging research in ADS and HAND populations individually have found common structural and functional aberrations in areas involved in attention, executive function, and sensory processing. For example, PWH and those with ADS tend to have diminished functional connectivity dispersed across the sensorimotor network (Becker et al., 2013; Cai et al., 2017; du Plessis et al., 2017; Koelewijn et al., 2017; Thomas et al., 2013; Zhou et al., 2017). PWH also exhibit reductions in gray matter volumes in sensorimotor regions (Lew et al., 2021; Schantell et al., 2021; Zhou et al., 2017). While regional hyper-activity in motor cortices is a critical feature of neuroHIV and ADS, movement-related neurological dysfunction in ADS appears to be more region dependent such that primary motor areas exhibit hyperexcitability while higher motor regions exhibit weaker activation (Albers et al., 2015; Vidoni et al., 2012; Wilson et al., 2013; Zhou et al., 2017). Though evidence from previous work suggests potential convergences and divergences in the neural aberrations serving motor control and processing in ADS and HAND (Casagrande et al., 2022; Meehan et al., 2022, 2023), no neuroimaging research has directly compared the aberrant movement-related neurophysiological signatures of ADS and HAND.
Numerous magnetoencephalographic (MEG) studies have examined the aberrant neural dynamics in ADS and HAND independently. Utilization of MEG allows direct measurements and signal decomposition of neural activity into multiple frequency bands supporting cognition, including beta (15–30 Hz) and gamma (30+ Hz), which are key cortical oscillations for movement control and processing (Barone and Rossiter, 2021; Heinrichs-Graham et al., 2018a; Khanna and Carmena, 2015; Muthukumaraswamy, 2010; Wilson et al., 2011a; Wilson et al., 2014, 2016). Such oscillations reflect shifts in the local synchronization of neural population-level activity within a specific, band-limited frequency range (e.g., theta, beta, gamma; Buzsáki et al., 2012; Buzsáki and Schomburg, 2015). Unfortunately, to date, most MEG studies of ADS and HAND (separately) have utilized resting-state designs, and to date, none have investigated disease-specific movement-related oscillatory activity, or how stimuli and response conflict affects motor processing in the brain. Previous work from our lab has quantified the oscillatory dynamics during motor control and found that PWH exhibit weaker perimovement beta responses in the primary motor cortices and hyper-responsivity in supplementary motor and frontal regions (Wilson et al., 2013), but this work did not probe the movement-related gamma synchronization response. Such beta responses are widely thought to reflect motor planning-related neural activity, while the movement-related gamma synchronization likely represents the movement execution signal (Wilson et al., 2016). While only one MEG study has probed motor processing in PWH, no MEG studies have examined the dynamic neural activity supporting motor control and processing in AD. Interestingly, however, an extensive number of studies have evaluated resting state EEG and MEG, which has shown that patients on the ADS exhibit weakened beta and stronger gamma activity (C. Huang et al., 2000; Koelewijn et al., 2017; Stam et al., 2002; van Deursen et al., 2008; Wiesman et al., 2021).
Herein, we examined the impact of cognitive interference on movement-related oscillatory activity in participants on the ADS and those with HAND using MEG. This work builds on our recent study that examined oscillatory differences in cognitive control networks in an overlapping group of participants with HAND and those on the ADS, which revealed critical differences in theta oscillations across multiple higher-level brain regions between patients and controls, as well as disease-specific findings (Meehan et al., 2023). Given the limited literature on motor control, our hypotheses were broadly framed and included disparate neural dynamics in ADS versus HAND in key cognitive control and motor processing and attention areas (e.g., frontoparietal and supplementary motor cortices). Specifically, we anticipated that the ADS group would have abnormal gamma and beta responses compared to those with HAND in the context of cognitive interference relative to no interference in frontoparietal and association motor regions, and that both clinical groups would exhibit aberrant motor-related interference oscillations in parietal and motor areas.
2. Materials and methods
2.1. Participants
Thirty-one amyloid-positive patients on the ADS with amnestic mild cognitive impairment or mild AD, as determined by a fellowship-trained neurologist specializing in memory disorders, were enrolled in this study. In addition, 25 cognitively impaired PWH who were receiving effective combination antiretroviral therapy were enrolled to comprise the HAND group. Finally, a control group of 31 older adults with normal cognition (17 amyloid-negative and 14 without PET) were also enrolled. Participants were between the ages of 51 and 79 years old. The groups were matched on all key demographics except age (i.e., the HAND group was slightly younger than healthy controls and ADS patients); thus, age was included as a nuisance covariate in all statistical modeling. Note that the data reported here was included in a previous publication focused on cognitive control processing (Meehan et al., 2023). However, the data were completely reanalyzed for the current investigation. Briefly, in the earlier paper, the onset of the arrow stimuli was time zero, which enabled us to examine the brain dynamics involved in cognitive control and interference suppression. In contrast, the onset of the motor response is time zero in the current study, which enables us to evaluate how brain responses time-locked to the behavioral motor response (i.e., button press) are affected by cognitive interference. This early difference in the processing pipeline dramatically changes the oscillatory neural responses that can be reliably detected.
Exclusion criteria included any medical illness affecting CNS function, any neurological disorder (other than AD/aMCI/HAND), history of head trauma, and current substance abuse. The local Institutional Review Board reviewed and approved this investigation. Written informed consent was obtained from each participant (and for ADS patients, from their informant, as well) following a detailed description of the study. In cases where a participant's capacity to consent was questionable, informed assent was obtained from the research participant, in addition to informed consent from a legally-authorized representative.
2.2. Neuropsychological testing
All participants underwent a battery of neuropsychological assessments, with raw scores for each participant being converted to demographically-adjusted z-scores using published normative data (Benedict et al., 1998; Brandt and Benedict, 2001; Heaton et al., 2004). This battery, which was developed in collaboration with a clinical neuropsychologist specializing in cognitive disorders, assessed multiple functional domains known to be impaired in patients with HAND and those on the AD spectrum (Table 1). Specifically, the cohort of PWH were assessed on the following cognitive domains per the Frascati criteria (Antinori et al., 2007): learning, memory, attention and executive function, motor, and processing speed. The ADS cohort completed a neuropsychological assessment that assessed: learning, memory, attention and executive function, language, and processing speed. In addition, we measured premorbid function and functional impairment in all participants, along with general cognitive status in the ADS group. Controls completed one of these two batteries depending on which patient cohort they were originally recruited with, although note that many assessments were included in both batteries (see Table 2). Using these assessments and the Lawton and Brody (1969) Activities of Daily Living (ADL), PWH were diagnosed with HAND according to the Frascati guidelines, including subgroups with asymptomatic neurocognitive impairment (ANI; i.e., having at least two cognitive domains one SD below the standardized mean, with no ADL deficits), mild neurocognitive disorder (MND; i.e., at least two cognitive domains one SD below the standardized mean, with ADL deficits), or HIV-associated dementia (HAD; i.e., having at least two cognitive domains two SDs below the standardized mean, with ADL deficits). Healthy controls were cognitively normal and did not meet the above criteria for neuropsychological impairment. For patients on the ADS, instrumental activities of daily living (IADLs) were measured (with an informant) using the Functional Activities Questionnaire (FAQ; Pfeffer et al., 1982). In addition to the neuropsychological battery, general cognitive status was measured using the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005) and the Mini-Mental State Examination (MMSE; Folstein et al., 1975). Finally, participants on the ADS completed the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) and the Quick Dementia Rating System (QDRS; Galvin, 2015) with an informant to better characterize clinical characteristics of ADS-specific impairment.
Table 1.
Participant demographics and disease characteristics.
| ADS (n = 22) |
HAND (n = 22) |
Healthy Controls (n = 30) |
|
|---|---|---|---|
| Age (years) | 69.41 (4.84) | 58.68 (6.11) | 65.21 (7.23) |
| Sex | 12 Females/10 Males | 12 Females/10 Males | 15 Females/15 Males |
| ADS Severity | 11 AD/11 MCI | – | – |
| QDRS - Total | 5.80 (2.15) | – | – |
| QDRS - Cognitive | 2.73 (0.97) | – | – |
| QDRS - Behavioral | 3.07 (1.44) | – | – |
| PSQI Total | 4.09 (2.71) | – | 5.40 (2.67) |
| HAND Severity | – | 17 ANI/1 MND/4 HAD | – |
| CD4 Nadir (cells/μL) | – | 221.41 (144.95) | – |
| Current CD4 (cells/μL) | – | 828.55 (376.61) | – |
| Years Since HIV Diagnosis | – | 14.36 (8.06) | – |
| Years on cART | – | 12.05 (6.70) | – |
| Years without cART Treatment | – | 2.32 (3.47) | – |
Note. Means and standard deviations are displayed for each continuous variable, and counts are shown for each categorical variable. ADS – Alzheimer's Disease Spectrum, AD – Alzheimer's Disease, MCI – Mild Cognitive Impairment, QDRS – Quick Dementia Rating System, PSQI – Pittsburgh Sleep Quality Index, HAND – HIV-associated Neurocognitive Disorders, ANI – Asymptomatic Neurocognitive Impairment, MND – Mild Neurocognitive Impairment, HAD – HIV-associated Dementia, cART – Combination antiretroviral therapy.
Table 2.
Neuropsychological domains and tests by cohort.
| Aging Cohort of PWH | AD Spectrum Cohort | ||||||
|---|---|---|---|---|---|---|---|
| Domain | Assessment |
HAND (n
= 22) |
Controls
(n = 14) |
Domain | Assessment |
ADS
(n = 22) |
Controls
(n = 16) |
| Learning | HVLT-R Learning Trials 1–3 1 | −1.77 (1.04) | 0.24 (0.72) | Learning | HVLT-R Learning Trials 1–3 1 | −1.71 (1.39) | −1.13 (1.65) |
| Memory | HVLT-R Delayed Recall 1 | −1.72 (1.10) | 0.25 (0.88) | WMS-IV Logical Memory I Recall1 | −1.23 (1.49) | −0.54 (1.63) | |
| HVLT-R Recognition Discriminability Index 1 | −1.13 (1.28) | 0.50 (0.58) | Memory | HVLT-R Delayed Recall 1 | −2.44 (1.33) | −1.72 (1.55) | |
| Phonemic verbal fluency 1 | −0.60 (0.99) | 0.14 (1.21) | HVLT-R Recognition Discriminability Index 1 | −2.44 (1.23) | −1.24 (1.68) | ||
| Executive Function | Semantic verbal fluency 1 | −0.31 (1.00) | 0.44 (1.09) | WMS-IV Logical Memory II Delayed Recall1 | −1.85 (1.43) | −1.02 (1.76) | |
| Trail Making Test Part B 1 | −0.23 (0.74) | 0.61 (0.81) | Attention and Executive Function | WMS-IV Logical Memory II Recognition1 | −0.54 (1.00) | −0.36 (1.04) | |
| Attention | Comalli Stroop Test Interference Trial1 | −1.29 (1.77) | 0.02 (1.30) | WAIS-IV Digit Span Forward, Backward, and Sequencing1 | −0.74 (1.26) | −0.28 (1.21) | |
| WAIS-III Symbol Search1 | −0.53 (0.69) | 1.33 (0.73) | Trail Making Test Part B 1 | −0.88 (1.52) | −0.40 (1.31) | ||
| Comalli Stroop Test Word Trial1 | −1.30 (1.40) | 0.13 (0.84) | Boston Naming Test1 | −0.32 (1.34) | 0.01 (1.31) | ||
| WAIS-III Digit Symbol Coding1 | −0.30 (0.71) | 1.67 (0.74) | Language | Phonemic verbal fluency 1 | −0.70 (1.04) | −0.75 (1.22) | |
| Processing Speed | Trail Making Test Part A 1 | −0.38 (0.67) | 0.46 (1.00) | Semantic verbal fluency 1 | −1.66 (1.59) | −1.09 (2.02) | |
| Comalli Stroop Test Color Trial1 | −0.91 (1.18) | 0.13 (0.84) | Processing Speed | WAIS-IV Digit Symbol Coding1 | −0.50 (1.57) | 0.16 (1.37) | |
| Motor | Grooved Pegboard – Dominant Hand1 | −1.15 (1.02) | 0.20 (0.95) | Trail Making Test Part A 1 | −1.21 (1.59) | −0.64 (1.57) | |
| Grooved Pegboard – Non-Dominant Hand1 | −1.35 (0.76) | 0.16 (0.70) | Functional Impairment | Functional Activities Questionnaire (FAQ)2 | 12.23 (5.79) | 0.19 (0.40) | |
| Functional Impairment | Modified version of the Lawton and Brody Instrumental Activities of Daily Living Scale Total Declines2 | 1.65 (1.97) | 1.00 (1.84) | General Cognitive Status | Montreal Cognitive Assessment (MoCA)2 | 24.95 (3.34) | 29.50 (0.73) |
| Mini-mental State Examination (MMSE)2 | 24.95 (3.34) | 29.50 (0.73) | |||||
| Premorbid Function | WRAT-4 Word Reading 1 | −0.82 (0.94) | 0.72 (1.05) | Premorbid Function | WRAT-4 Word Reading 1 | 0.35 (0.91) | 1.11 (0.80) |
Note. Mean demographically corrected z-scores and standard deviations of neuropsychological performance on the assessments included in each study battery by group. Assessments that are shared across both cohorts are in bold. PWH – People with HIV, ADS – Alzheimer's disease spectrum, HVLT-R – Hopkins Verbal Learning Test – Revised, WMS-IV – Wechsler Memory Scale, 4th Edition, WAIS-III – Wechsler Adult Intelligence Scale, 3rd Edition, WAIS-IV – Wechsler Adult Intelligence Scale, 4th Edition, WRAT-4 – Wide Range Achievement Test.
z-score.
Raw score.
2.3. Florbetapir 18F positron emission tomography
Combined PET/CT data using 18F-florbetapir (Amyvid™, Eli Lilly) and a GE Discovery MI digital scanner (Waukesha, WI) were collected following the standard procedures described by the Society of Nuclear Medicine and Molecular Imaging (3D acquisition; single intravenous slow-bolus <10 mL; dose = 370 MBq; waiting period = 30–50 min; acquisition = 10 min; Minoshima et al., 2016). Images were attenuation corrected using the CT data, reconstructed in MIMNeuro (slice thickness = 2 mm; Joshi et al., 2012), converted to voxel standardized uptake values based on body weight (SUVbw), and normalized into MNI space. Each scan was over-read by a fellowship-trained neuroradiologist blinded to group assignment and assessed as being “amyloid-positive” or “amyloid-negative” using established clinical criteria (Joshi et al., 2012). At this stage, patients who were amyloid-negative were excluded from the AD spectrum group.
2.4. MEG experimental paradigm and Behavioral analysis
During MEG recording, participants were seated in a nonmagnetic chair within a magnetically-shielded room. Participants performed 200 total trials of an arrow-based Eriksen flanker task (Eriksen and Eriksen, 1974; Lew et al., 2018, 2020; McDermott et al., 2017; Schantell et al., 2022a) while seated in the magnetically shielded room. Each trial began with a fixation that was presented for 1450 to 1550 milliseconds (ms), followed by a row of 5 arrows for 2500 ms. Participants indicated, with their right hand, whether the middle arrow was pointing to the left (index finger) or right (middle finger). Trials were pseudorandomized and equally divided between congruent and incongruent conditions, with left and right arrows being equally represented in each of the conditions. Responses with a reaction time 2.5 standard deviations (SDs) above or below the participant's mean were excluded prior to averaging. Group by condition 2 × 2 ANCOVAs, controlling for the effect of age, were used to probe for differences in reaction time and accuracy. We first compared the behavioral metrics in healthy controls and patients (ADS + HAND) to examine shared behavior in ADS and HAND relative to controls and then followed-up with a disease-focused analysis examining ADS versus HAND group comparisons.
2.5. MEG data acquisition
MEG data acquisition, structural coregistration, preprocessing, and sensor–/source-level analyses followed a similar pipeline as a number of previous manuscripts from our laboratory (Meehan et al., 2021; Spooner et al., 2019; Wiesman and Wilson, 2020). All recordings took place in a one-layer magnetically-shielded room with active shielding engaged for environmental noise compensation. A 306-sensor Elekta/MEGIN MEG system (Helsinki, Finland), equipped with 204 planar gradiometers and 102 magnetometers, was used to sample neuromagnetic responses continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz. Such planar gradiometers are more sensitive to magnetic fields generated by local sources (e.g., the brain), whereas magnetometers are equally sensitive to magnetic fields generated by local and distant (e.g., subways) sources. Participants were monitored by a real-time audio-video feed from inside the shielded room during MEG data acquisition. Each MEG dataset was individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (MaxFilter v2.2; correlation limit: 0.950; correlation window duration: 6 s; Taulu and Simola, 2006). Only the gradiometer data was used in further analyses.
2.6. Structural MRI processing and MEG coregistration
Prior to MEG acquisition, four coils were attached to the participants' heads and localized, together with the three fiducial points and scalp surface, using a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once positioned in the MEG, the coils were driven with an electrical current at a unique frequency and the magnetic fields associated with these currents were localized in reference to the MEG sensors array. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant's MEG data were co-registered with structural T1-weighted MRI data using BESA MRI (Version 2.0) prior to source-space analysis. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Following source analysis (i.e., beamforming), each participant's 4.0 × 4.0 × 4.0 mm functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
2.7. MEG preprocessing time-frequency transformation and sensor-level statistics
Cardiac and blink artifacts were identified in the raw MEG data and removed with signal-space projection (SSP), which was subsequently accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was then bandpass filtered between 0.5 and 200 Hz, plus a 60 Hz notch filter, and divided into 4000 ms epochs, with the baseline extending from −1800 to −1000 ms prior to movement onset (time: 0 ms). Epochs containing artifacts were rejected using a fixed threshold method, supplemented with visual inspection. Briefly, in MEG, the raw signal amplitude is strongly affected by the distance between the brain and the MEG sensors, as the magnetic field strength falls off sharply as the distance from the current source increases. To account for this source of variance across participants, as well as other sources of variance, we used an individually-determined threshold based on the within-subject signal distribution for both amplitude and gradient to reject artifacts. Across all participants, the average amplitude threshold for rejecting artifacts was 1132.43 (SD = 381.77) fT/cm and the average gradient threshold was 228.72 (SD = 136.79) fT/(cm*ms). Across all groups, an average of 167.66 (SD = 16.41) out of 200 possible trials per participant were used for further analysis in this experiment, including an average of 83.88 (SD = 8.09) out of 100 trials per participant in the incongruent condition and 83.78 (SD = 9.07) out of 100 trials per participant in the congruent condition. Importantly, our comparisons between groups and conditions were not affected by differences in the number of accepted trials per group, as this metric did not significantly differ as a function of condition (p = .96) or group (p = .27).
Complex demodulation (Kovach and Gander, 2016; Papp and Ktonas, 1977) was used to transform the artifact-free epochs into the time-frequency domain and the resulting spectral power estimations were averaged per sensor to generate time-frequency plots of mean spectral density. The time-frequency analysis was performed with a frequency-step of 2 Hz and a time-step of 25 ms between 4 and 100 Hz. These sensor-level data were then normalized by each respective bin's baseline power for visualization purposes, calculated as the mean power during the −1800 to −1000 ms baseline period.
The specific time-frequency windows used for source imaging were determined by statistical analysis of the sensor-level spectrograms across both conditions, all participants, and the entire array of gradiometers. Each data point in each sensor-level spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, paired sample t-tests against baseline were conducted on each data point and the output spectrogram of t-values was thresholded at p < .05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins (per sensor) that were also above the threshold (p < .05), and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, time-frequency windows within significant clusters were identified and used to guide source-level imaging. Cluster-based permutation testing on the sensor-array was performed in BESA Statistics (v2.1).
2.8. MEG source imaging
MEG data were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001; Hillebrand et al., 2005; Van Veen et al., 1997), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images were derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. In principle, the beamformer operator generates a spatial filter for each grid point that passes signals without attenuation from the given neural region, while suppressing activity in all other brain areas. The filter properties arise from the forward solution (lead field) for each location on a volumetric grid specified by input voxel space, and from the MEG covariance matrix. Basically, for each voxel, a set of beamformer weights is determined, which amounts to each MEG sensor being allocated a sensitivity weighting for activity in the particular voxel. Following convention, the source power in these images was normalized per participant using a pre-stimulus period (i.e., baseline) of equal duration and bandwidth (Hillebrand et al., 2005). MEG preprocessing and imaging were conducted using the Brain Electrical Source Analysis (version 7.0) software.
2.9. Statistical analysis
Normalized source power was computed for the selected time frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Each participant's functional images were transformed into standardized space using the transform that was previously applied to the structural images and then spatially resampled. The resulting 3D maps of brain activity were averaged across all participants to assess the anatomical basis of the significant oscillatory responses identified through the sensor-level analysis. Whole-brain flanker interference maps were computed by subtracting the congruent from the incongruent condition for each frequency band independently per participant. Using the resulting interference maps, we employed an ANCOVA approach, with age as a nuisance covariate. In our first level analyses, we collapsed across patient groups (ADS + HAND) and aimed to identify regions where neural oscillatory responses differed between patients and controls. Brain regions where significant group differences were found were then probed to identify disease specific effects (i.e., ADS versus HAND). Bayes Factors (BF01) were also computed to evaluate the probability of the null for non-significant group comparisons between ADS and HAND. Similarly, our second level analyses compared the ADS and HAND groups directly at the whole-brain level using interference maps of each oscillatory response and an ANCOVA approach with age as a nuisance covariate. Our goals were to identify differences in regional oscillatory activity during motor processing between the two clinical groups. To account for multiple comparisons, a significance threshold of p < .005 was used for the identification of significant clusters in all whole-brain statistical maps, accompanied by a cluster (k) threshold of at least 10 contiguous voxels (i.e., 640 mm3 of brain tissue) based on the theory of Gaussian random fields (Poline et al., 1995; Worsley et al., 1996, 1999). Age-adjusted unstandardized residual values for each variable of interest were generated by regressing such variables on age. The resulting residuals were used in figures to depict relationships in analyses of which age was adjusted for.
3. Results
3.1. Behavioral analysis
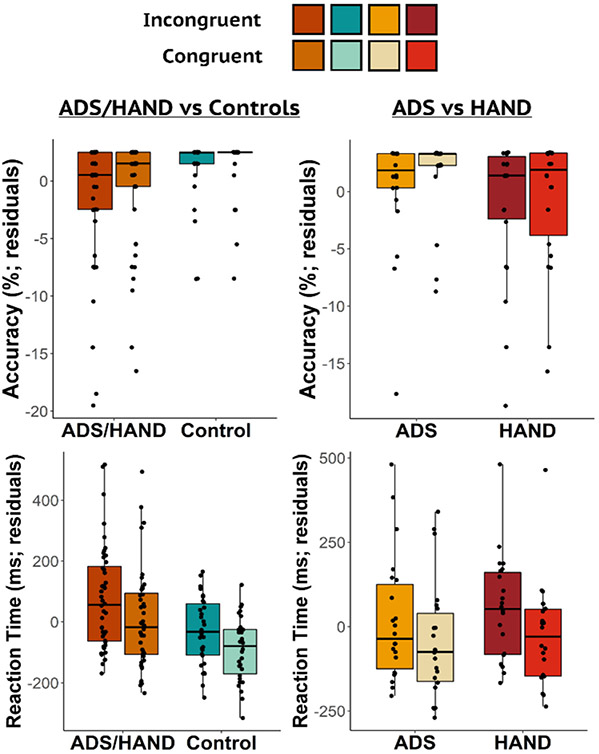
Thirteen participants were excluded from the final sample. Nine participants in the ADS group and two in the HAND group performed very poorly on the task (i.e., accuracy <60%) and two other participants (one HAND and one control) were removed due to poor MEG data quality. Thus, the final sample consisted of 22 participants on the ADS, 22 with HAND, and 30 healthy controls (refer to Table 1 for demographic and clinical characteristics). The remaining participants performed well on the task with high accuracy in both the incongruent (M = 97.14%, SD = 4.78%) and congruent (M = 97.82%, SD = 4.18%) conditions, and reasonable reaction times (incongruent: M = 762.83 ms, SD = 157.46 ms; congruent: M = 696.01 ms, SD = 148.53 ms). ANCOVAs with age as a nuisance covariate were used to assess group differences in behavior (i.e., accuracy and reaction time) during the Flanker task (Fig. 1). Primary analyses collapsing across both clinical groups (ADS + HAND) revealed worse performance relative to controls. Specifically, ADS + HAND participants were less accurate (F1,71 = 4.04, p = .048) and responded slower (F1,71 = 8.86, p = .004) than controls (Fig. 1). Comparisons between ADS and HAND groups revealed no differences in reaction time (p = .47) nor accuracy (p = .082).
Fig. 1.
Flanker Task Behavioral Results. Residuals of accuracy (top) and reaction time (bottom) controlling for the effect of age are shown on the y-axis for each condition with group on the x-axis. The impaired groups (ADS/HAND) were less accurate and slower to respond than controls. However, the two clinical groups did not differ from each other.
3.2. Neuropsychological assessment results
All participants underwent a battery of neuropsychological assessments (Table 2), which broadly showed that participants with HAND and those on the ADS performed worse than healthy controls. Further, those with HAND reported non-significantly higher levels of functional impairment than controls (t(34) = 0.99, p = .329), while those on the ADS reported greater functional impairment relative to healthy controls (t(36) = −8.27, p < .001).
3.3. MEG sensor-level analyses
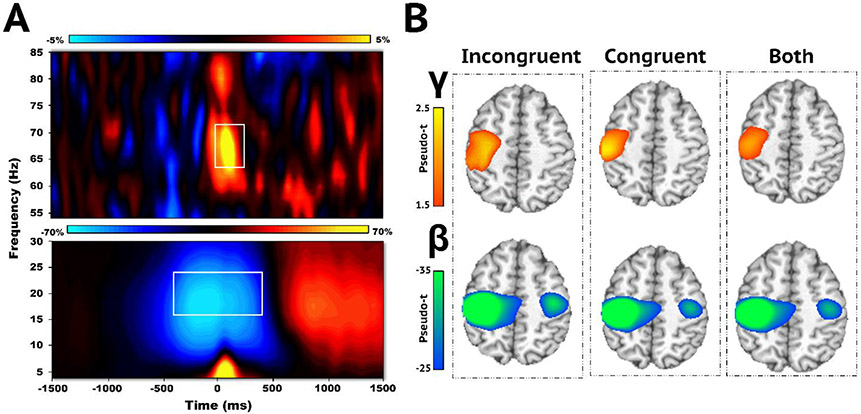
Sensor-level analyses across both conditions and all participants revealed two significant oscillatory responses consistent with previous MEG studies using this task (Embury et al., 2019; Heinrichs-Graham et al., 2018b; Spooner et al., 2021; Fig. 2A). Robust oscillatory decreases in the beta range (16–24 Hz) from −400 to 400 ms (0 ms = movement onset; p < .001, corrected) were observed, as well as significant increases relative to baseline in the gamma band (64–72 Hz) from −50 to 200 ms (p < .001, corrected).
Fig. 2.
Sensor-level Time-frequency Spectrograms and Whole-brain Average Maps. (A) Time-frequency spectrograms depicting movement-related oscillatory activity averaged across all trials, conditions, and participants. Time (ms) is presented on the x-axis and frequency (Hz) is on the y-axis. The color scale bar is shown above each spectrogram and indicates the percent change in amplitude from baseline. Strong increases in gamma (64–72 Hz; −50 to 200 ms) and decreases in peri-movement beta (16–24 Hz, −400 to 400 ms) activity were observed in a sensor near the contralateral primary motor cortex (MEG0443). Both oscillatory responses significantly differed from baseline (p < .001, corrected) and are denoted with white boxes. (B) Each brain image depicts the average maps across all participants for the following trial conditions: incongruent, congruent, and both conditions for each neural response. Gamma oscillations were identified in the contralateral primary motor cortex, while peri-movement beta activity was generated by populations of neurons in the bilateral motor cortices. Color scale bars to the left of each row indicates oscillatory response amplitude per voxel.
3.4. MEG source-level analyses
We then examined the cortical origins of the significant beta and gamma oscillatory responses identified in the time-frequency analysis. Whole-brain images were computed for each condition (i.e., incongruent and congruent) and averaged across all participants (Fig. 2B). Robust decreases in beta activity were observed in the bilateral primary motor cortices, while increases in gamma were more lateralized to the contralateral primary motor cortex.
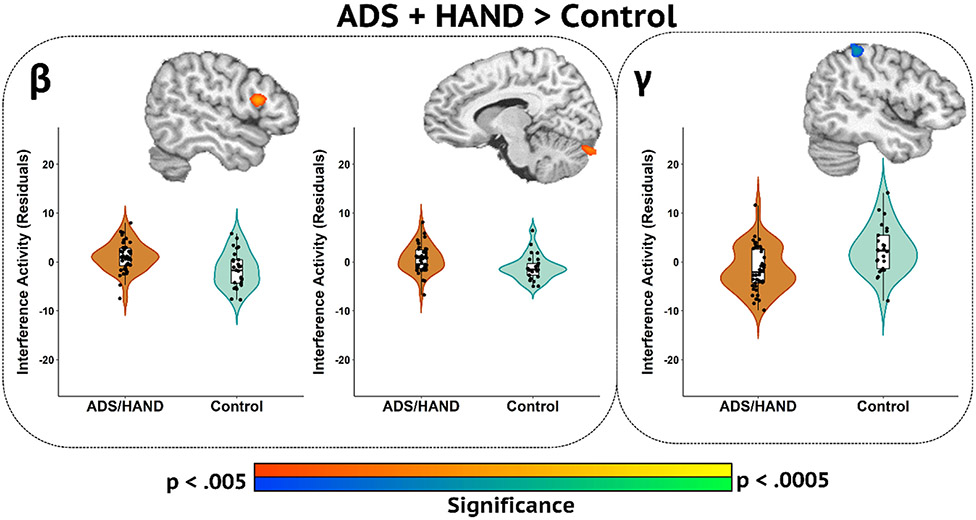
Next, flanker interference (incongruent – congruent) maps were computed and these served as the main input for statistical analyses. First, we collapsed across ADS and HAND groups and conducted ANCOVAs between patients (ADS + HAND) and controls. Our main findings indicated weaker beta interference (i.e., less negative) responses in patients relative to controls in the right inferior frontal gyrus and left cerebellum (Fig. 3; p < .005, corrected), as well as weaker gamma interference activity in the right superior parietal cortex in patients compared to controls (Fig. 3, p < .005, corrected). Post-hoc analyses of gamma and beta interference activity revealed that while ADS and HAND groups differed from controls, the two clinical groups did not differ from each other. Follow-up Bayesian analyses provided moderate evidence for there being no group differences between ADS and HAND groups for beta in the right inferior frontal gyrus (BF01 = 2.91), while there was weak evidence for there being no group differences in beta activity in the left cerebellum (BF01 = 0.98) and the right superior parietal cortex gamma response (BF01 = 1.62).
Fig. 3.
Whole-brain Group Difference Maps Between Participants with ADS or HAND and Controls. Whole-brain group difference maps of beta and gamma interference activity are displayed accompanied by violin plots for the peak voxel in each significant cluster. Beta interference group differences (p < .005, corrected) were revealed in the right inferior frontal gyrus (left) and left cerebellum (middle). Gamma interference differences between ADS /HAND and controls were found in the right superior parietal cortex (right). Residuals of amplitude interference values controlling for age are presented with each corresponding brain slice.
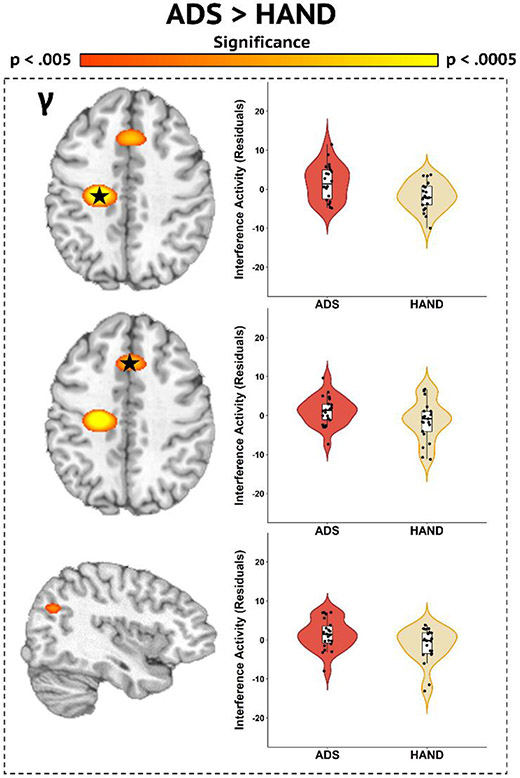
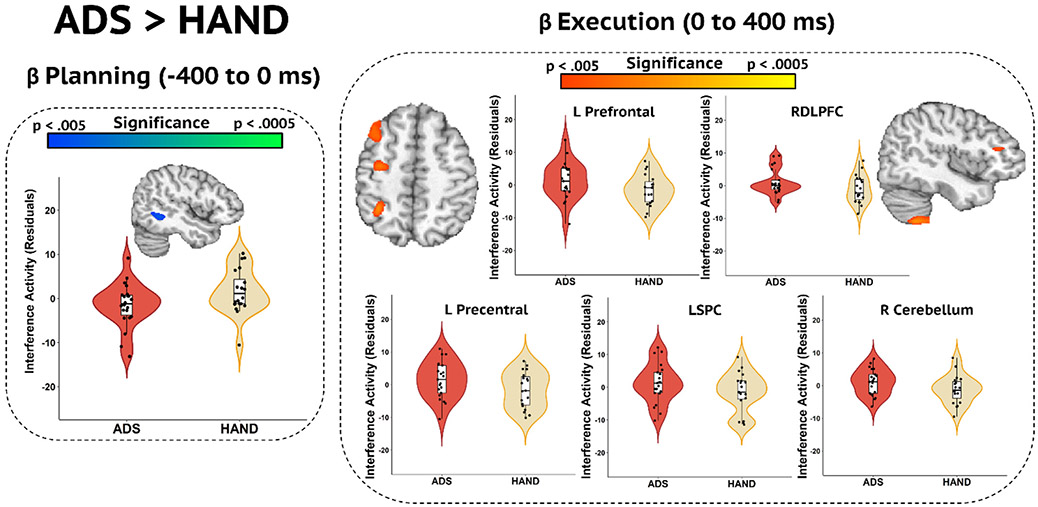
Following the initial analyses, we used a whole-brain approach to identify the regions in which ADS and HAND groups differed in oscillatory activity. Participants on the ADS exhibited stronger interference activity in the gamma band compared to those with HAND in the left premotor cortex, right pre-supplementary motor area, and right inferior parietal cortex (Fig. 4; p < .005, corrected). No differences were found in beta band activity. However, previous studies investigating the mechanisms supporting motor control have examined two distinct subprocesses of movement: planning and execution (Heinrichs-Graham et al., 2020; Heinrichs-Graham and Wilson, 2015; Kurz et al., 2014). Thus, we conducted an additional analysis to probe for differences in the subcomponents of motor processing by dissecting the beta (16–24 Hz) response into two separate 400 ms time bins prior to (i.e., planning) and following the movement onset (i.e., execution). The ADS group exhibited stronger beta interference activity during the motor planning period (−400 to 0 ms) than the HAND group in the right superior temporal area (Fig. 5; p < .005, corrected), which was followed by weaker interference responses in ADS during the motor execution window (0 to 400 ms) in the left precentral gyrus, left prefrontal cortex, superior parietal cortex, right inferior frontal gyrus, and right cerebellum (Fig. 5; p < .005, corrected).
Fig. 4.
Whole-brain Gamma Differences between ADS and HAND groups. Disease-specific gamma interference differences (p < .005, corrected) were observed in the left premotor cortex (top), right pre-supplementary motor area (middle), and right inferior parietal cortex (bottom). Residuals of interference amplitude values controlling for age are presented to the right of each image.
Fig. 5.
Whole-brain Beta Differences in the Motor Planning and Execution Phases. (Left) Group effects in the motor planning period (−400 to 0 ms; p < .005, corrected) were observed in the right superior temporal area. (Right) Group beta differences during the motor execution period (0 to 400 ms; p < .005, corrected) were identified in the left prefrontal, left precentral, left superior parietal cortex (LSPC), right cerebellar cortices, and the right dorsolateral prefrontal cortex (RDLPFC). Residuals of interference amplitude values controlling for age are presented near the corresponding brain slices, and labeled for those in the right panel.
3.5. Neuro-behavioral correlations
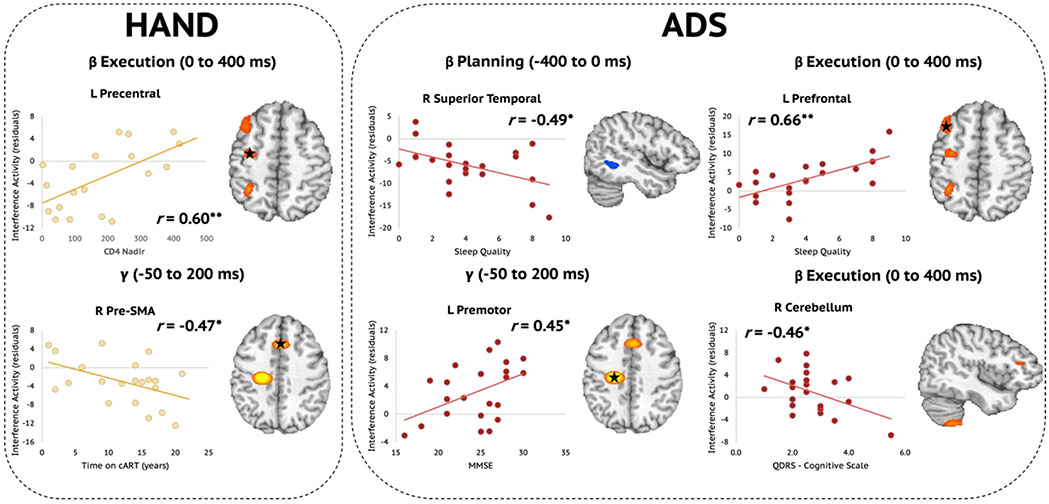
Next, we wanted to explore how these distinct neural interference responses were related to disease-specific clinical indices of HAND (i.e., CD4 nadir, current CD4 counts, duration on cART, disease duration) and ADS (i.e., MMSE, MoCA, FAQ, Quick Dementia Rating Scale (QDRS), and Pittsburgh Sleep Quality Index (PSQI)). Pseudo-t values were extracted from peak voxels identified in the whole-brain analyses comparing interference effects among those with HAND and those on the ADS and subjected to additional testing. Regarding clinical HIV metrics, we found that lower CD4 nadir counts (cells/μL) were associated with stronger gamma interference effects in the left precentral cortex (r = 0.60, p = .007; Fig. 6, top left). Conversely, we found that weaker gamma interference effects in the right pre-SMA were associated with being on cART for a longer period of time (r = −0.47, p = .033; Fig. 6, bottom left). Among participants on the ADS, stronger (i.e., more negative) oscillatory beta interference effects during the motor planning period in the right superior temporal areas were associated with poorer sleep quality, measured using the PSQI (r = −0.49, p = .020; Fig. 6, top middle). However, during the movement execution period, poorer sleep quality was associated with weaker (i.e., more positive) beta interference effects in the left prefrontal cortex (r = 0.66, p = .001; Fig. 6, top right) of those on the ADS. Further, greater cognitive impairment as reported by participants' informants was associated with stronger (i.e., more negative) beta interference effects in the right cerebellum during the movement execution period (r = −0.46, p = .037; Fig. 6, bottom right). Finally, stronger gamma interference effects in the left premotor cortex were associated with better performance on the MMSE (r = 0.45, p = .035; Fig. 6, bottom middle).
Fig. 6.
Relationships Between Disease-specific Metrics and Oscillatory Beta and Gamma Interference Effects. (Left) Among participants with HAND, a lower CD4 nadir was associated with stronger (i.e., more negative) beta interference during the motor execution period in the left precentral gyrus (top). Interestingly, however, the longer those with HAND were on combination antiretroviral therapy (cART), the weaker their oscillatory gamma interference effects were in the right pre-supplementary motor area (SMA; bottom). (Right) Among participants in the ADS group, stronger (i.e., more negative) oscillatory beta interference effects during the motor planning period in the right superior temporal area were associated with poorer sleep quality, as measured by the Pittsburgh Sleep Quality Index (PSQI). Conversely, during the movement execution period, poorer sleep quality was associated with weaker (i.e., more positive) beta interference effects in the left prefrontal cortex of those on the ADS. Further, greater informant-reported cognitive impairment was associated with stronger (i.e., more negative) beta interference effects in the right cerebellum during the movement execution period. Finally, stronger gamma interference effects in the left premotor cortex were associated with more optimal MMSE scores. *p < .05, **p < .01.
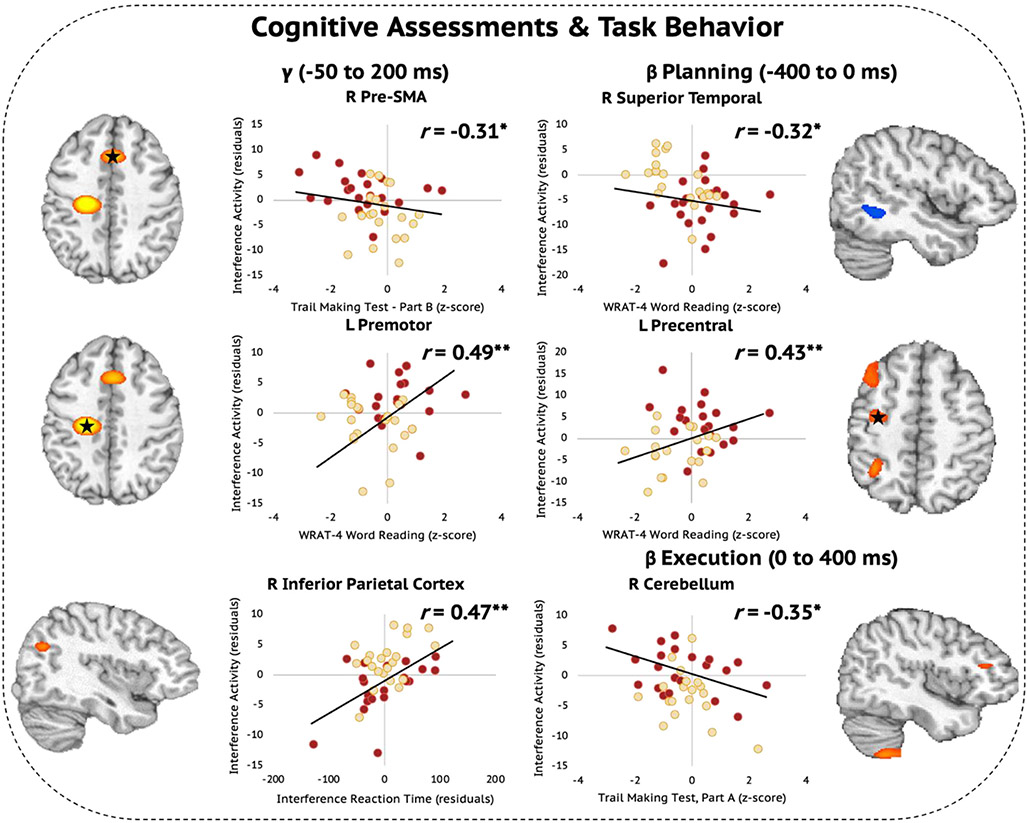
In addition, to index the relationships between cognitive functioning and the regional oscillatory interference responses that differentiated those with HAND compared to ADS, we conducted exploratory analyses using the extracted pseudo-t values, performance measures on the flanker task during the MEG, and the demographically corrected z-scores from the common neuropsychological assessments shared across the HAND and ADS cohorts. We identified several relationships between oscillatory interference effects and neuropsychological performance. Specifically, we found that weaker oscillatory gamma interference effects in the right pre-SMA was associated with better performance (i.e., higher z-scores) on the Trail Making Test, Part B (r = −0.31, p = .045; Fig. 7, top left). Additionally, stronger gamma interference effects in the left premotor cortex scaled with higher premorbid function measured using the WRAT-4 Word Reading test (r = 0.49, p = .001; Fig. 7, middle left). Stronger gamma interference effects in the right inferior parietal cortex were associated with greater behavioral interference to the flanker task in terms of reaction time (in ms; r = 0.47, p = .002; Fig. 7, bottom left). Further, higher WRAT-4 Word Reading scores were associated with stronger (i.e., more negative) beta interference effects in the right superior temporal area during the motor planning period (r = −0.32, p = .001; Fig. 7, top right), with the inverse of this relationship observed in the left precentral cortex where weaker (i.e., more positive) beta interference effects during the motor planning period were associated with higher premorbid function (r = 0.43, p = .006; Fig. 7, middle right). Next, we found that better performance on the Trail Making Test, Part A scaled with stronger beta interference effects in the right cerebellum during the motor execution period (r = −0.35, p = .026; Fig. 7, bottom right). Finally, because of floor effects on the learning, delayed recall, and percent retained trials of the HVLT-R, these data were heavily skewed among the HAND and ADS groups. Thus, we did not explore relationships between neural oscillatory interference effects and the results of these assessments.
Fig. 7.
Oscillatory Beta and Gamma Interference Effects Scale with Cognitive Assessments Among Those with HAND and ADS. (Left) Weaker oscillatory gamma interference effects in the right pre-SMA were associated with better performance on the Trail Making Test, Part B. Higher premorbid function measured using the WRAT-4 Word Reading test was associated with stronger gamma interference effects in the left premotor cortex. Stronger gamma interference effects in the right inferior parietal cortex scaled with greater behavioral interference in terms of reaction time (in ms). (Right) Better WRAT-4 Word Reading scores were associated with stronger (i.e., more negative) beta interference effects in the right superior temporal area during the motor planning period, but the inverse of this relationship was observed in the left precentral cortex in which weaker (i.e., more positive) beta interference effects during the motor planning period were associated with higher premorbid function. Finally, better performance on the Trail Making Test, Part A scaled with stronger beta interference effects in the right cerebellum during the motor execution period. *p < .05, **p < .01.
4. Discussion
Prolongment of life expectancy in PWH has resulted in patients becoming increasingly at risk for developing age-related neurodegenerative diseases such as AD. Examining AD in the context of HIV is greatly understudied and delineating the neurophysiological properties that may be shared or specific to each disease is critical to understanding the mechanisms underlying each disorder. In this context, we examined how cognitive interference affected motor control networks in participants with HAND, those on the ADS, and healthy controls using high-density MEG, which enabled the underlying spatiotemporal oscillatory dynamics to be closely examined, and thereby expanded upon the neural parameters that can be quantified by other modalities. Our results revealed ADS and HAND groups exhibit disease nonspecific differences relative to controls in the beta and gamma range across prefrontal and parietal cortices, as well as disease-specific gamma differences across multiple cortical regions and temporally-dependent effects in the beta range. We expand upon these findings and discuss the implications in greater detail below.
Previous studies have reported robust motor-related beta and gamma oscillations during the Flanker task (Embury et al., 2019; Heinrichs-Graham et al., 2018a; Spooner et al., 2021), and that such activity is highly sensitive to the healthy aging process (Heinrichs-Graham et al., 2018b; Heinrichs-Graham and Wilson, 2016). However, far fewer studies have probed motor-related beta and gamma activity in ADS and HAND populations independently, and none have studied motor processing differences among these clinical groups. Movement-related oscillatory responses in participants with ADS are not well understood, but many task-based and resting state studies have identified abnormal gamma and beta activity and connectivity in ADS (Güntekin et al., 2013; Jafari et al., 2020; Wiesman et al., 2021). Previous MEG work in PWH reported aberrant beta responses in motor and higher order cognitive processing regions (Spooner et al., 2022; Wilson et al., 2013, 2019). Likewise, abnormal gamma responses have also been noted in PWH (Arif et al., 2020; Groff et al., 2020; Spooner et al., 2018; Wiesman et al., 2018). Thus, common gamma and beta aberrations in HAND and ADS relative to controls, particularly within motor and cognitive processing areas, were expected.
Patients in both groups exhibited weaker gamma interference activity than controls in the right superior parietal cortex, a key perceptual-motor integration and processing area (Wolpert et al., 1998). Reduced beta interference activity was also observed in the ADS + HAND group compared to controls in important motor and higher order processing areas. High-frequency gamma activity has previously been linked to motor execution (Muthukumaraswamy, 2010) and is commonly affected by cognitive interference (Gaetz et al., 2013; Grent-’t-Jong et al., 2013; Heinrichs-Graham et al., 2018a; Wiesman et al., 2020), while perimovement beta ERD activity is a key contributor to motor planning and selection (Grent-’t-Jong et al., 2014; Heinrichs-Graham et al., 2016; Heinrichs-Graham et al., 2018a; Heinrichs-Graham and Wilson, 2015; Tzagarakis et al., 2010; Wiesman et al., 2020). Thus, weaker gamma and beta interference responses in such higher-order association cortices may reflect general deficiencies in top-down control of motor processing during cognitive interference in the clinical groups.
In addition to these disease non-specific differences, we also found disease-specific alterations in interference activity between ADS and HAND groups. Gamma interference responses were differentially impacted by ADS and HAND in motor and parietal regions, which are known to contribute to several of the subprocesses of cognitive motor control. Specifically, the premotor cortex and pre-supplementary motor area are key drivers of cognitive integration, temporal organization, and updating of cognitive plans of motor behavior (Chouinard and Paus, 2006; Freund, 1990; Ikeda et al., 1999; Nachev et al., 2007), and gamma activity in the premotor cortex has been implicated in superadditive cognitive interference during motor processing (Wiesman et al., 2020). Similarly, the inferior parietal cortex supports sensory-motor integration during intentional movement as well as sensorimotor preparation (Desmurget and Sirigu, 2012; Jubault et al., 2007).
Interestingly, disease-specific alterations in interference activity scaled with disease-specific metrics. In particular, those with HAND exhibited stronger beta interference effects in the left precentral cortex with lower CD4 nadir counts, which may reflect that those with the most severe legacy effects and/or weaker immune systems may utilize more compensatory mechanisms to overcome interference serving motor control (Schantell et al., 2022b). Further, those who have been on cART the longest had stronger gamma interference effects in the right pre-SMA, which may reflect the legacy effects of earlier antiretroviral treatment, which had more severe side effects, or the cumulative effects of cART exposure (Schantell et al., 2022b). Among those on the ADS, poorer sleep quality was associated with stronger beta interference effects in the right superior temporal cortex during the planning period, along with weaker beta interference effects in the left prefrontal cortex during the movement execution period. Such findings may suggest that sleep quality differentially impacts interference effects in higher-order cognitive regions during the motor planning and execution periods of motor control processes. We also found that stronger gamma interference activity in the left premotor cortex was associated with higher MMSE scores, suggesting that higher functioning patients on the ADS were able to better adapt during the interference trials.
Disease-specific alterations in the oscillatory activity serving motor control also scaled with cognitive functioning across participants with HAND and those on the ADS. Specifically, we found that premorbid functioning was associated with interference activity in several regions including gamma activity in the left premotor cortex, and beta activity during the planning period in the left precentral cortex and right superior temporal cortex. These finding may indicate that better premorbid functioning has a protective effect against disease-specific alterations in interference activity. Additionally, performance on the Trail Making Test Parts A and B were differentially associated with motor-related interference activity, with better performance on the Trail Making Test Part A being associated with stronger beta interference effects in the right cerebellum during the movement execution period, which suggests that such activity may support faster processing speed and low-level attention. However, worse performance on the Trail Making Test Part B scaled with stronger gamma interference effects in the right pre-SMA across both patient groups, which may link gamma-specific alterations in gamma interference activity to deficits in executive function.
Alterations in gamma interference activity may be attributed to differences in GABA concentration and neural inhibition in ADS and HAND pathologies. GABA, the principal inhibitory neurotransmitter in the brain, has been strongly linked to gamma activity in the visual and sensorimotor domains (Gaetz et al., 2011; Groth et al., 2021; Magazzini et al., 2016; Muthukumaraswamy et al., 2009; Nowak et al., 2017). Patients on the ADS are known to exhibit oscillatory gamma dysfunction and reduced GABA levels, which have been linked to cognitive impairment and typically precede amyloid plaque formation (Colgin and Moser, 2010; Y. Huang and Mucke, 2012; Xu et al., 2020). Additionally, neuroinflammation in PWH has been shown to lead to upregulation of GABAergic signaling (Green and Thayer, 2019). Thus, gamma interference aberrations in ADS and HAND within these regions suggest disease-specific neural mechanisms during cognitive interference suppression which, in turn, may impact motor control.
We also identified temporally-specific (e.g., planning and execution) effects in perimovement beta interference activity between the two clinical groups in primary and secondary motor cortices, frontoparietal, and temporal regions. Stronger planning-related beta interference activity in the right superior temporal area in those on the ADS relative to HAND suggests disease-specific effects during integration of spatial information processing, as the superior temporal has been linked to spatial coding and movement planning (Hanakawa et al., 2008; Shah-Basak et al., 2018). Conversely, participants on the ADS exhibited weaker beta interference oscillations during execution than those with HAND in areas supporting the planning, selection, and top-down cognitive control of voluntary movement such as the right cerebellum, left precentral gyrus, and bilateral frontoparietal cortices. Of note, beta responses in the contralateral precentral gyrus have been associated with motor planning and movement selection (Heinrichs-Graham et al., 2016; Heinrichs-Graham et al., 2018a; Heinrichs-Graham and Wilson, 2015; Wilson et al., 2010; Wilson et al., 2011b). Beta activity in frontoparietal regions such as the prefrontal cortex, inferior frontal gyrus, and superior parietal cortex have been previously linked to higher-order modulation of movement and cognition (Buschman and Miller, 2007; Richter et al., 2017; Salazar et al., 2012).
Although limited data exist regarding the role of beta oscillations in relation to ADS and HAND pathological mechanisms, both neurological disorders display executive function and cognitive control impairments. Beta oscillations are thought to be a top-down disinhibitory neural rhythm during motor and cognitive processing, particularly underlying voluntary movement (Barone and Rossiter, 2021; Khanna and Carmena, 2015). Thus, these findings may suggest disease-specific alterations in the disinhibition of top-down control during motor processing. Differing directionality of the temporally-distinct interference beta aberrations between patients on the ADS and people with HAND may also indicate disease-specific compensatory mechanisms to resolve cognitive interference during motor planning and execution. Considering the lack of behavioral differences between the clinical groups, it may be inferred that those on the ADS recruit more neural resources during planning to compensate for deficiencies during execution, whereas patients with HAND may compensate for disease-specific motor impairments during planning by generating accentuated responses during execution.
Collectively, the findings of this study have incredible novelty and potential for furthering research on the neurophysiology of ADS and HAND pathologies, although they are not without limitations. First, the sample sizes of the patient groups were relatively small, and thus, future work is necessary to corroborate the results identified in the present study. Next, we did not have AD biomarker data (i.e., amyloid beta and tau measures) for all participants. Future studies comparing ADS and HAND directly should include biomarker quantification (via blood plasma, CSF, and/or PET) for all participants, such that these biomarker data may be used in a multimodal approach and linked to various neuroimaging metrics (e.g., MEG markers in each disease; see Wiesman et al., 2022). Third, we did not have a comparison group with hallmark movement-related symptoms, such as those with Parkinson's disease. Such an additional group would provide significant insight in distinguishing primary cognitive and motor symptomatology and should be a focus of future work. Additionally, the neuropsychological battery slightly differed between the groups and future work comparing ADS and HAND should use identical neuropsychological assessments. Finally, we found aberrations of motor-related beta and gamma oscillations that were modulated by cognitive interference. Other movement-related cognitive processes have been shown to modulate beta and gamma activity in controls, such as movement complexity, certainty, and cueing (Heinrichs-Graham et al., 2016; Heinrichs-Graham and Wilson, 2015; Tzagarakis et al., 2010). An interesting direction would be to investigate how these different cognitive processes supporting motor control are altered in ADS and HAND. Beyond motor control, future studies using visual processing tasks should examine posterior-to-anterior information flow and whether that distinguishes different types of cognitive impairment.
To close, we found disease nonspecific movement-related oscillatory aberrations in participants with ADS or HAND compared to controls in the gamma and beta range. Altered gamma interference activity was identified in the superior parietal cortex, while disparate beta interference responses were found in frontal and cerebellar cortices in the clinical groups (ADS + HAND) relative to controls. Our results also indicated disease-specific aberrations across various regions essential for different sub-features supporting cognitive-motor processing. Differences in the gamma band were observed in cognitive-motor control regions, while temporally distinct beta interference effects were located in key areas for top-down cognitive control of motor coordination and control, which scaled with disease-specific clinical parameters and neuropsychological measures. This is the first study to directly examine similarities and differences in movement-related oscillatory activity in ADS and HAND populations, and our findings suggest both disease-specific and nonspecific effects that may inform both diagnostic and mechanistic studies in the future and lead to major breakthroughs in understanding cognitive decline in older PWH who are at the greatest risk of developing ADS disorders.
Acknowledgements
We would like to thank the participants for volunteering their time to participate in the study, as well as our staff and local collaborators for their contributions to the work. We would also like to specifically thank Nichole Knott and Katie Losh for assistance with the MEG recordings.
Funding
This research was supported by grants R01-MH116782 (TWW), R01-DA056223 (TWW), R01-MH118013 (TWW), P20-GM144641 (TWW), F31-AG055332 (AIW), F32-NS119375 (AIW), F31-DA056296 (MS), R36-DA059323 (MS), and F30-AG076259 (SDS) from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CRediT authorship contribution statement
Chloe E. Meehan: Data curation, Formal analysis, Writing - original draft. Mikki Schantell: Data curation, Formal analysis, Project administration, Writing - review & editing. Seth D. Springer: Formal analysis, Writing – original draft. Alex I. Wiesman: Data curation, Project administration, Writing - review & editing. Sara L. Wolfson: Data curation, Project administration, Writing - review & editing. Jennifer O'Neill: Data curation, Project administration, Writing - review & editing. Daniel L. Murman: Writing – review & editing. Sara H. Bares: Writing – review & editing. Pamela E. May: Writing – review &editing. Craig M. Johnson: Writing – review &editing. Tony W. Wilson: Supervision, Conceptualization, Formal analysis, Writing – review &editing, Writing - original draft, Project administration, Resources.
Sara Bares reports competing interests with Gilead Sciences, ViiV Healthcare, and Janssen. All other authors declare no conflicts of interest.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Data availability
Data will be made available on request.
References
- Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, Boxer AL, Buchman AS, Cruickshanks KJ, Devanand DP, Duffy CJ, Gall CM, Gates GA, Granholm A-C, Hensch T, Holtzer R, Hyman BT, Lin FR, McKee AC, Zhang LI, 2015. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 11 (1), 70–98. 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Wojna VE, 2007. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69 (18), 1789–1799. 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif Y, Wiesman AI, O’Neill J, Embury C, May PE, Lew BJ, Schantell MD, Fox HS, Swindells S, Wilson TW, 2020. The age-related trajectory of visual attention neural function is altered in adults living with HIV: A cross-sectional MEG study. EBioMedicine 61, 103065. 10.1016/j.ebiom.2020.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone J, Rossiter HE, 2021. Understanding the role of sensorimotor Beta oscillations. Front. Syst. Neurosci 15. https://www.frontiersin.org/article/10.3389/fnsys.2021.655886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KM, Heinrichs-Graham E, Fox HS, Robertson KR, Sandkovsky US, O’Neill J, Swindells S, Wilson TW, 2013. Decreased MEG beta oscillations in HIV-infected older adults during the resting-state. J. Neuro-Oncol 19 (6), 586–594. 10.1007/s13365-013-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J, 1998. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol 12 (1), 43–55. 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- Brandt J, Benedict RHB, 2001. Hopkins Verbal Learning Test– Revised: Professional Manual. Psychological Assessment Resources. [Google Scholar]
- Buschman TJ, Miller EK, 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science (New York, N.Y.) vol. 315 (5820), 1860–1862. 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28 (2), 193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Schomburg EW, 2015. What does gamma coherence tell us about interregional neural communication? Nat. Neurosci 18 (4), 484–489. 10.1038/nn.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C, 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci 13 (6), 407–420. 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Chong T, Peng Y, Shen W, Li J, von Deneen KM, Huang L, Alzheimer’s Disease Neuroimaging Initiative, 2017. Altered functional brain networks in amnestic mild cognitive impairment: A resting-state fMRI study. Brain Imaging Behav. 11 (3), 619–631. 10.1007/s11682-016-9539-0. [DOI] [PubMed] [Google Scholar]
- Calcagno A, Celani L, Trunfio M, Orofino G, Imperiale D, Atzori C, Arena V, d’Ettorre G, Guaraldi G, Gisslen M, Di Perri G, 2021. Alzheimer dementia in people living with HIV. Neurol. Clin. Pract 11 (5), e627–e633. 10.1212/CPJ.0000000000001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande CC, Wiesman AI, Schantell M, Johnson HJ, Wolfson SL, O’Neill J, Johnson CM, May PE, Swindells S, Murman DL, Wilson TW, 2022. Signatures of somatosensory cortical dysfunction in Alzheimer’s disease and HIV-associated neurocognitive disorder. Brain Communicat. 4 (4), fcac169. 10.1093/braincomms/fcac169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Paus T, 2006. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist 12 (2), 143–152. 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, 2010. Gamma oscillations in the hippocampus. Physiology (Bethesda) 25 (5), 319–329. 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Sirigu A, 2012. Conscious motor intention emerges in the inferior parietal lobule. Curr. Opin. Neurobiol 22 (6), 1004–1011. 10.1016/j.conb.2012.06.006. [DOI] [PubMed] [Google Scholar]
- du Plessis L, Paul RH, Hoare J, Stein DJ, Taylor PA, Meintjes EM, Joska JA, 2017. Resting-state functional magnetic resonance imaging in clade C HIV: within-group association with neurocognitive function. J. Neuro-Oncol 23 (6), 875–885. 10.1007/s13365-017-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury CM, Heinrichs-Graham E, Lord GH, Drincic AT, Desouza CV, Wilson TW, 2019. Altered motor dynamics in type 1 diabetes modulate behavioral performance. NeuroImage: Clin. 24, 101977 10.1016/j.nicl.2019.101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW, 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys 16 (1), 143–149. 10.3758/BF03203267. [DOI] [Google Scholar]
- Ernst MD, 2004. Permutation methods: A basis for exact inference. Stat. Sci 19 (4), 676–685. 10.1214/088342304000000396. [DOI] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12 (3), 189–198. 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freund HJ, 1990. Premotor area and preparation of movement. Rev. Neurol 146 (10), 543–547. [PubMed] [Google Scholar]
- Fulop T, Witkowski JM, Larbi A, Khalil A, Herbein G, Frost EH, 2019. Does HIV infection contribute to increased beta-amyloid synthesis and plaque formation leading to neurodegeneration and Alzheimer’s disease? J. Neuro-Oncol 25 (5), 634–647. 10.1007/s13365-019-00732-3. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TPL, 2011. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. NeuroImage 55 (2), 616–621. 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Liu C, Zhu H, Bloy L, Roberts TPL, 2013. Evidence for a motor gamma-band network governing response interference. NeuroImage 74, 245–253. 10.1016/j.neuroimage.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, 2015. The quick dementia rating system (QDRS): A rapid dementia staging tool. Alzheimer’s & Dementia : Diagnosis, Assess. & Disease Monit 1 (2), 249–259. 10.1016/j.dadm.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MV, Thayer SA, 2019. HIV gp120 upregulates tonic inhibition through α5-containing GABAARs. Neuropharmacology 149, 161–168. 10.1016/j.neuropharm.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P, 2013. Oscillatory dynamics of response competition in human sensorimotor cortex. NeuroImage 83, 27–34. 10.1016/j.neuroimage.2013.06.051. [DOI] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P, 2014. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J. Neurophysiol 112 (2), 224–232. 10.1152/jn.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff BR, Wiesman AI, Rezich MT, O’Neill J, Robertson KR, Fox HS, Swindells S, Wilson TW, 2020. Age-related visual dynamics in HIV-infected adults with cognitive impairment. Neurology(R) Neuroimmunol. & Neuroinflammat 7 (3), e690 10.1212/NXI.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R, 2001. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl. Acad. Sci 98 (2), 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth CL, Singh A, Zhang Q, Berman BD, Narayanan NS, 2021. GABAergic modulation in movement related oscillatory activity: A review of the effect pharmacologically and with aging. Tremor and Other Hyperkinetic Movements (New York, N.Y.) 11, 48. 10.5334/tohm.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntekin B, Emek-Savaş DD, Kurt P, Yener GG, Başar E, 2013. Beta oscillatory responses in healthy subjects and subjects with mild cognitive impairment. NeuroImage: Clin 3, 39–46. 10.1016/j.nicl.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M, 2008. Motor planning, imagery, and execution in the distributed motor network: A time-course study with functional MRI. Cereb. Cortex 18 (12), 2775–2788. 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I, 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Heinrichs-Graham E, Wilson TW, 2015. Coding complexity in the human motor circuit. Hum. Brain Mapp 36 (12), 5155–5167. 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, 2016. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage 134, 514–521. 10.1016/j.neuroimage.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Arpin DJ, Wilson TW, 2016. Cue-related temporal factors modulate movement-related Beta oscillatory activity in the human motor circuit. J. Cogn. Neurosci 28 (7), 1039–1051. 10.1162/jocn_a_00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Hoburg JM, Wilson TW, 2018a. The peak frequency of motor-related gamma oscillations is modulated by response competition. NeuroImage 165, 27–34. 10.1016/j.neuroimage.2017.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, McDermott TJ, Mills MS, Wiesman AI, Wang YP, Stephen JM, Calhoun VD, Wilson TW, 2018b. The lifespan trajectory of neural oscillatory activity in the motor system. Dev. Cogn. Neurosci 30, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Taylor BK, Wang Y-P, Stephen JM, Calhoun VD, Wilson TW, 2020. Parietal oscillatory dynamics mediate developmental improvement in motor performance. Cereb. Cortex 30 (12), 6405–6414. 10.1093/cercor/bhaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR, 2005. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp 25 (2), 199–211. 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdle GC, Quidé Y, Kassem MS, Johnson K, Rae CD, Brew BJ, Cysique LA, 2020. Brain amyloid in virally suppressed HIV-associated neurocognitive disorder. Neurol. - Neuroimmunol. Neuroinflammat 7 (4) 10.1212/NXI.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L, 2012. Alzheimer mechanisms and therapeutic strategies. Cell 148 (6), 1204–1222. 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V, 2000. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clin. Neurophysiol 111 (11), 1961–1967. 10.1016/s1388-2457(00)00454-5. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, Nagamine T, Taki W, Kimura J, Shibasaki H, 1999. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain 122 (5), 915–931. 10.1093/brain/122.5.915. [DOI] [PubMed] [Google Scholar]
- Jafari Z, Kolb BE, Mohajerani MH, 2020. Neural oscillations and brain stimulation in Alzheimer’s disease. Prog. Neurobiol 194, 101878 10.1016/j.pneurobio.2020.101878. [DOI] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM, Investigators, the F. F. 18 S, 2012. Performance characteristics of amyloid PET with Florbetapir F 18 in patients with Alzheimer’s Disease and cognitively Normal subjects. J. Nucl. Med 53 (3), 378–384. 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- Jubault T, Ody C, Koechlin E, 2007. Serial Organization of Human Behavior in the inferior parietal cortex. J. Neurosci 27 (41), 11028–11036. 10.1523/JNEUROSCI.1986-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Carmena JM, 2015. Neural oscillations: Beta band activity across motor networks. Curr. Opin. Neurobiol 32, 60–67. 10.1016/j.conb.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Koelewijn L, Bompas A, Tales A, Brookes MJ, Muthukumaraswamy SD, Bayer A, Singh KD, 2017. Alzheimer’s disease disrupts alpha and beta-band resting-state oscillatory network connectivity. Clin. Neurophysiol 128 (11), 2347–2357. 10.1016/j.clinph.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Gander PE, 2016. The demodulated band transform. J. Neurosci. Methods 261, 135–154. 10.1016/j.jneumeth.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW, 2014. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol 56 (11), 1072–1077. 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM, 1969. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9 (3), 179–186. [PubMed] [Google Scholar]
- Lew BJ, McDermott TJ, Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology 91 (20), e1860–e1869. 10.1212/WNL.0000000000006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, O’Neill J, Rezich MT, May PE, Fox HS, Swindells S, Wilson TW, 2020. Interactive effects of HIV and ageing on neural oscillations: Independence from neuropsychological performance. Brain Communicat. 2 (1), fcaa015. 10.1093/braincomms/fcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, Schantell MD, O’Neill J, Morsey B, Wang T, Ideker T, Swindells S, Fox HS, Wilson TW, 2021. Reductions in Gray matter linked to epigenetic HIV-associated accelerated aging. Cerebral Cortex (New York, N.Y.: 1991) 31 (8),3752–3763. 10.1093/cercor/bhab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazzini L, Muthukumaraswamy SD, Campbell AE, Hamandi K, Lingford-Hughes A, Myers JFM, Nutt DJ, Sumner P, Wilson SJ, Singh KD, 2016. Significant reductions in human visual gamma frequency by the gaba reuptake inhibitor tiagabine revealed by robust peak frequency estimation. Hum. Brain Mapp 37 (11), 3882–3896. 10.1002/hbm.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164 (1), 177–190. 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- McDermott TJ, Wiesman AI, Proskovec AL, Heinrichs-Graham E, Wilson TW, 2017. Spatiotemporal oscillatory dynamics of visual selective attention during a flanker task. NeuroImage 156, 277–285. 10.1016/j.neuroimage.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan CE, Wiesman AI, Spooner RK, Schantell M, Eastman JA, Wilson TW, 2021. Differences in rhythmic neural activity supporting the temporal and spatial cueing of attention. Cereb. Cortex bhab 132. 10.1093/cercor/bhab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan CE, Embury CM, Wiesman AI, Schantell M, Wolfson SL, O’Neill J, Swindells S, Johnson CM, May PE, Murman DL, Wilson TW, 2022. Convergent and divergent oscillatory aberrations during visuospatial processing in HIV-related cognitive impairment and Alzheimer’s disease. Cerebral Cortex (New York, N.Y.: 1991) bhac268. 10.1093/cercor/bhac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan CE, Schantell M, Wiesman AI, Wolfson SL, O’Neill J, Bares SH, Johnson CM, May PE, Murman DL, Wilson TW, 2023. Oscillatory markers of neuroHIV-related cognitive impairment and Alzheimer’s disease during attentional interference processing. Aging 15 (2), 524–541. 10.18632/aging.204496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanini B, Valcour V, 2017. Differentiating HIV-associated neurocognitive disorders from Alzheimer’s Disease: an emerging issue in geriatric neuro HIV. Curr. HIV/AIDS Rep 14 (4), 123–132. 10.1007/s11904-017-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, Mathis CA, McConathy J, Nordberg A, Sabri O, Seibyl JP, Stokes MK, Laere KV, 2016. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J. Nucl. Med 57 (8), 1316–1322. 10.2967/jnumed.116.174615. [DOI] [PubMed] [Google Scholar]
- Mohamed M, Skolasky RL, Zhou Y, Ye W, Brasic JR, Brown A, Pardo CA, Barker PB, Wong DF, Sacktor N, 2020. Beta-amyloid (Aβ) uptake by PET imaging in older HIV+ and HIV− individuals. J. Neuro-Oncol 26 (3), 382–390. 10.1007/s13365-020-00836-1. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, 2010. Functional properties of human primary motor cortex gamma oscillations. J. Neurophysiol 104 (5), 2873–2885. 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD, 2009. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. U. S. A 106 (20), 8356–8361. 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C, 2007. The role of the pre-supplementary motor area in the control of action. NeuroImage 36, T155–T163. 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namagga JK, Rukundo GZ, Voss JG, 2019. Prevalence and risk factors of HIV-associated neurocognitive disorders in rural southwestern Uganda. J. Assoc. Nurses in AIDS Care : JANAC 30 (5), 531–538. 10.1097/JNC.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, 2005. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 53 (4), 695–699. 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nowak M, Hinson E, van Ede F, Pogosyan A, Guerra A, Quinn A, Brown P, Stagg CJ, 2017. Driving human motor cortical oscillations leads to behaviorally relevant changes in local GABAA inhibition: A tACS-TMS study. J. Neurosci 37 (17), 4481–4492. 10.1523/JNEUROSCI.0098-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P, 1977. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed. Sci. Instrum 13, 135–145. [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S, 1982. Measurement of functional activities in older adults in the community. J. Gerontol 37 (3), 323–329. 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Holmes AP, Frackowiak RS, Friston KJ, 1995. Estimating smoothness in statistical parametric maps: variability of p values. J. Comput. Assist. Tomogr 19 (5), 788–796. 10.1097/00004728-199509000-00017. [DOI] [PubMed] [Google Scholar]
- Richter CG, Thompson WH, Bosman CA, Fries P, 2017. Top-down Beta enhances bottom-up gamma. J. Neurosci 37 (28), 6698–6711. 10.1523/JNEUROSCI.3771-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar RF, Dotson NM, Bressler SL, Gray CM, 2012. Content-specific frontoparietal synchronization during visual working memory. Science (New York, N.Y.) vol. 338 (6110), 1097–1100. 10.1126/science.1224000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Taylor BK, Lew BJ, O’Neill JL, May PE, Swindells S, Wilson TW, 2021. Gray matter volumes discriminate cognitively impaired and unimpaired people with HIV. NeuroImage: Clin. 31, 102775 10.1016/j.nicl.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Springer SD, Arif Y, Sandal ME, Willett MP, Johnson HJ, Okelberry HJ, O’Neill JL, May PE, Bares SH, Wilson TW, 2022a. Regular cannabis use modulates the impact of HIV on the neural dynamics serving cognitive control. J. Psychopharmacol. (Oxford, England) 36 (12), 1324–1337. 10.1177/02698811221138934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Taylor BK, Spooner RK, May PE, O’Neill J, Morsey BM, Wang T, Ideker T, Bares SH, Fox HS, Wilson TW, 2022b. Epigenetic aging is associated with aberrant neural oscillatory dynamics serving visuospatial processing in people with HIV. Aging (Albany NY) 14 (24), 9818–9831. 10.18632/aging.204437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Basak PP, Chen P, Caulfield K, Medina J, Hamilton RH, 2018. The role of the right superior temporal gyrus in stimulus-centered spatial processing. Neuropsychologia 113, 6–13. 10.1016/j.neuropsychologia.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Mills MS, O’Neill J, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. NeuroImage. Clin 20, 85–91. 10.1016/j.nicl.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, Heinrichs-Graham E, Wilson TW, 2019. Rhythmic spontaneous activity mediates the age-related decline in somatosensory function. Cereb. Cortex 29 (2), 680–688. 10.1093/cercor/bhx349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Arif Y, Taylor BK, Wilson TW, 2021. Movement-related gamma synchrony differentially predicts behavior in the presence of visual interference across the lifespan. Cereb. Cortex 31 (11), 5056–5066. 10.1093/cercor/bhab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Taylor BK, Ahmad IM, Dyball K, Emanuel K, O’Neill J, Kubat M, Swindells S, Fox HS, Bares SH, Stauch KL, Zimmerman MC, Wilson TW, 2022. Mitochondrial redox environments predict sensorimotor brain-behavior dynamics in adults with HIV. Brain Behav. Immun 107, 265–275. 10.1016/j.bbi.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, van Cappellen van Walsum AM, Pijnenburg YAL, Berendse HW, de Munck JC, Scheltens P, van Dijk BW, 2002. Generalized synchronization of MEG recordings in Alzheimer’s Disease: evidence for involvement of the gamma band. J. Clin. Neurophysiol 19 (6), 562–574. 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol 51 (7), 1759–1768. 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM, 2013. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology 80 (13), 1186–1193. 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G, 2016. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimer’s & Dement.: Diagnosis, Assessment & Disease Monitoring 4, 1–5. 10.1016/j.dadm.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G, 2010. Beta-band activity during motor planning reflects response uncertainty. J. Neurosci 30 (34), 11270–11277. 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ, 1997. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput 35 (2), 135–140. 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- van Deursen JA, Vuurman EFPM, Verhey FRJ, van Kranen-Mastenbroek VHJM, Riedel WJ, 2008. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J. Neural Transmiss. (Vienna, Austria: 1996) 115 (9), 1301–1311. 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen BD, Van Drongelen W, Yuchtman M, Suzuki A, 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng 44 (9), 867–880. 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Thomas GP, Honea RA, Loskutova N, Burns JM, 2012. Evidence of altered Corticomotor system connectivity in early-stage Alzheimer’s Disease. J. Neurol. Phys. Ther 36 (1), 8–16. 10.1097/NPT.0b013e3182462ea6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW, 2020. Attention modulates the gating of primary somatosensory oscillations. NeuroImage 211, 116610. 10.1016/j.neuroimage.2020.116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain : J. Neurol 141 (6), 1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Koshy SM, Heinrichs-Graham E, Wilson TW, 2020. Beta and gamma oscillations index cognitive interference effects across a distributed motor network. NeuroImage 213, 116747. 10.1016/j.neuroimage.2020.116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Murman DL, May PE, Schantell M, Losh RA, Johnson HJ, Willet MP, Eastman JA, Christopher-Hayes NJ, Knott NL, Houseman LL, Wolfson SL, Losh KL, Johnson CM, Wilson TW, 2021. Spatio-spectral relationships between pathological neural dynamics and cognitive impairment along the Alzheimer’s disease spectrum. Alzheimer’s & Dementia (Amsterdam, Netherlands) 13 (1), e12200. 10.1002/dad2.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Murman DL, Losh RA, Schantell M, Christopher-Hayes NJ, Johnson HJ, Willett MP, Wolfson SL, Losh KL, Johnson CM, May PE, Wilson TW, 2022. Spatially resolved neural slowing predicts impairment and amyloid burden in Alzheimer’s disease. Brain: A J. Neurol 145 (6), 2177–2189. 10.1093/brain/awab430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC, 2010. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 73 (2), 75–84. 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Fleischer A, Archer D, Hayasaka S, Sawaki L, 2011a. Oscillatory MEG Motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil. Neural Repair 25 (2), 188–193. 10.1177/1545968310378511. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC, 2011b. Abnormal gamma and Beta MEG activity during finger movements in early-onset psychosis. Dev. Neuropsychol 36 (5), 596–613. 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O’Neill J, Knott NL, Fox HS, Swindells S, 2013. Functional brain abnormalities during finger-tapping in HIV-infected older adults: A magnetoencephalography study. J. Neuroimmune Pharmacol.: Off. J. Soc. NeuroImmune Pharmacol 8 (4) 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM, 2014. Circadian modulation of motor-related beta oscillatory responses. NeuroImage 102 (Pt 2), 531–539. 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Proskovec AL, McDermott TJ, 2016. Neuroimaging with magnetoencephalography: A dynamic view of brain pathophysiology. Translat. Res.: J. Laborat. Clin. Med 175, 17–36. 10.1016/j.trsl.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Lew BJ, Spooner RK, Rezich MT, Wiesman AI, 2019. Aberrant brain dynamics in neuroHIV: evidence from magnetoencephalographic (MEG) imaging. Prog. Mol. Biol. Transl. Sci 165, 285–320. 10.1016/bs.pmbts.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M, 1998. Maintaining internal representations: the role of the human superior parietal lobe. Nat. Neurosci 1 (6) 10.1038/2245. Article 6. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC, 1996. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp 4 (1), 58–73. . [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC, 1999. Detecting changes in nonisotropic images. Hum. Brain Mapp 8 (2–3), 98–101. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhao M, Han Y, Zhang H, 2020. GABAergic inhibitory interneuron deficits in Alzheimer’s Disease: implications for treatment. Front. Neurosci 14. https://www.frontiersin.org/article/10.3389/fnins.2020.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li R, Wang X, Miao H, Wei Y, Ali R, Qiu B, Li H, 2017. Motor-related brain abnormalities in HIV-infected patients: A multimodal MRI study. Neuroradiology 59 (11), 1133–1142. 10.1007/s00234-017-1912-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.