Abstract
Rationale and Objectives:
Cannabis use is often associated with the use of other psychoactive substances, which is subsequently linked to an increased risk for addiction. While there is a growing body of neuroimaging literature investigating the cognitive effect of long-term cannabis use, very little is known about the potential additive effects of cannabis polysubstance use.
Methods:
Fifty-six adults comprised of 18 polysubstance users (i.e., cannabis plus at least one other illicit substance), 19 cannabis-only users, and 19 nonusers completed a visuospatial attention task while undergoing magnetoencephalography. A data-driven approach was used to identify oscillatory neural responses, which were imaged using a beamforming approach. The resulting cortical regions were probed for group differences and used as seeds for whole-brain connectivity analysis.
Results:
Participants exhibited robust theta, alpha, beta, and gamma responses during visuospatial processing. Statistical analyses indicated that the cannabis-only group had weaker occipital theta relative to the nonusers, and that both polysubstance and cannabis-only users had reduced spontaneous gamma in the occipital cortices during the pre-stimulus baseline period relative to nonusers. Finally, functional connectivity analyses revealed that polysubstance users had sharply reduced beta connectivity between occipital and prefrontal, as well as occipital and left temporal cortices.
Conclusions:
Cannabis use should be considered in a polysubstance context, as our correlational design suggests differences in functional connectivity among those who reported cannabis-only versus polysubstance use in occipital to prefrontal pathways critical to visuospatial processing and attention function. Future work should distinguish the effect of different polysubstance combinations and use more causal designs.
Keywords: Magnetoencephalography, MEG, Substance use disorder, Drug, Gamma, Theta, Beta, Oscillations
Introduction
With an increase internationally in the legalization of cannabis, both recreational and medicinal use is becoming more prevalent worldwide (World Health Organization 2016). Importantly, cannabis is often associated with the use of other substances (Barrett et al. 2006; Davis et al. 2019), including cocaine, hallucinogens, and prescription stimulants for non-medicinal purposes (Olthuis et al. 2013). Such polysubstance use is associated with greater risk for addiction, and polysubstance users often report higher cannabis dependence severity than cannabis-only users (Connor et al. 2013). Despite the ubiquity of polysubstance use with cannabis, few studies have investigated the potential differences relative to cannabis-only use, and this is especially true in the context of human neuroimaging.
Studies examining the neurocognitive impairments observed in chronic cannabis users alone have shown deficits in cognitive flexibility, visual attention, short- and long-term memory, and other functions (for reviews, see Figueiredo et al. 2020; Lichenstein et al. 2022). Specifically, impaired performance in chronic cannabis users relative to non-users has been shown in inhibitory control (Solowij 2002; Behan et al. 2014; Sagar et al. 2015) and visual attention regions (Hunault et al. 2009; Filbey et al. 2009; Charboneau et al. 2013; Karoly et al. 2019). In addition to such behavioral work, many neuroimaging studies have investigated chronic cannabis use across different cognitive domains. For example, studies using functional MRI (fMRI) have shown increased activation in prefrontal cortices and impaired performance in chronic cannabis users during an attentional control task (Chang et al. 2006; Abdullaev et al. 2010). More recently, several magnetoencephalography (MEG) studies have investigated the association between chronic cannabis use and altered neural oscillatory dynamics serving distinct domains of attention function. For example, one recent study showed that cannabis users exhibit stronger theta oscillations in multiple regions of the prefrontal cortices compared to demographically-matched non-users during an attentional reorientation task (Springer et al. 2021). Rangel-Pacheco et al. (2021) also found altered theta activity in chronic cannabis users during a visual selective attention task, although in their study, the strongest differences were in the occipital cortices during early visual processing (i.e., 0–250 ms). Interestingly, this study also showed aberrantly reduced functional connectivity in the theta range between occipital and prefrontal cortices in chronic cannabis users (Rangel-Pacheco et al. 2021). In contrast to these theta findings, a recent MEG study focusing on the interaction of chronic cannabis use and HIV-infection in the context of visual attention function reported that cannabis use was associated with sharp declines in spontaneous gamma activity within the occipital cortices of both user groups (Christopher-Hayes et al. 2021), with relatively normal theta oscillations in the cannabis users who were not HIV positive. Such decreases in spontaneous gamma prior to stimulus onset is in agreement with work in the somatosensory system, where Arif et al. (2021) showed aberrantly reduced spontaneous gamma in the primary somatosensory cortex and impaired sensory gating in chronic cannabis users relative to controls. While these studies significantly advanced our understanding of how cannabis use affects brain function, polysubstance use was an exclusion criterion in all of the MEG studies and most of the fMRI work. Thus, any additive or interactive effects on brain function in the presence of polysubstance use remains poorly understood.
While the neuroimaging literature is limited, there is an extensive collection of neuropsychological studies examining cannabis polysubstance use. Such work has shown that cannabis use is the main predictor of impairment among MDMA and cocaine polysubstance users in a cognitive flexibility task (Verdejo-García et al. 2005), and that MDMA and methamphetamine polysubstance use in cannabis users is associated with greater impairment in perceptual reasoning than cannabis use alone (Banks et al. 2019). In addition to cognitive effects, cannabis polysubstance use has been associated with a significantly increased risk of developing mental health disorders (Salom et al. 2016), and more specifically psychosis compared to cannabis use alone (Jones et al. 2017). However, it should be noted that the data on neuropsychological function is not entirely consistent in this area. For example, a mega-analysis investigating response inhibition tasks found no increased impairments in polysubstance users, and in fact, only lifetime cannabis use was associated with suboptimal performance in inhibition tasks (Liu et al. 2019). While the latter finding is surprising, it could simply reflect that inhibitory processing is less susceptible to the effects of cannabis polysubstance use.
As mentioned above, only a handful of studies have examined associations between cannabis polysubstance use and alterations in brain structure and function. Recent studies using electroencephalography (EEG) have shown that cannabis polysubstance users exhibit increased midline theta activity during working memory processing (Binkowska et al. 2021a), as well as increased late parietal activity during a visual learning recognition task (Binkowska et al. 2021b). Interestingly, both of these EEG studies found differences in polysubstance users compared to non-users, with the cannabis only users not differing from either group. In another recent study, chronic cannabis users were divided based on whether they met criteria for alcohol use disorder (AUD). The key findings of this MEG study included greater oscillatory alpha activity in the occipital cortices during an attention task in the cannabis users with AUD compared to those without AUD (Lew et al. 2021a). Thus, the limited literature on brain function in the context of cannabis polysubstance use suggests the potential for additive negative effects over cannabis use alone, but the data is far from definitive, and many questions remain.
In the current study, we use MEG imaging to examine the association between cannabis polysubstance use and aberrant oscillatory neural dynamics during visuospatial processing. Visuospatial attention function is central to activities of daily living (Konstantopoulos et al. 2010), and hence, critical to overall cognitive function. To assess the differences between cannabis-only and cannabis polysubstance use, we focused our study on three groups: chronic cannabis-only users, polysubstance users (i.e., cannabis plus regular use of at least one other illicit substance), and nonuser controls. Based on the previous works reviewed above, we hypothesized that both cannabis and polysubstance users would exhibit differences relative to controls in the oscillatory dynamics serving visuospatial processing, with these differences being predominantly in theta and gamma responses within occipital cortices. In addition, we hypothesized that polysubstance users would exhibit aberrant functional connectivity between occipital cortices and prefrontal regions critical for attention during visuospatial processing.
Materials and Methods
Participants
Participants between the ages of 19–60 years who recreationally used cannabis and other substances were recruited from the community using a convenience sampling method. Nonuser controls were selected from a large cohort of participants enrolled in an ongoing neuroimaging study of healthy and pathological aging (R01-MH103220) based on their reported current and past substance use history during a standardized medical and demographic interview. Those who did not report current, or history of substance use and had completed the MEG and structural MRI components of the study were included as nonuser controls. For both the cannabis and the polysubstance groups, participants were enrolled if they reported using cannabis at least 2–4 times per month over the past six months on the first question of the Cannabis Use Disorder Identification Test-Revised (CUDIT-R; Adamson et al. 2010). Cannabis users and polysubstance users were subsequently grouped according to the participants’ responses on Module E of the Structured Clinical Interview for the Diagnostic and Statistical Manual – Fifth Edition (SCID-5). Participants who reported using cannabis at least 2–4 times per month and also used at least one other substance (e.g., sedatives, stimulants, opioids, inhalants, phencyclidine, hallucinogens, etc.), not including alcohol or tobacco, at least once a month over the past 12 months were classified as polysubstance users. Ultimately, the study sample was comprised of 21 nonusers, 21 cannabis-only users, and 22 polysubstance users. Additionally, we collected tobacco use information for the cannabis and polysubstance use groups utilizing the NIDA Quick Screen questionnaire (National Institute on Drug Abuse 2022). Exclusionary criteria for all groups included any medical illness affecting CNS function such as HIV, any diagnosed neurological or psychiatric disorder, history of head trauma, and ferromagnetic implants contraindicated for MRI. In order to avoid the acute effects of cannabis or other substances, participants were asked to abstain from using any non-prescription substances (e.g., alcohol) prior to participation on the day of study. Our institutional review board approved this investigation, and each participant provided written informed consent prior to participation in the study.
Experimental Paradigm
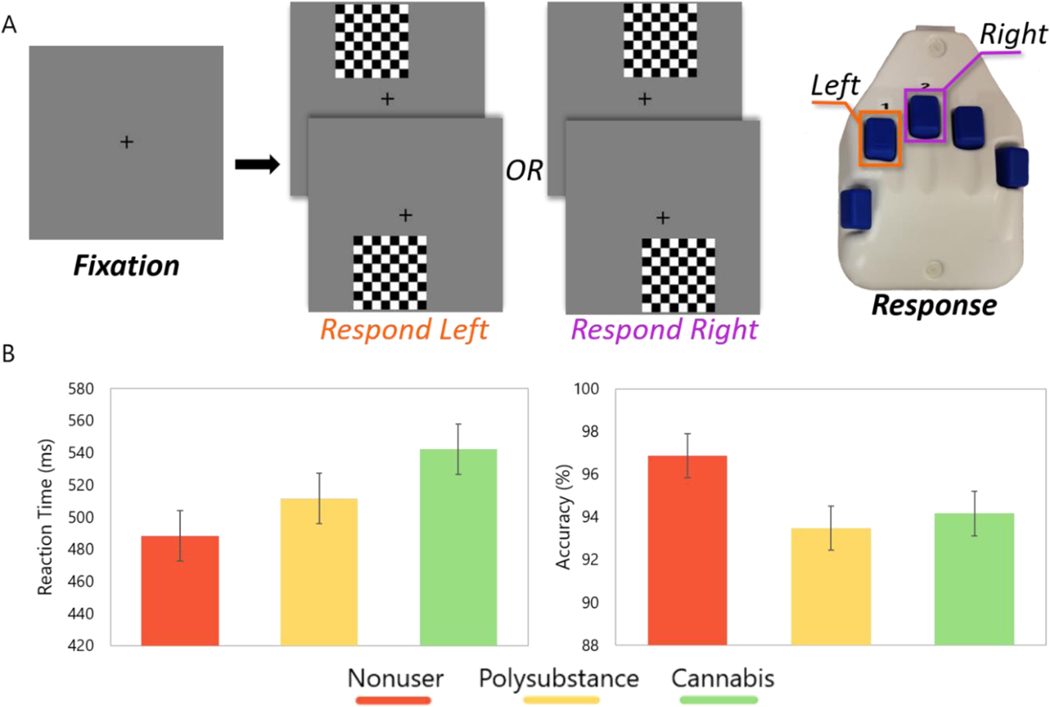
Participants completed an established visuospatial discrimination task termed VisAttend (Wiesman et al. 2017a; Wiesman and Wilson 2019a). During the task, participants were seated in a magnetically shielded room, and were instructed to fixate on a centrally-presented fixation cross. The task consisted of an 8 × 8 checkerboard appearing for 800 ms after a variable interstimulus interval (ISI; range: 1900 – 2100 ms) at one of four positions offset by 75% relative to the fixation cross (Figure 1A). Participants indicated whether the checkerboard was located on the left (index finger) or right (middle finger) side and ignored the top/bottom parameter on each of the trials. Each participant completed 240 trials (60 per location) in a pseudorandomized order, which took about 14 minutes to complete.
Fig. 1.
(A) An illustration of the visuospatial task paradigm. Each trial was comprised of a fixation period lasting about 2000 ms (variable interstimulus interval: 1900 – 2100 ms) and a stimulus-presentation period lasting 800 ms, which consisted of the appearance of a checkered grid in one of four locations. Participants indicated the location of the checkerboard (i.e., left or right) with their index (left) or middle (right) finger. (B) Groupwise behavioral performance data on the visuospatial task. Reaction time (in ms) is displayed on the y-axis in the panel on the left, while accuracy (in % correct) appears on the y-axis in the right panel. Groups are defined in the color legend displayed below the graphs. There were no statistical differences between groups in reaction time or accuracy metrics. Error bars display standard error of mean
MEG Data Acquisition Recording and Structural MRI Coregistration
Our MEG data acquisition, structural coregistration, preprocessing, and sensor-/source-level analyses closely followed the analysis pipeline of previous manuscripts (Wiesman et al. 2017b; Spooner et al. 2018, 2019; Kurz et al. 2018; Wiesman and Wilson 2020). All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged for environmental noise compensation. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1– 330 Hz using a 306-sensor MEGIN MEG system (Helsinki, Finland) equipped with 204 planar gradiometers and 102 magnetometers. Participants were monitored during data acquisition via real-time audio-video feeds from inside the shielded room. Each MEG dataset was individually corrected for head motion using continuous head localization data (see below) and subjected to noise reduction using the signal space separation method with a temporal extension (correlation limit: .950; correlation window duration: 6 s; Taulu and Simola, 2006). Only data from the gradiometers were used for further analysis.
Structural MRI Processing & MEG Coregistration
Preceding MEG measurement, four head position indicator (HPI) coils were attached to the participant’s head and localized, together with the three fiducial points and scalp surface, using a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for MEG recording, an electric current with a unique frequency label was fed to each of the HPI coils throughout the MEG data collection session. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors for each data point (i.e., 1 kHz sampling rate) throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant’s MEG data were co-registered with structural T1-weighted MRI data using BESA MRI (Version 3.0) prior to source-space analysis. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Following source analysis (i.e., beamforming), each participant’s 4.0 × 4.0 × 4.0 mm functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
MEG Data Pre-Processing, Time-Frequency Transformation, & Sensor-Level Statistics
Cardiac and eyeblink artifacts were removed from the data using the signal-space projection approach (SSP; Uusitalo and Ilmoniemi 1997). The continuous magnetic time series was then filtered 0.5 – 200 Hz and split into epochs of 2700 ms (−500 – 2200 ms), with 0 ms defined as the stimulus onset (checkerboard appeared) and the −400 to 0 ms time window serving as the baseline period. Epochs containing artifacts were then removed using a fixed threshold method, which was supplemented by visual inspection. Briefly, the raw signal amplitude in MEG is strongly affected by the distance between the brain and the MEG sensor array, as the magnetic field strength falls off sharply as the distance from the current source and the detector increases. To account for this source of variance across participants, as well as actual variance in neural response amplitude, we used an individually-determined threshold based on the signal distribution for both amplitude and gradient to reject artifacts. Across all participants, the average amplitude threshold was 1044.91 (SD = 271.69) fT and the average gradient threshold was 191.33 (SD = 105.64) fT/s. Across all groups there was an average of 202.47 trials accepted (SD = 16.89) and a one-way ANOVA revealed no significant difference between the average accepted trials by group (F[2,53] = 0.035, p = 0.97).
The epochs remaining after artifact rejection were transformed into the time-frequency domain using complex demodulation (Kovach and Gander 2016; 2.0 Hz, 25 ms; range 4 – 100 Hz), and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These plots were then normalized by the baseline power of each respective bin, which was calculated as the mean power during the −400 to 0 ms time-period. The time-frequency windows used for the beamformer analysis were then determined using a data-driven, two-stage statistical approach.
Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, paired-sample t-tests were conducted to test for differences against the baseline at each data point and the output spectrogram of t-values was thresholded at p < .05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the threshold (p < .05), and a cluster value was derived by summing all the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst 2004; Maris and Oostenveld 2007; Oostenveld et al. 2011; Embury et al. 2018, 2019; Veniero et al. 2021; Killanin et al. 2022; Schantell et al. 2022; Gutteling et al. 2022; van Es et al. 2022; Springer et al. 2022; Spooner and Wilson 2022). For each comparison, 10,000 permutations were computed to build a distribution of cluster values, and the time-frequency windows that were non-exchangeable with baseline across all participants according to these permutation analyses were used to guide subsequent oscillatory source level analysis.
MEG Source Imaging and Statistical Analyses
Cortical activity was imaged through an extension of the linearly constrained minimum variance vector beamformer known as the dynamic imaging of coherent sources (DICS) approach (Van Veen et al. 1997; Gross et al. 2001). This beamformer calculates single images based on cross-spectral densities of all combinations of MEG gradiometers averaged over the selected time-frequency range and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged pre-stimulus noise period of equal duration and bandwidth (Hillebrand et al. 2005). These images are usually referred to as pseudo-t maps, where the units (pseudo-t) reflect noise-normalized power differences per voxel between a baseline or passive period and an active task-based period. As stated above, these 4.0 × 4.0 × 4.0 mm functional images were transformed into standardized space using the transform that was applied to the participant’s structural images and spatially resampled. Images were then grand-averaged across all participants to identify the peak voxels per time-frequency window.
Time series (i.e., virtual sensors) data were then extracted from these peak voxels per participant by applying the sensor-weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded a time series for the specific coordinate in source space. These virtual sensor data were then decomposed into time-frequency space and averaged across the previously identified time-frequency extents (i.e., the passbands used in the beamformer analysis) for each response per participant. This resulted in estimates of the power envelope for each time-frequency domain response, and we computed both relative (baseline-corrected) and absolute power time series for each peak voxel to probe oscillatory and spontaneous differences, respectively, between groups. Since we did not have laterality hypotheses, we collapsed across hemispheres in the case of bilateral responses in homologue cortices by averaging left and right values from each participant. In order to examine the task-related oscillatory responses, we utilized the relative time series envelopes and averaged the values across the active time periods used in the beamforming analyses. To investigate spontaneous activity, values from the absolute power time series envelopes were averaged across the baseline period (−400 to 0 ms) for each peak. Finally, to control for the possible confounding effects of age and alcohol use, ANCOVAs were used to identify group differences on the parameters of interest (i.e., the effects of age and alcohol use were partialed out). We also ran an ANCOVA including a full range of potential confounders and report these as well, although we did not have tobacco use estimates on all participants and thus could not include this in the ANCOVA models. Throughout the process, any value ±2.5 SD from the mean was considered an outlier and removed prior to statistical analyses.
Functional Connectivity Analyses
To examine functional connectivity, peak voxels in the averaged maps described above were used as seeds for computation of whole-brain cortico-cortical coherence using the DICS approach (Gross et al. 2001). The resulting images reflect time-frequency-resolved connectivity between these seeds and all other voxels in the brain. Similar to the power maps described above, coherence maps computed for active periods were normalized to coherence maps from passive (i.e., baseline) periods, resulting in whole-brain estimates of percent-change in coherence (i.e., connectivity) from baseline for each participant per peak. These whole-brain cortico-cortical coherence images were compared voxel-wise using ANCOVAs, controlling for peak-voxel power estimates for possible amplitude confounds in MEG measures of functional connectivity (e.g., coherence; (Schoffelen and Gross 2009) Thus, all reported clusters for the coherence analysis are significant above and beyond the effects of source power. The resulting maps were controlled for multiple comparisons using a spatial extent threshold of at least 5 contiguous voxels (i.e., ≥ 320mm3). To avoid concerns with signal leakage, we did not consider coherence within four cm of the seed in further analysis (Brookes et al. 2011). Lastly, similar to the time-series peak-voxel approach, we computed an ANCOVA, controlling for the effect of age and alcohol use, to examine group differences in coherence. Additionally, we computed a secondary ANCOVA that included a full range of potential confounders.
Results
Demographics and Substance Use Characteristics
As stated in the Participants section, the study sample was comprised of 21 nonusers, 21 cannabis-only users, and 22 polysubstance users. During data processing and analysis, a total of eight participants were excluded due to poor behavioral performance or excessively noisy MEG data (two nonusers, two cannabis users, and four polysubstance users). Thus, the final sample included 19 nonusers (13 males), 19 cannabis-only users (9 males), and 18 polysubstance users (15 males). The final polysubstance group used a variety of substances in addition to cannabis, including prescription stimulants, cocaine, sedatives, and hallucinogens within the past year. Detailed information on their polysubstance use is provided in Supplementary Table S1. Of note, seven of the 18 polysubstance users used multiple drugs from more than one of the named classes.
Quantitative demographic data for the final sample is provided in Table 1 and indicated that the three groups differed in mean age and alcohol consumption based on the Alcohol Use Disorders Identification Test - Concise (AUDIT-C). Specifically, a one-way ANOVA on age was significant (F[2,53] = 4.93, p = 0.01) and indicated that the nonusers (p = 0.04) and the cannabis-only users (p = 0.01) were significantly older than the polysubstance users. Likewise, an ANOVA on AUDIT-C scores was also significant (F[2,53] = 10.48, p < 0.001). Post-hoc tests revealed that the polysubstance users had a significantly higher AUDIT-C score compared to nonusers (p < 0.001) and cannabis-only users (p = 0.002). Chi-square tests were conducted to assess group differences in demographics of sex, education, income, and race. Pairwise comparisons were conducted, and none of the pairs were significantly different (p > 0.05). Finally, we conducted independent t-tests on CUDIT-R and NIDA Quick Screen scores. CUDIT-R scores between cannabis (M = 11.33, SD = 4.43) and cannabis polysubstance groups (M = 13.78, SD = 4.56) were not significant (t[35] = 1.63, p = 0.11). NIDA Quick Screen for tobacco use scores between cannabis (M = 2.05, SD = 1.90) and cannabis polysubstance use (M = 2.55, SD = 1.54) groups were also not significantly different (t[35] = 0.91, p = 0.37). Thus, to control for these differences in age and alcohol use, all group-wise analyses used an ANCOVA approach.
Table 1.
Demographic and Substance Use Data
| Nonusers M (SD) | Cannabis Users M (SD) | Polysubstance Users M (SD) | p | |
|---|---|---|---|---|
| N | 19 | 19 | 18 | - |
| Age | 32.8 (9.4) | 34.22 (8.91) | 26.27 (5.12) | 0.01 |
| Sex (M/F) | 13/6 | 9/10 | 15/3 | n.s. |
| Race* | 16/3/0/0 | 16/2/0/1 | 13/3/2/0 | n.s. |
| Ethnicity# | 15/4 | 13/6 | 15/3 | n.s. |
| AUDIT-C | 3.90 (1.6) | 3.38 (2.74) | 5.89 (2.17) | < 0.001 |
| CUDIT-R | - | 11.33 (4.43) | 13.78 (4.56) | n.s |
| NIDA Quick Scan | - | 2.05 (1.90) | 2.55 (1.54) | n.s. |
| Education (years) | 17.21 (1.69) | 15.56 (1.82) | 14.47 (1.9) | n.s. |
| Income+ | 210% | 180% | 218% | n.s. |
AUDIT-C: Alcohol Use Disorders Identification Test-Concise
CUDIT-R: Cannabis Use Disorders Identification Test – Revised
n.s.: Not significant at p < .05
Race depicted as white/Black/American Indian/Asian
Income level is measured in % above the federal poverty line.
Ethnicity is depicted as non-Hispanic/Hispanic
Behavioral Results
Performance on the visuospatial attention task was evaluated for group differences in reaction time (RT; F[2,51] = 2.00; p = 0.18) and accuracy (F[2,51] = 1.4; p = 0.25), but neither differed by group while controlling for age and alcohol use (Figure 1B).
Sensor-Level Time-Frequency Analyses
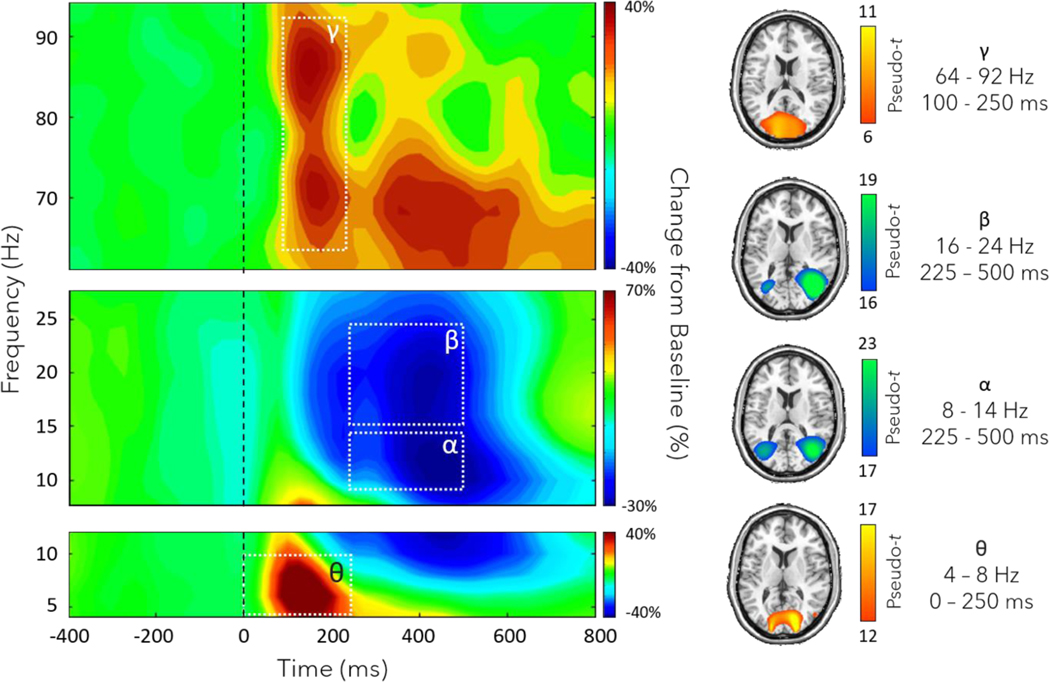
Statistical analysis of the sensor-level time-frequency spectrograms were computed across all participants and gradiometers using all artifact-free trials where participants responded correctly to the location of the stimulus. These analyses indicated strong increases or synchronizations in the theta (4 – 8 Hz; 0 – 250 ms) and gamma range (64 – 92 Hz; 100 – 250 ms) in an overlapping group of sensors near the occipital and parietal cortices, as well as desynchronizations or decreases in power from baseline in the alpha (8 – 14 Hz; 225 – 500 ms) and beta range (16 – 24 Hz; 225 – 500 ms) in sensors near occipito-parietal cortices (Figure 2). All four of these time-frequency windows were significant following permutation testing (p < 0.001, corrected).
Fig. 2.
(Left) Grand-averaged time-frequency spectrograms of MEG sensors exhibiting significant oscillatory responses, including gamma (64 – 92 Hz, 100 – 250 ms; top), beta (16 – 24 Hz, 225 – 500 ms; middle), alpha (8 – 14 Hz, 225 – 500 ms; middle), and theta (4 – 8 Hz, 0 – 250 ms; bottom). In each spectrogram, frequency (Hz) is shown on the y-axis and time (ms) on the x-axis. Signal power data are expressed as a percent difference from the baseline period with the color scale bar shown to the right of each spectrogram. (Right) Grand-averaged beamformer images (pseudo-t) across all participants for each time-frequency window. Note that the windows stop at 500 ms because this corresponds to the earliest reaction times, and we were interested in visuospatial processing and not decision processes or the motor response
Functional Mapping Analysis
The time-frequency windows identified through the sensor-level analyses were imaged using a beamformer and these maps were grand-averaged across groups per oscillatory response to identify their spatial origin. To examine the temporal evolution of these responses, virtual sensors were then extracted from the peak voxel in each cluster. In the case of homologue responses, these virtual sensor time series were averaged across hemisphere since we did not have laterality hypotheses. From these time series, we computed mean absolute power to estimate spontaneous activity during the baseline period and mean relative power to estimate oscillatory activity during stimulus processing. Finally, these values per time-frequency responses were subjected to ANCOVAs controlling for age and alcohol use, and then a follow-up ANCOVA with other potential confounders to ensure our findings were not biased by factors (environmental and demographic) that did not differ by group.
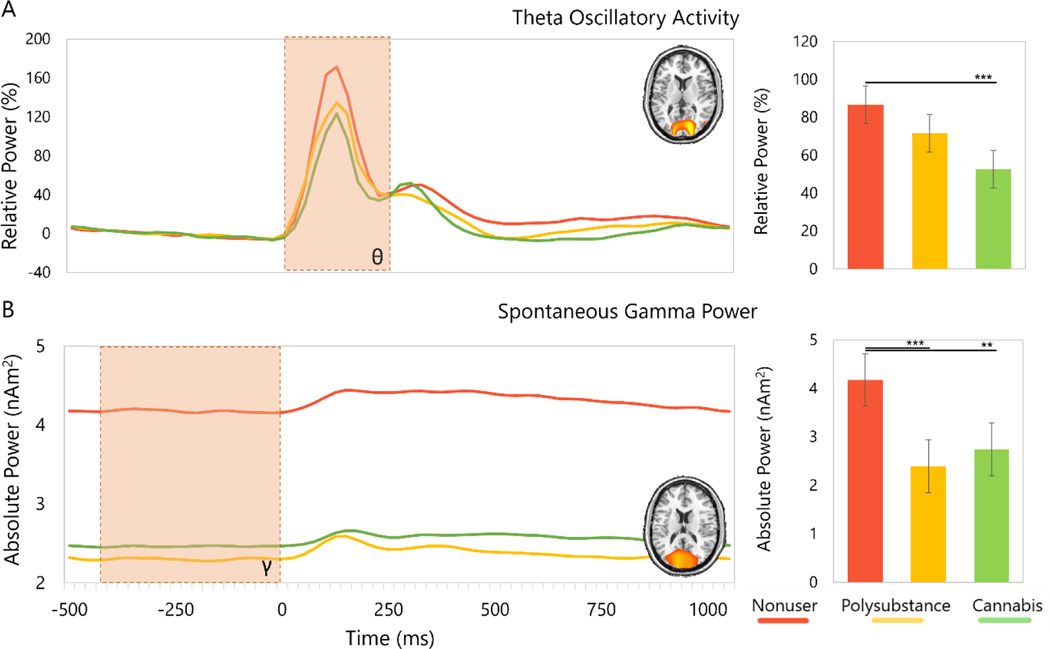
Our results indicated significant group differences in theta oscillatory responses during visuospatial processing (F[2,47] = 9.02; p < 0.001), with nonusers having significantly stronger theta power than cannabis-only users (p < 0.001) and marginally stronger than polysubstance users (p = 0.08). The cannabis-only and polysubstance users did not differ in theta power (p = 0.21; Fig 3A). There were also significant group effects in spontaneous gamma activity (F[2,48] = 8.50; p < 0.005 Fig. 3B), with post-hoc tests revealing significantly diminished spontaneous activity during the baseline period in the polysubstance users (p < 0.001) and the cannabis-only users (p = 0.008) relative to the nonusers. The two user groups did not differ in spontaneous gamma activity (p = 0.30) and we did not observe group differences in alpha or beta activity. Additionally, we performed ANCOVAs with the non-significantly different demographic variables (e.g., sex, income, education, race, ethnicity) to ensure these factors were not biasing our results. All group effects remained significant in these larger ANCOVA models, which are fully reported in Supplementary Table S2. Lastly, to evaluate whether RT differences were associated with the strength of the theta peak, and the same for accuracy and spontaneous gamma, we computed linear regressions and ANOVA analyses. Briefly, we computed a linear regression in both cases with RT as a predictor of the theta peak and accuracy as a predictor of spontaneous gamma activity; both showed no significant associations (theta: [t = −-1.01, p= 0.32, R2 = 0.018]; gamma: [t = 0.55, p = 0.59, R2 = −0.014]). Following that, we extracted the model residuals and computed an ANOVA comparing the three groups. These showed significant group effects for both theta [F(2,49) = 7.47, p = 0.002] and gamma [F(2,50) = 4.76, p = 0.01]. The theta residuals showed increased power in the non-user group relative to the cannabis group (p = 0.001), while the gamma residuals showed significantly stronger power in non-user group compared to both user groups (polysubstance: p = 0.01; cannabis only: p = 0.02). Therefore, the non-significant group differences in RT and accuracy did not underlie our main group effects for the theta oscillatory response or spontaneous gamma activity. Supplementary Figure S1 depicts violin plots by group of the extracted residuals.
Fig. 3.
(A, Left) Peak voxel time series of theta oscillatory activity in the occipital cortices collapsed across hemisphere by group with relative theta power (%) on the y-axis and time (ms) on the x-axis. (A, Right) Mean theta oscillatory response power by group over the time window used in the beamformer analysis, with relative power (%) on the y-axis and group on the x-axis. (B, Left) Peak voxel time series of spontaneous gamma activity in the occipital cortex collapsed across hemisphere during the baseline period with absolute power (nAm2) on the y-axis and time (ms) on the x-axis. (B, Right) Mean spontaneous gamma activity during the baseline period (−400 to 0 ms) with absolute power (nAm2) on the y-axis and group on the x-axis. Error bars display standard error of mean. ‘**’ = p < 0.01, ‘***’ = p < 0.005
Functional Connectivity
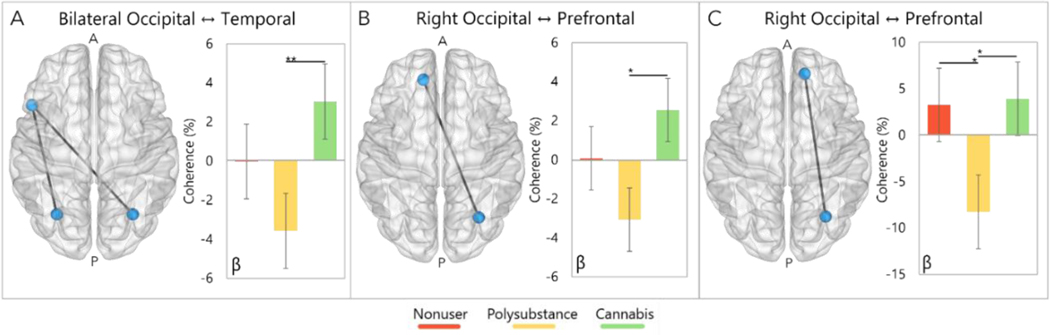
Next, we examined functional connectivity differences between groups using the peak voxels of each oscillatory response as seeds. Briefly, we computed whole-brain voxel-wise coherence per oscillatory response and participant and subjected these maps to ANCOVA analyses, controlling for the effects of age, alcohol use, and source amplitude. These analyses revealed three networks with significant group differences in the beta band. First, we observed differences in beta connectivity between bilateral occipital cortices and the left temporal cortices (F[2,50] = 5.67; p = 0.006), with follow-up analyses indicating that cannabis-only users had significantly increased coherence compared to polysubstance users (p = 0.004), but not nonusers (p = 0.24; Fig. 4A). Similar group differences were also observed in beta connectivity between the right occipital seed and the left prefrontal cortices (F[2,50] = 4.16; p = 0.02), with post-hoc testing showing increased connectivity in the cannabis-only group compared to the polysubstance group (p = 0.01), but not the nonusers (p = 0.23; Fig. 4B). Finally, group differences in beta coherence were also observed between right occipital and right prefrontal cortices (F[2,50] = 5.31; p = 0.008). Here, follow-up analyses showed polysubstance users having significantly decreased connectivity compared to cannabis-only users (p = 0.01) and nonusers (p = 0.01; Fig. 4C). Similar to the functional mapping results, we ran secondary ANCOVA analyses with other demographic variables included (e.g., sex, income, education, race, ethnicity) and the main effect of group remained significant in all three models, of which the results are reported in Supplementary Table S2.
Fig. 4.
(A, B, C) 3D representations showing pathways where group differences in beta coherence were detected. (A) Cannabis-only users exhibited significantly stronger beta connectivity between bilateral occipital and temporal cortices relative to polysubstance users. (B) Cannabis-only users also exhibited stronger beta connectivity between right occipital and left prefrontal cortices relative to polysubstance users. (C) Polysubstance users exhibited weaker beta connectivity between right occipital and right prefrontal cortices compared to nonusers and cannabis-only users. All bar graphs show the change in functional connectivity compared to baseline in percentage units on the y-axis, with group denoted on the x-axis. The group legend is at the bottom of the graph. Error bars display standard error of mean. ‘*’ = p = 0.01, ‘**’ = p < 0.005
Discussion
In this study, we examined potential differences in the association between regular cannabis use and cannabis polysubstance use on the neural dynamics underlying visuospatial processing. Our main findings indicate stronger oscillatory theta responses in the bilateral visual cortices in nonusers compared to cannabis-only users and marginally polysubstance users, as well as differences in spontaneous gamma activity during the baseline period between nonusers and both polysubstance and cannabis-only users in overlapping regions of the occipital cortices. Additionally, functional connectivity analysis indicated decreased beta connectivity between the right occipital and left prefrontal cortices in polysubstance users compared to cannabis-only users, as well as decreased beta connectivity between right occipital and right prefrontal cortices compared to both nonusers and cannabis-only users. Finally, polysubstance users also exhibited decreased beta connectivity relative to cannabis-only users among bilateral occipital and the left temporal cortices. Consistent with other neuroimaging literature investigating single-substance use, we did not find groupwise differences in accuracy or reaction time (Tomasi et al. 2007; Jager et al. 2008; Tomasi et al. 2011; Rangel-Pacheco et al. 2021; Springer et al. 2021; Lew et al. 2021a) and follow-up analyses indicated that non-significant group differences in behavior did not significantly affect the group differences observed in our neural data. Below, we discuss the implications of these findings for understanding the unique effects of cannabis use and cannabis polysubstance use on brain dynamics and connectivity.
Occipital theta oscillations have been found to play an essential role in spatial processing and orientation in the occipital cortex (Dugué et al. 2015; Yan et al. 2017), and past studies have reported decreased theta responses in regular cannabis users compared to nonusers during such processing (Edwards et al. 2009; Rangel-Pacheco et al. 2021; Springer et al. 2021; Christopher-Hayes et al. 2021). Thus, our findings of weaker theta oscillatory responses in cannabis users compared to nonusers closely aligns with the previous literature. In contrast, several studies investigating use of other single substances, including opioids, methylphenidate, and cocaine have found increased neural responses compared to nonusers (Tomasi et al. 2007, 2011; Minnerly et al. 2019). For example, Minnerly et al. (2019) found increases in theta power during the resting-state in occipital and frontal EEG sensors in opioid users compared to nonusers. Other work has found increased activation in parietal and frontal regions during visual processing using fMRI in methylphenidate users (Tomasi et al. 2011), as well as increased activation in the occipital cortex of cocaine users (Tomasi et al. 2007). Thus, our work closely corresponds to past studies showing reduced theta responses in cannabis only users compared to nonusers, while the data on polysubstance users is more complicated. Essentially, the studies above showed that the use of other substances was generally associated with increased neural activity, including theta activity (Minnerly et al., 2019). Given the effects of cannabis use alone, the marginal decreases we observed in the polysubstance group relative to the nonusers could reflect that cannabis has a dampening effect on neural activity in this group that at least partially offsets that of other substance(s). Alternatively, polysubstance users may exhibit a differential pattern of cannabis consumption, which could alter the net impact of cannabis on cognition and brain function. Such differential patterns of use may be affected by previous experience with other substances, as well as the greater or lesser addictiveness of other substances, relative to cannabis (Degenhardt et al. 2010; Davis et al. 2019; Crummy et al. 2020). Future studies are needed to clarify among these possible interpretations and to help identify whether the effect is specific to particular substances. In addition, it will be important for future studies to clarify the degree to which their substance use groups also consume cannabis to facilitate interpretation of effects.
Another major finding of the current investigation was that spontaneous gamma activity during the pre-stimulus baseline period was significantly attenuated in the cannabis only and polysubstance groups. Gamma activity is known to be essential for the detailed encoding of visual stimuli, which is associated with top-down processing (Müller et al. 2000; Jensen et al. 2014; Fries 2015; Wiesman et al. 2017b). Our findings were restricted to the pre-stimulus baseline period, which may be involved with neural tuning of bottom-up processing (Buzsáki and Wang 2012) and/or anticipation of upcoming stimuli. Therefore, this finding may suggest a difference in bottom-up processing during task performance, which is not reflected in the behavioral response data. This finding is also consistent with results from another study that showed that cannabis users exhibit significantly weaker spontaneous gamma activity during a visuospatial processing task when compared to non-users (Christopher-Hayes et al. 2021). In contrast, studies examining substances other than cannabis (e.g., opioids) have reported elevated spontaneous gamma activity across the cortex in users relative to non-users (Wang et al. 2016). This finding of elevated spontaneous gamma activity being pathological is consistent with studies of aging and HIV-associated cognitive impairment (Spooner et al. 2018, 2019, 2020, 2023; Wiesman et al. 2018; Wiesman and Wilson 2019b; Casagrande et al. 2021). Altogether, these data suggest that cannabis may have a largely dampening effect on spontaneous gamma activity, which significantly offsets any elevations caused by the use of other substances (e.g., in the polysubstance group). Such an effect could suggest that cannabis may have beneficial effects on brain function in those with cognitive impairment, advancing age, and/or other conditions where brain inflammation is thought to be central to changes in cognition (Lew et al. 2018, 2021b; Wilson et al. 2019; Spooner et al. 2021, 2023). Cannabidiol (CBD), a passive (i.e., non-psychoactive) ingredient in cannabis (Burstein 2015), has previously been shown to have antioxidant and anti-inflammatory properties (Gallily et al. 2018; Atalay et al. 2019), therefore possibly reducing inflammation in certain pathologies mentioned above. However, whether the same anti-inflammatory properties are embodied by cannabis more broadly (Hampson et al. 2000; Marsicano et al. 2002; Centonze et al. 2007) will be important to identify in future studies.
In addition to the group differences in oscillatory theta responses and spontaneous gamma activity in the visual cortex, we conducted whole-brain connectivity analyses and found differential occipito-frontal and occipito-temporal functional connectivity differences in the beta band in polysubstance users. Occipital beta oscillations have been associated with changes in visual attention (Kinsey et al. 2011), while weaker beta responses with difficulty in sustaining attention (Gola et al. 2013), and with visual perceptual decision-making (Chand et al. 2016). Generally, beta oscillations in the coherence regions of the frontal lobe have been found to play a role in regulation of cognitive control and visual attention (Stoll et al. 2016; Chand et al. 2016) and thus may indicate aberrant attentional processes in the current context. In regular cannabis users, previous studies have found stronger functional connectivity compared to nonusers (Orr et al. 2013; Filbey and Dunlop 2014), including a report of increased occipito-frontal connectivity specifically in cannabis users, with some suggesting this increased connectivity to be compensatory in nature (Harding et al., 2012). Cocaine users, on the other hand, have been found to exhibit lower connectivity in midbrain areas during a sustained attention task (Tomasi et al. 2011), which broadly agrees with resting-state work in polysubstance users where decreased connectivity has been observed across various networks (Copersino et al. 2016; Müller et al. 2018), including an executive control network (Rakesh et al. 2021) involving multiple regions reported in the current study. Our results generally agree with these findings, as the polysubstance users exhibited reduced connectivity across multiple pathways relative to the cannabis only users and both groups in the right occipital to right prefrontal pathway that is critical for attention function. Additionally, our results suggest that the stronger connectivity in cannabis users may be compensatory in nature, as suggested by previous studies (e.g., Harding et al. 2012; Springer et al. 2021), whereas the hypoconnectivity in the polysubstance group may point to a reorganization of neural networks (Wang et al. 2015; Binkowska et al. 2021b). Interestingly, such differences in brain connectivity have been observed across multiple conditions. For example, decreased whole-brain connectivity has been identified in children with fetal alcohol syndrome (Wozniak et al. 2013, 2017; Fan et al. 2017), as well as attention-deficit/hyperactivity disorder (ADHD; Konrad and Eickhoff 2010; Sato et al. 2012). Both, fetal alcohol syndrome and ADHD are major risk factors for addiction in and of themselves, which alternatively could explain the current findings (Wilens et al. 1997, 2011; Dodge et al. 2019; Flannigan et al. 2020). However, future studies are needed to further clarify the underlying mechanisms and the precise implications for attention function.
Finally, it is important to discuss the limitations of the current study. First, the methods of administration, the potency of substances, and the exact type of substance varied considerably across our sample and intra-individually across time. Whether such differences would have an impact on brain and cognitive changes related to substance use are not fully understood. Our participants also used various amounts of substances, which again often varied intra-individually over time, and we were not able to integrate this variance into the analysis. Future studies could improve this aspect, although there are limitations in what can be achieved in this regard due to poor recollection of substance use history in many participants and the fact that many users have limited information about the substances they are using (e.g., the actual potency). It should also be noted that substance use in our participants was relatively modest and future work should focus on those with more severe substance use characteristics. Future studies should aim to compare different types of polysubstance users (e.g., cocaine + cannabis versus opioids + cannabis) to ascertain the potentially additive effects of different substances. This would require much larger samples but would provide critical information. Future studies should also collect more detailed information on tobacco use and fully integrate this into the statistical models. Given the known differences in tobacco between those who use illicit substances and those who do not, this will be especially critical to consider in future work and major caveat to the findings reported herein. Lastly, literature suggests that non-drug environmental predispositions as well as genetics may play a role in substance use (Pasman et al. 2020; Lopez-Leon et al. 2021; Sanchez-Roige et al. 2022). For example, a large body of literature has shown that environmental factors, such as socio-economic status, unique to the individual play an important role in exposure and initial use of substance (Merikangas and McClair 2012). Further, specific genes have been linked to substance use disorders and some of these are also risk factors for depression and other psychiatric conditions (Kendler et al. 2003); thus, some of the group differences observed in the current study could reflect these genes and/or emergent psychiatric conditions. Parental exposure to drugs is also a risk factor for addiction and exposure during pregnancy and/or indirect exposure during childhood can also affect brain function and could potentially explain some or our findings. Thus, future studies should include genotyping as well as more detailed demographic and socio-economic variables (e.g., parental factors, personality traits, exposure to substances in utero, etc.) to gain further insight into possible causes and/or contributors to substance use.
In conclusion, our results provide insight into the commonalities and differences between cannabis and polysubstance users in association to the neural oscillatory dynamics and connectivity serving visuospatial processing. Specifically, our results demonstrate that polysubstance use adds a complex variable that is important to consider. We showed that cannabis and polysubstance users share commonalities through weaker spontaneous gamma activity than nonusers in the occipital cortex during visuospatial processing. However, differences between the user groups were identified in functional brain connectivity in the beta band between occipito-frontal and occipito-temporal cortices. Lastly, we replicated previous studies that demonstrated weaker oscillatory theta power in cannabis users compared to nonusers during visuospatial processing. In sum, the current MEG investigation provided critical new data on the differences of cannabis and cannabis polysubstance use of the neural dynamics and processes serving visuospatial function in adults.
Supplementary Material
Funding:
This study was supported by the National Institutes of Health through grants R01-DA047828 (TWW), R03-DA041917 (TWW), R01-DA056223 (TWW), R01-MH103220 (TWW), R01-MH116782 (TWW), R01-MH118013 (TWW), and P20-GM144641. The funders had no role in the study design, collection, analysis, or interpretation of data, nor did they influence writing the report or the decision to submit this work for publication. The data presented in this manuscript have not been published or presented elsewhere.
Footnotes
Conflicts: There are no financial or other types of conflicts of interest to disclose.
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ (2010) Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res 215:45–57. 10.1016/j.bbr.2010.06.023 [DOI] [PubMed] [Google Scholar]
- Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. (2010) An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend 110:137–143. 10.1016/j.drugalcdep.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Arif Y, Spooner RK, Heinrichs-Graham E, Wilson TW (2021) High-definition transcranial direct current stimulation modulates performance and alpha/beta parieto-frontal connectivity serving fluid intelligence. J Physiol 599:5451–5463. 10.1113/JP282387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalay S, Jarocka-Karpowicz I, Skrzydlewska E (2019) Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 9:21. 10.3390/antiox9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks DE, Hershberger AR, Pemberton T, et al. (2019) Poly-use of cannabis and other substances among juvenile-justice involved youth: variations in psychological and substance-related problems by typology. Am J Drug Alcohol Abuse 45:313–322. 10.1080/00952990.2018.1558450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO (2006) Patterns of simultaneous polysubstance use in drug using university students. Hum Psychopharmacol 21:255–263. 10.1002/hup.766 [DOI] [PubMed] [Google Scholar]
- Behan B, Connolly CG, Datwani S, et al. (2014) Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology 84:131–137. 10.1016/j.neuropharm.2013.05.027 [DOI] [PubMed] [Google Scholar]
- Binkowska AA, Jakubowska N, Gaca M, et al. (2021a) Not Just a Pot: Visual Episodic Memory in Cannabis Users and Polydrug Cannabis Users: ROC and ERP Preliminary Investigation. Front Hum Neurosci 15:677793. 10.3389/fnhum.2021.677793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkowska AA, Jakubowska N, Krystecka K, et al. (2021b) Theta and Alpha Oscillatory Activity During Working Memory Maintenance in Long-Term Cannabis Users: The Importance of the Polydrug Use Context. Front Hum Neurosci 15:740277. 10.3389/fnhum.2021.740277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Hale JR, Zumer JM, et al. (2011) Measuring functional connectivity using MEG: Methodology and comparison with fcMRI. NeuroImage 56:1082–1104. 10.1016/j.neuroimage.2011.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S (2015) Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem 23:1377–1385. 10.1016/j.bmc.2015.01.059 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J (2012) Mechanisms of Gamma Oscillations. Annu Rev Neurosci 35:203–225. 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande CC, Lew BJ, Taylor BK, et al. (2021) Impact of HIV-infection on human somatosensory processing, spontaneous cortical activity, and cortical thickness: A multimodal neuroimaging approach. Hum Brain Mapp 42:2851–2861. 10.1002/hbm.25408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agrò A, Bernardi G, Maccarrone M (2007) The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol Sci 28:180–187. 10.1016/j.tips.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Chand GB, Lamichhane B, Dhamala M (2016) Face or House Image Perception: Beta and Gamma Bands of Oscillations in Brain Networks Carry Out Decision-Making. Brain Connect 6:621–631. 10.1089/brain.2016.0421 [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T (2006) Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129:1096–1112. 10.1093/brain/awl064 [DOI] [PubMed] [Google Scholar]
- Charboneau EJ, Dietrich MS, Park S, et al. (2013) Cannabis cue-induced brain activation correlates with drug craving in limbic and visual salience regions: Preliminary results. Psychiatry Res Neuroimaging 214:122–131. 10.1016/j.pscychresns.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher-Hayes NJ, Lew BJ, Wiesman AI, et al. (2021) Cannabis use impacts pre-stimulus neural activity in the visual cortices of people with HIV. Hum Brain Mapp 42:5446–5457. 10.1002/hbm.25634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Gullo MJ, Chan G, et al. (2013) Polysubstance Use in Cannabis Users Referred for Treatment: Drug Use Profiles, Psychiatric Comorbidity and Cannabis-Related Beliefs. Front Psychiatry 4:. 10.3389/fpsyt.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Price JS, Frost KH, et al. (2016) Default Mode Network Functional Reorganization During Early Abstinence in Polysubstance-Using Emerging Adults Treated for Opioid Dependence. J Neuropsychiatry Clin Neurosci 28:325–327. 10.1176/appi.neuropsych.15090240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crummy EA, O’Neal TJ, Baskin BM, Ferguson SM (2020) One Is Not Enough: Understanding and Modeling Polysubstance Use. Front Neurosci 14:569. 10.3389/fnins.2020.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, Slutske WS, Martin NG, et al. (2019) Identifying subtypes of cannabis users based on simultaneous polysubstance use. Drug Alcohol Depend 205:107696. 10.1016/j.drugalcdep.2019.107696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Dierker L, Chiu WT, et al. (2010) Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend 108:84–97. 10.1016/j.drugalcdep.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Jacobson SW (2019) Effects of Fetal Substance Exposure on Offspring Substance Use. Pediatr Clin North Am 66:1149–1161. 10.1016/j.pcl.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Marque P, VanRullen R (2015) Theta Oscillations Modulate Attentional Search Performance Periodically. J Cogn Neurosci 27:945–958. 10.1162/jocn_a_00755 [DOI] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, Steinmetz AB, et al. (2009) Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behav Neurosci 123:894–904. 10.1037/a0016328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury CM, Mcdermott TJ, Wiesman AI, et al. (2018) Altered Neural Dynamics during a Flanker Attention Task in Patients with Type 1 Diabetes. Diabetes 67:1594-P. 10.2337/db18-1594-P [DOI] [Google Scholar]
- Embury CM, Wiesman AI, Proskovec AL, et al. (2019) Neural dynamics of verbal working memory processing in children and adolescents. NeuroImage 185:191–197. 10.1016/j.neuroimage.2018.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD (2004) Permutation Methods: A Basis for Exact Inference. Stat Sci 19:. 10.1214/088342304000000396 [DOI] [Google Scholar]
- Fan J, Taylor PA, Jacobson SW, et al. (2017) Localized reductions in resting-state functional connectivity in children with prenatal alcohol exposure. Hum Brain Mapp 38:5217–5233. 10.1002/hbm.23726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo PR, Tolomeo S, Steele JD, Baldacchino A (2020) Neurocognitive consequences of chronic cannabis use: a systematic review and meta-analysis. Neurosci Biobehav Rev 108:358–369. 10.1016/j.neubiorev.2019.10.014 [DOI] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J (2014) Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend 140:101–111. 10.1016/j.drugalcdep.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, et al. (2009) Marijuana craving in the brain. Proc Natl Acad Sci U S A 106:13016–13021. 10.1073/pnas.0903863106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannigan K, Coons-Harding KD, Anderson T, et al. (2020) A Systematic Review of Interventions to Improve Mental Health and Substance Use Outcomes for Individuals with Prenatal Alcohol Exposure and Fetal Alcohol Spectrum Disorder. Alcohol Clin Exp Res 44:2401–2430. 10.1111/acer.14490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P (2015) Rhythms for Cognition: Communication through Coherence. Neuron 88:220–235. 10.1016/j.neuron.2015.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallily R, Yekhtin Z, Hanuš LO (2018) The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res 3:282–290. 10.1089/can.2018.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M, Magnuski M, Szumska I, Wróbel A (2013) EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int J Psychophysiol 89:334–341. 10.1016/j.ijpsycho.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, et al. (2001) Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci 98:694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteling TP, Sillekens L, Lavie N, Jensen O (2022) Alpha oscillations reflect suppression of distractors with increased perceptual load. Prog Neurobiol 214:102285. 10.1016/j.pneurobio.2022.102285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Lolic M, et al. (2000) Neuroprotective antioxidants from marijuana. Ann N Y Acad Sci 899:274–282 [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, et al. (2012) Functional Connectivity in Brain Networks Underlying Cognitive Control in Chronic Cannabis Users. Neuropsychopharmacology 37:1923–1933. 10.1038/npp.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, et al. (2005) A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25:199–211. 10.1002/hbm.20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, Böcker KBE, et al. (2009) Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology (Berl) 204:85–94. 10.1007/s00213-008-1440-0 [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MML, van der Tweel I, et al. (2008) Assessment of Cognitive Brain Function in Ecstasy Users and Contributions of Other Drugs of Abuse: Results from an fMRI Study. Neuropsychopharmacology 33:247–258. 10.1038/sj.npp.1301415 [DOI] [PubMed] [Google Scholar]
- Jensen O, Gips B, Bergmann TO, Bonnefond M (2014) Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci 37:357–369. 10.1016/j.tins.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Jones JD, Calkins ME, Scott JC, et al. (2017) Cannabis Use, Polysubstance Use, and Psychosis Spectrum Symptoms in a Community-Based Sample of U.S. Youth. J Adolesc Health 60:653–659. 10.1016/j.jadohealth.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Schacht JP, Meredith LR, et al. (2019) Investigating a novel fMRI cannabis cue reactivity task in youth. Addict Behav 89:20–28. 10.1016/j.addbeh.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killanin AD, Embury CM, Picci G, et al. (2022) Trauma moderates the development of the oscillatory dynamics serving working memory in a sex-specific manner. Cereb Cortex N Y N 1991 bhac008. 10.1093/cercor/bhac008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey K, Anderson SJ, Hadjipapas A, Holliday IE (2011) The role of oscillatory brain activity in object processing and figure–ground segmentation in human vision. Int J Psychophysiol 79:392–400. 10.1016/j.ijpsycho.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB (2010) Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 31:904–916. 10.1002/hbm.21058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantopoulos P, Chapman P, Crundall D (2010) Driver’s visual attention as a function of driving experience and visibility. Using a driving simulator to explore drivers’ eye movements in day, night and rain driving. Accid Anal Prev 42:827–834. 10.1016/j.aap.2009.09.022 [DOI] [PubMed] [Google Scholar]
- Kovach CK, Gander PE (2016) The demodulated band transform. J Neurosci Methods 261:135–154. 10.1016/j.jneumeth.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM, Wilson TW (2018) Children with Cerebral Palsy Hyper-Gate Somatosensory Stimulations of the Foot. Cereb Cortex 28:2431–2438. 10.1093/cercor/bhx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, McDermott TJ, Wiesman AI, et al. (2018) Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology 91:e1860–e1869. 10.1212/WNL.0000000000006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, Salimian A, Wilson TW (2021a) Occipital neural dynamics in cannabis and alcohol use: independent effects of addiction. Sci Rep 11:22258. 10.1038/s41598-021-01493-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, Schantell MD, O’Neill J, et al. (2021b) Reductions in Gray Matter Linked to Epigenetic HIV-Associated Accelerated Aging. Cereb Cortex 31:3752–3763. 10.1093/cercor/bhab045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein SD, Manco N, Cope LM, et al. (2022) Systematic review of structural and functional neuroimaging studies of cannabis use in adolescence and emerging adulthood: evidence from 90 studies and 9441 participants. Neuropsychopharmacology 47:1000–1028. 10.1038/s41386-021-01226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, van den Wildenberg WPM, de Graaf Y, et al. (2019) Is (poly-) substance use associated with impaired inhibitory control? A mega-analysis controlling for confounders. Neurosci Biobehav Rev 105:288–304. 10.1016/j.neubiorev.2019.07.006 [DOI] [PubMed] [Google Scholar]
- Lopez-Leon S, González-Giraldo Y, Wegman-Ostrosky T, Forero DA (2021) Molecular genetics of substance use disorders: An umbrella review. Neurosci Biobehav Rev 124:358–369. 10.1016/j.neubiorev.2021.01.019 [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, et al. (2002) Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem 80:448–456. 10.1046/j.0022-3042.2001.00716.x [DOI] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL (2012) Epidemiology of substance use disorders. Hum Genet 131:779–789. 10.1007/s00439-012-1168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerly C, Bressler SL, Shokry IM, Tao R (2019) Estimating Mental Health Conditions of Patients with Opioid Use Disorder. J Addict 2019:8586153. 10.1155/2019/8586153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Dolder PC, Schmidt A, et al. (2018) Altered network hub connectivity after acute LSD administration. NeuroImage Clin 18:694–701. 10.1016/j.nicl.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Gruber T, Keil A (2000) Modulation of induced gamma band activity in the human EEG by attention and visual information processing. Int J Psychophysiol 38:283–299. 10.1016/S0167-8760(00)00171-9 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2022) Resource guide: screening for drug use in general medical settings [Google Scholar]
- Olthuis JV, Darredeau C, Barrett SP (2013) Substance use initiation: The role of simultaneous polysubstance use: Simultaneous polysubstance use. Drug Alcohol Rev 32:67–71. 10.1111/j.1465-3362.2012.00470.x [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M (2011) FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput Intell Neurosci 2011:1–9. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Morioka R, Behan B, et al. (2013) Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse 39:372–381. 10.3109/00952990.2013.848213 [DOI] [PubMed] [Google Scholar]
- Pasman JA, Verweij KJH, Abdellaoui A, et al. (2020) Substance use: Interplay between polygenic risk and neighborhood environment. Drug Alcohol Depend 209:107948. 10.1016/j.drugalcdep.2020.107948 [DOI] [PubMed] [Google Scholar]
- Rakesh D, Lv J, Zalesky A, et al. (2021) Altered resting functional connectivity patterns associated with problematic substance use and substance use disorders during adolescence. J Affect Disord 279:599–608. 10.1016/j.jad.2020.10.051 [DOI] [PubMed] [Google Scholar]
- Rangel-Pacheco A, Lew BJ, Schantell MD, et al. (2021) Altered fronto-occipital connectivity during visual selective attention in regular cannabis users. Psychopharmacology (Berl) 238:1351–1361. 10.1007/s00213-020-05717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar KA, Dahlgren MK, Gönenç A, et al. (2015) The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cogn Neurosci 16:84–92. 10.1016/j.dcn.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salom CL, Betts KS, Williams GM, et al. (2016) Predictors of comorbid polysubstance use and mental health disorders in young adults—a latent class analysis. Addiction 111:156–164. 10.1111/add.13058 [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S, Kember RL, Agrawal A (2022) Substance use and common contributors to morbidity: A genetics perspective. eBioMedicine 83:104212. 10.1016/j.ebiom.2022.104212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato JR, Hoexter MQ, Castellanos XF, Rohde LA (2012) Abnormal brain connectivity patterns in adults with ADHD: a coherence study. PloS One 7:e45671. 10.1371/journal.pone.0045671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Arif Y, Springer S, et al. (2022) P33. Regular Cannabis Use Modulates the Impact of HIV on the Neural Dynamics Serving Cognitive Control in the Dorsolateral Prefrontal Cortex. Biol Psychiatry 91:S101. 10.1016/j.biopsych.2022.02.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen J-M, Gross J (2009) Source connectivity analysis with MEG and EEG. Hum Brain Mapp 30:1857–1865. 10.1002/hbm.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N (2002) Cognitive Functioning of Long-term Heavy Cannabis Users Seeking Treatment. JAMA 287:1123. 10.1001/jama.287.9.1123 [DOI] [PubMed] [Google Scholar]
- Spooner RK, Taylor BK, Ahmad IM, et al. (2023) Mitochondrial redox environments predict sensorimotor brain-behavior dynamics in adults with HIV. Brain Behav Immun 107:265–275. 10.1016/j.bbi.2022.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Taylor BK, Moshfegh CM, et al. (2021) Neuroinflammatory profiles regulated by the redox environment predicted cognitive dysfunction in people living with HIV: A cross-sectional study. EBioMedicine 70:103487. 10.1016/j.ebiom.2021.103487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Mills MS, et al. (2018) Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. NeuroImage Clin 20:85–91. 10.1016/j.nicl.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, O’Neill J, et al. (2020) Prefrontal gating of sensory input differentiates cognitively impaired and unimpaired aging adults with HIV. Brain Commun 2:fcaa080. 10.1093/braincomms/fcaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, et al. (2019) Rhythmic Spontaneous Activity Mediates the Age-Related Decline in Somatosensory Function. Cereb Cortex 29:680–688. 10.1093/cercor/bhx349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wilson TW (2022) Spectral specificity of gamma-frequency transcranial alternating current stimulation over motor cortex during sequential movements. Cereb Cortex bhac 423. 10.1093/cercor/bhac423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SD, Spooner RK, Schantell M, et al. (2021) Regular recreational Cannabis users exhibit altered neural oscillatory dynamics during attention reorientation. Psychol Med 1–10. 10.1017/S0033291721002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SD, Wiesman AI, May PE, et al. (2022) Altered visual entrainment in patients with Alzheimer’s disease: magnetoencephalography evidence. Brain Commun 4:fcac198. 10.1093/braincomms/fcac198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll FM, Wilson CRE, Faraut MCM, et al. (2016) The Effects of Cognitive Control and Time on Frontal Beta Oscillations. Cereb Cortex 26:1715–1732. 10.1093/cercor/bhv006 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, et al. (2007) Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res 155:189–201. 10.1016/j.pscychresns.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, et al. (2011) Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. NeuroImage 54:3101–3110. 10.1016/j.neuroimage.2010.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ (1997) Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35:135–140. 10.1007/BF02534144 [DOI] [PubMed] [Google Scholar]
- van Es MWJ, Marshall TR, Spaak E, et al. (2022) Phasic modulation of visual representations during sustained attention. Eur J Neurosci 55:3191–3208. 10.1111/ejn.15084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen BD, Van Drongelen W, Yuchtman M, Suzuki A (1997) Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Veniero D, Gross J, Morand S, et al. (2021) Top-down control of visual cortex by the frontal eye fields through oscillatory realignment. Nat Commun 12:1757. 10.1038/s41467-021-21979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García, López-Torrecillas, Aguilar de Arcos, Pérez-García (2005) Differential effects of MDMA, cocaine, and cannabis use severity on distinctive components of the executive functions in polysubstance users: A multiple regression analysis. Addict Behav 30:89–101. 10.1016/j.addbeh.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Wang GY, Kydd RR, Russell BR (2016) Quantitative EEG and Low-Resolution Electromagnetic Tomography (LORETA) Imaging of Patients Undergoing Methadone Treatment for Opiate Addiction. Clin EEG Neurosci 47:180–187. 10.1177/1550059415586705 [DOI] [PubMed] [Google Scholar]
- Wang Z, Suh J, Li Z, et al. (2015) A hyper-connected but less efficient small-world network in the substance-dependent brain. Drug Alcohol Depend 152:102–108. 10.1016/j.drugalcdep.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, Proskovec AL, et al. (2017a) Oscillations during observations: Dynamic oscillatory networks serving visuospatial attention. Hum Brain Mapp 38:5128–5140. 10.1002/hbm.23720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, Proskovec AL, et al. (2017b) Oscillations during observations: Dynamic oscillatory networks serving visuospatial attention. Hum Brain Mapp 38:5128–5140. 10.1002/hbm.23720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, O’Neill J, Mills MS, et al. (2018) Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain 141:1678–1690. 10.1093/brain/awy097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW (2019a) The impact of age and sex on the oscillatory dynamics of visuospatial processing. NeuroImage 185:513–520. 10.1016/j.neuroimage.2018.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW (2020) Attention modulates the gating of primary somatosensory oscillations. NeuroImage 211:116610. 10.1016/j.neuroimage.2020.116610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW (2019b) The impact of age and sex on the oscillatory dynamics of visuospatial processing. NeuroImage 185:513–520. 10.1016/j.neuroimage.2018.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Mick E, et al. (1997) Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis 185:475–482. 10.1097/00005053-199708000-00001 [DOI] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, et al. (2011) Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry 50:543–553. 10.1016/j.jaac.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Lew BJ, Spooner RK, et al. (2019) Aberrant brain dynamics in neuroHIV: Evidence from magnetoencephalographic (MEG) imaging. Prog Mol Biol Transl Sci 165:285–320. 10.1016/bs.pmbts.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016) The health and social effects of nonmedical cannabis use. World Health Organization, Geneva [Google Scholar]
- Wozniak JR, Mueller BA, Bell CJ, et al. (2013) Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:748–756. 10.1111/acer.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Mattson SN, et al. (2017) Functional connectivity abnormalities and associated cognitive deficits in fetal alcohol Spectrum disorders (FASD). Brain Imaging Behav 11:1432–1445. 10.1007/s11682-016-9624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Feng Y, Liu T, et al. (2017) Theta Oscillations Related to Orientation Recognition in Unattended Condition: A vMMN Study. Front Behav Neurosci 11:166. 10.3389/fnbeh.2017.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.