Abstract
Study Objectives:
To evaluate 6-month efficacy and safety of low-sodium oxybate in people with idiopathic hypersomnia during an open-label extension period (OLE) of a phase 3 clinical trial.
Methods:
Efficacy measures included the Epworth Sleepiness Scale (ESS), Idiopathic Hypersomnia Severity Scale (IHSS), Patient Global Impression of Change (PGIc), Functional Outcomes of Sleep Questionnaire, short version (FOSQ-10), and Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP). Treatment-emergent adverse events were collected throughout the OLE.
Results:
The OLE population included 106 participants. Most were female (71%) and White (83%), and the mean (SD) age was 41.0 (13.8) years. ESS scores decreased (improved) during the OLE (mean [SD], study baseline: 16.3 [2.8]; OLE week 2: 6.7 [4.7]; OLE end: 5.3 [3.7]), and IHSS total scores trended toward a decrease (study baseline: 32.6 [7.3]; OLE week 2: 16.2 [8.9]; OLE end: 14.8 [8.6]. Median (minimum, maximum) paired differences from OLE week 2 to OLE end were ESS, −1.0 (−20, 7; nominal P = .012); IHSS, −1.0 (−31, 19; nominal P = .086). The proportion of participants reporting PGIc ratings of “very much improved” increased from 36.7% at OLE week 2 to 53.8% at the OLE end. The FOSQ-10 and WPAI:SHP scores remained stable during OLE. The incidence of newly reported treatment-emergent adverse events decreased over the duration of the OLE.
Conclusions:
Efficacy and safety of low-sodium oxybate were maintained or improved during the 6-month OLE, supporting long-term treatment with low-sodium oxybate in adults with idiopathic hypersomnia.
Clinical Trial Registration: Registry: ClinicalTrials.gov; Name: A Multicenter Study of the Efficacy and Safety of JZP-258 in the Treatment of Idiopathic Hypersomnia (IH) With an Open-label Safety Extension; URL: https://clinicaltrials.gov/study/NCT03533114; Identifier: NCT03533114 and Registry: EU Clinical Trials; Name: A Double-blind, Placebo-controlled, Randomized Withdrawal, Multicenter Study of the Efficacy and Safety of JZP-258 in the Treatment of Idiopathic Hypersomnia (IH) with an Open-label Safety Extension; URL: https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-001311-79/results; Identifier: 2018-001311-79.
Citation:
Morse AM, Dauvilliers Y, Arnulf I, et al. Long-term efficacy and safety of low-sodium oxybate in an open-label extension period of a placebo-controlled, double-blind, randomized withdrawal study in adults with idiopathic hypersomnia. J Clin Sleep Med. 2023;19(10):1811–1822.
Keywords: sleepiness, quality of life, sleep inertia, work productivity
BRIEF SUMMARY
Current Knowledge/Study Rationale: Idiopathic hypersomnia is a debilitating sleep disorder associated with symptoms such as excessive daytime sleepiness, sleep inertia, and cognitive impairment that can lead to impaired quality of life. Low-sodium oxybate, which is approved in the United States for the treatment of adults with idiopathic hypersomnia, showed efficacy and safety in a phase 3 trial, but long-term efficacy and safety are not known.
Study Impact: In a 6-month, open-label extension of the phase 3 trial, efficacy and safety of low-sodium oxybate treatment were maintained, or improved, after the main study. Efficacy measures were consistent across subgroup analyses of participants with varying demographics and baseline medications.
INTRODUCTION
Idiopathic hypersomnia is a debilitating, chronic, neurologic sleep disorder1,2 characterized by excessive daytime sleepiness (EDS), sleep inertia, cognitive impairment, long and unrefreshing naps, and prolonged nighttime sleep in many patients.1,3 Most individuals with idiopathic hypersomnia report difficulty with functioning and work due to their idiopathic hypersomnia symptoms,3–5 and report overall lower quality of life compared with healthy controls.6 Low-sodium oxybate (LXB; calcium, magnesium, potassium, and sodium oxybates; Xywav, Jazz Pharmaceuticals, Palo Alto, CA) is an oxybate medication with the same active moiety as high-sodium oxybate (SXB) and 92% less sodium.7–10 LXB is approved in the United States for the treatment of idiopathic hypersomnia in adults11 and for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy. LXB is currently the only treatment for idiopathic hypersomnia approved by the US Food and Drug Administration.12
The efficacy and safety of LXB in patients with idiopathic hypersomnia were established in a phase 3 trial, and the primary results have been published.13 LXB treatment resulted in clinically meaningful improvement in EDS, overall idiopathic hypersomnia symptoms, and sleep inertia, and reduced the impact of idiopathic hypersomnia symptoms on quality of life and functioning.13 Efficacy on the primary endpoint (change in Epworth Sleepiness Scale [ESS] from the end of a stable-dose period [SDP] to the end of a double-blind randomized withdrawal period [DBRWP]) was similar across subgroups of participants stratified by sex, baseline idiopathic hypersomnia treatment, LXB dosing regimen, and idiopathic hypersomnia phenotype (with and without long sleep time).13 The safety profile of LXB for the treatment of idiopathic hypersomnia was consistent with that reported for LXB in the treatment of narcolepsy.13 This clinical trial was the first study to include the Idiopathic Hypersomnia Severity Scale (IHSS), a recent validated measure that assesses the frequency, severity, and consequences of idiopathic hypersomnia symptoms, as well as the response to treatment.14 In this study, IHSS total scores declined (improved) with open-label LXB treatment, then worsened significantly in participants randomized to placebo compared with LXB during the DBRWP.13
The aim of these analyses was to evaluate the 6-month efficacy of LXB during an open-label extension period (OLE) of the LXB study,13 including efficacy on measures of EDS, overall idiopathic hypersomnia symptoms, patient-reported change in symptom severity, quality of life, and functioning. In addition to efficacy in the overall population, efficacy in subgroups of participants defined by sex, age, baseline treatment, and LXB dosing regimen was examined for some outcomes. Finally, the long-term safety of LXB was evaluated (treatment-emergent adverse events [TEAEs] during the OLE).
METHODS
Study design and participants
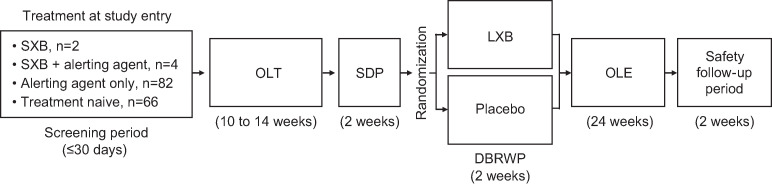
This was a phase 3, double-blind, placebo-controlled, randomized withdrawal study13 that consisted of a screening period (≤ 30 days), an open-label titration and optimization period (OLT; 10–14 weeks), SDP (2 weeks), DBRWP (2 weeks), an OLE (24 weeks), and a safety follow-up period (2 weeks) (Figure 1).13 This study was performed in line with the International Conference on Harmonization Guideline for Good Clinical Practice and the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Figure 1. Timeline of the entire study.
DBRWP = double-blind randomized withdrawal period, LXB = low-sodium oxybate, OLE = open-label extension, OLT = open-label titration and optimization period, SDP = stable-dose period, SXB = high-sodium oxybate. Modified from Dauvilliers Y, Arnulf I, Foldvary-Schaefer N, et al. Safety and efficacy of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomized withdrawal study. Lancet Neurol. 2022;21(1):53-65. doi:10.1016/S1474-4422(21)00368-9, with permission from Elsevier.
Eligible participants were 18–75 years of age with a primary diagnosis of idiopathic hypersomnia (International Classification of Sleep Disorders, second edition or International Classification of Sleep Disorders, third edition criteria) and average nocturnal total sleep time ≥ 7 hours.3,15 Participants could be taking medications for the treatment of idiopathic hypersomnia symptoms, including alerting agents (stimulants or wake-promoting agents), SXB, or both, or could be treatment naive. Participants taking alerting agents were on a stable dose for ≥ 2 months and agreed to remain on the same dose throughout the study. Participants not taking SXB had to have an ESS score ≥ 11 at screening and baseline, and participants taking SXB at study entry had to have had prior clinical improvement in EDS with SXB per the investigator’s clinical judgment. Key exclusion criteria were hypersomnia due to another medical, behavioral, sleep, or psychiatric disorder and evidence of untreated or inadequately treated sleep-disordered breathing, known or suspected respiratory difficulty, or any condition that might compromise breathing.
Study procedures
Participants who entered the study taking SXB (n = 6) were transitioned to LXB with the same regimen and dose, gram-for-gram. Participants who were oxybate naive could initiate LXB on a once-nightly or twice-nightly dosing regimen at the investigator’s discretion; all participants could also titrate to a thrice-nightly regimen. The maximum nightly starting dose for participants who were oxybate naive at study entry was 3 g (for once-nightly regimens) or 4.5 g (split into 2 nightly doses). Doses and regimens could be optimized as needed during the OLT, to a maximum dose of 6 g (once nightly) or 9 g (split into 2 nightly doses). Participants maintained that optimized stable dose for 2 weeks during the SDP. During the DBRWP, participants were randomly assigned to be withdrawn to placebo or maintain their LXB treatment.
Participants who completed the DBRWP entered an OLE, in which all participants took open-label LXB. Participants started the OLE at a dose of LXB no higher than the dose they were taking at the end of the SDP (a lower starting dose was allowed at the discretion of the investigator). If titration was required during the OLE, it proceeded at a rate of ≤ 1.5 g per night per week, not to exceed a maximum total dose of 9 g/night. Participants were permitted to initiate, discontinue, or change doses of concomitant alerting agents as needed during the OLE. Scheduled clinic visits occurred at OLE week (W) 2, W6, W14, and end of the OLE (OLE W24 or early termination).
OLE assessments and outcomes
The ESS, IHSS, and Patient Global Impression of Change (PGIc) were administered at OLE W2, W6, W14, and end of the OLE. The ESS contains 8 items; and the range of possible scores is 0–24, with higher scores indicating greater EDS.16 The IHSS comprises 14 items; and the total score range is 0–50, with higher scores indicating more severe or frequent symptoms.14 The individual component scores consisted of the following: day/performance component (7 items), with a total score range of 0–27; night/inertia component (5 items), with a total score range of 0–16; and napping component (2 items), with a total score range of 0–7. PGIc ratings during the OLE were relative to baseline (OLT day 1).
The Functional Outcomes of Sleep Questionnaire, short version (FOSQ-10),17 and Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP)18 were administered at OLE W6, W14, and end of the OLE (but not at OLE W2). The FOSQ-10 total score range is 5–20, with lower scores indicating increased impact of sleepiness on the ability to conduct daily activities (ie, poorer functional status). The WPAI:SHP for idiopathic hypersomnia consists of 4 components: percentage of work time missed due to idiopathic hypersomnia (absenteeism), percentage of impairment while working due to idiopathic hypersomnia (presenteeism), percentage of overall work impairment due to idiopathic hypersomnia (absenteeism plus presenteeism), and percentage of activity impairment due to idiopathic hypersomnia. Items relating to work productivity were completed only by employed participants; the item relating to activity impairment was completed by all participants. On all WPAI:SHP items, higher percentages indicate increased impairment. Adverse events were collected continuously throughout the study.
Statistical analyses
The intention-to-treat (ITT) population, which comprised all participants who were randomly assigned, was modified to include only those who took at least 1 dose of study medication during the DBRWP, and who had at least 1 set of postrandomization assessments for ESS or IHSS or PGIc (ie, mITT population). For the efficacy analyses during the OLE, participants taking SXB at study entry were excluded (n = 6 entered the study; n = 5 entered the OLE). The safety analysis population included all participants who received at least 1 dose of study drug. The OLE population included all participants who enrolled in the OLE. In the analyses of LXB dosing changes between the DBRWP and the OLE, the LXB dose during the SDP was used for participants randomized to placebo; for participants randomized to LXB, the LXB dose during the DBRWP was equivalent to the dose during the SDP.
For analysis of ESS and IHSS total scores, paired differences were obtained by subtracting scores at OLE W2 from scores at end of the OLE for each participant to assess whether scores were equal at the 2 time points. Analysis of covariance was used for normal distributions, whereas a Wilcoxon signed-rank test was used for nonnormal distributions. For analysis of PGIc, a McNemar test was used to assess percent change of “very much improved” between OLE W2 and end of the OLE in paired participants. ESS, IHSS, and PGIc scores were analyzed in subgroups by sex, age (above/below median, ≤ 39 or > 39 y), baseline treatment (alerting agents only or treatment naive), and LXB dosing regimen (once nightly or twice nightly). For these subgroup analyses of ESS and IHSS scores, least square (LS) means, standard errors (SEs), LS mean differences, 95% confidence intervals, and P values were obtained from a mixed model including change in ESS or IHSS total score from OLT day 1 as the response variable; covariates included baseline value, sex, age group, visit time, and baseline treatment. For PGIc, all subgroup analyses were conducted using Fisher’s exact test. IHSS components scores throughout the study were summarized with descriptive statistics. Six IHSS items are scored using a 3-point Likert scale, and 8 items are scored on a 4-point Likert scale.14 To assess IHSS improvement upon return to LXB treatment from placebo, the proportions of participants in the placebo group who reported a rare occurrence or low severity of symptoms (ie, scoring a “0” or “1”) on each IHSS item were compared from end of the DBRWP to OLE W2 and end of the OLE (from participants who had available scores at all 3 time points) using a McNemar test. P values are nominal; no adjustments for multiplicity were performed. Safety data were summarized with descriptive statistics.
RESULTS
Participants
Of 154 participants who entered the study and took LXB (safety population), 106 participants completed the DBRWP and entered the OLE (OLE safety population). Of the 106 participants who entered OLE, 11 participants did not complete the OLE (reasons were lack of efficacy, n = 1; protocol deviation, n = 1; TEAE, n = 3; withdrawal by participant, n = 5; lost to follow-up, n = 1).13 The modified ITT population in the OLE comprised 101 participants, after excluding 5 who took SXB at study entry. Baseline characteristics of the OLE safety population were similar to those of the entire study safety population (Table 1); most were female (71%) and White (83%), with a mean (SD) age of 41.0 (13.8) years.
Table 1.
Baseline demographics and disease characteristics.
| Characteristic | Safety Populationa | OLE Safety Populationb |
|---|---|---|
| (n = 154) | (n = 106) | |
| Age, mean (SD), y | 40.3 (13.7) | 41.0 (13.8) |
| Female, n (%) | 105 (68) | 75 (71) |
| Body mass index, mean (SD), kg/m2 | 27.4 (7.4) | 27.7 (8.2) |
| Race, n (%) | ||
| White | 129 (84) | 88 (83) |
| Black or African-American | 9 (6) | 6 (6) |
| Otherc | 16 (10) | 12 (11) |
| Treatment at study entry, n (%) | ||
| Alerting agentd | 82 (53) | 60 (57) |
| Treatment naive | 66 (43) | 41 (39) |
| SXB | 2 (1) | 2 (2) |
| SXB + alerting agentd | 4 (3) | 3 (3) |
| Idiopathic hypersomnia diagnosis, n (%) | ||
| With long sleep | 31 (20) | 21 (19.8) |
| Without long sleep | 123 (80) | 85 (80.2) |
| Baseline ESS score, mean (SD) | 16.1 (3.6) | 15.8 (3.7) |
| Baseline IHSS total score, mean (SD) | 32.1 (8.0) | 31.6 (8.2) |
aIncludes all participants who took at least 1 dose of study drug. bIncludes all participants who received at least 1 dose of study drug during OLE. cIncludes declined to state. dAlerting agents include stimulants and wake-promoting agents. ESS = Epworth Sleepiness Scale, IHSS = Idiopathic Hypersomnia Severity Scale, OLE = open-label extension period, SXB = high-sodium oxybate.
LXB dosing and concomitant alerting agents during OLE
At the end of the SDP, at OLE initiation, and at end of the OLE, the overall mean (SD) LXB dose (g/night) was 6.7 (1.7), 6.5 (1.7), and 6.7 (1.6), respectively. Between the DBRWP and start of the OLE, 87.7% of participants (93/106) maintained the LXB dose (and all maintained the regimen) that had been established during the OLT. Thirteen of 106 participants (12.3%) reduced their LXB dose from the DBRWP to OLE initiation (mean [SD] change: −0.2 [0.7] g/night). The majority of participants maintained their LXB dose (74/106; 69.8%) or nightly regimen (93.4%) from start of the OLE to end of the OLE. From OLE initiation to end of the OLE, 6 of 106 participants (5.7%) reduced, and 26 of 106 (24.5%) increased, their LXB dose (mean [SD] change: 0.3 [0.9] g/night). Of the 106 participants who entered the OLE, 22 (20.8%), 83 (78.3%), and 1 (0.9%) were taking LXB once, twice, or thrice nightly, respectively. Seven (6.6%) participants changed their dosing regimen during the OLE. Two participants changed from once nightly to twice nightly. Three participants changed from twice to once nightly, and 2 changed from twice to thrice nightly (with 1 participant later reverting back to twice nightly). During the OLE, 63 (59.4%) participants took alerting agents at least some of the time (including some who took > 1 alerting agent or had dose and regimen variability throughout the OLE).
Excessive daytime sleepiness
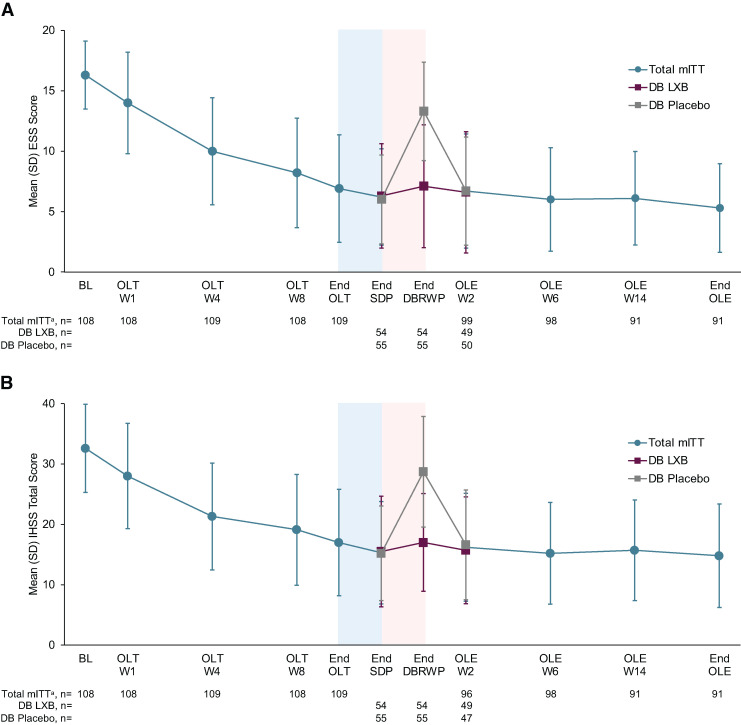
During the OLE, mean ESS scores slightly decreased, reflecting the improvement observed during the OLT and SDP in the main study (Figure 2A). Mean (SD) ESS scores at OLE W2, W6, W14, and end of the OLE were 6.7 (4.7), 6.0 (4.3), 6.1 (3.9), and 5.3 (3.7), respectively. Mean (SD) changes in ESS scores from study baseline to OLE W2, W6, W14, and end of the OLE were −9.7 (4.8), −10.3 (4.7), −10.3 (4.3), and −11.0 (4.2), respectively. The mean (SD) paired difference for ESS change from OLE W2 to end of the OLE (n = 90) was −1.2 (4.1), and the median (minimum, maximum) paired difference was −1.0 (−20, 7; P = .012). In participants who had taken placebo during the DBRWP, mean ESS scores increased during the DBRWP, then returned to the level seen during the SDP13,19 by OLE W2. Changes in ESS scores from study baseline to end of the OLE were similar in subgroups defined by sex, age, baseline treatment, and LXB dosing regimen (all comparisons, P > .05).
Figure 2. ESS (A) and IHSS (B) scores throughout the study.
Higher scores indicate greater levels of sleepiness (ESS) or idiopathic hypersomnia symptom severity (IHSS). The blue-shaded portions represent the 2-week SDP, and the pink-shaded portions represent the 2-week DBRWP. amITT population excludes participants taking high-sodium oxybate at study entry. Time points prior to OLE include the total mITT population. DB = double-blind, DBRWP = double-blind randomized withdrawal period, ESS = Epworth Sleepiness Scale, IHSS = Idiopathic Hypersomnia Severity Scale, LXB = low-sodium oxybate, mITT = modified intent-to-treat, OLE = open-label extension period, OLT = open-label titration and optimization period, SDP = stable-dose period, W = week.
Overall idiopathic hypersomnia symptoms
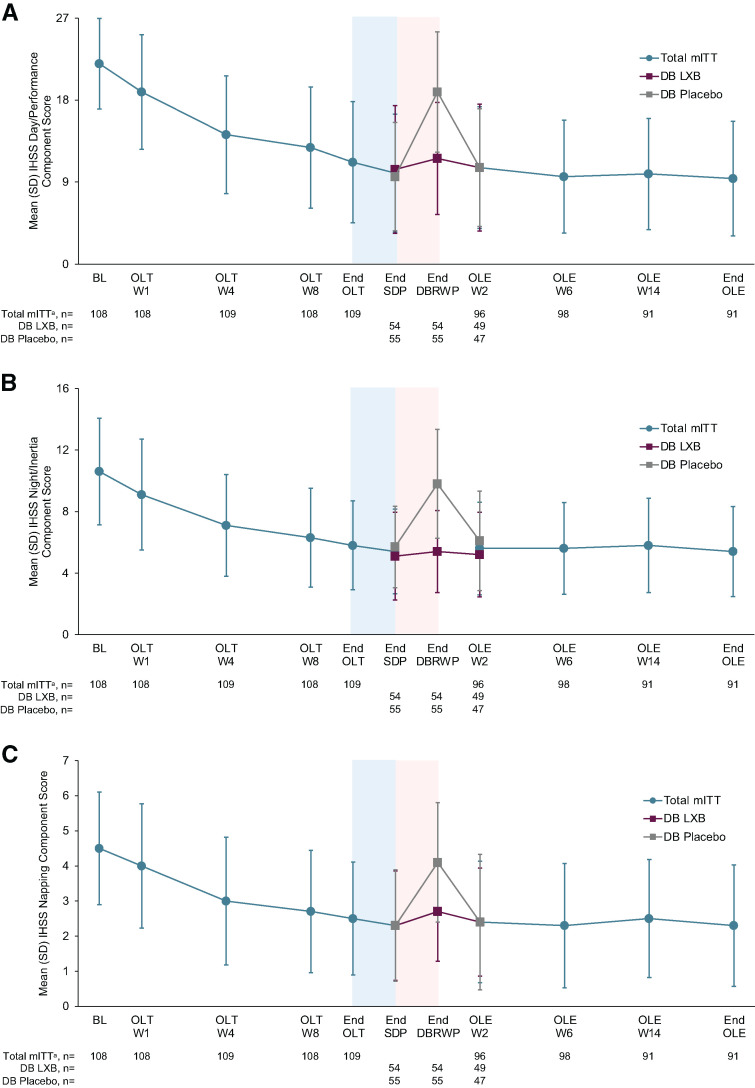
Following the improvements that were observed during the main study,13 mean IHSS total scores were maintained and further trended toward a decrease during the OLE (Figure 2B). Mean (SD) IHSS total scores at OLE W2, W6, W14, and end of the OLE were 16.2 (8.9), 15.2 (8.4), 15.7 (8.4), and 14.8 (8.6), respectively. Mean (SD) changes in IHSS total scores from study baseline to OLE W2, W6, W14, and end of the OLE were −16.3 (9.3), −17.1 (9.6), −17.0 (9.1), and −17.8 (9.6), respectively. The mean (SD) paired difference for IHSS total score change from OLE W2 to end of the OLE (n = 87) was −1.3 (6.6), and the median (minimum, maximum) paired difference was −1.0 (−31, 19; P = .086). In participants who had taken placebo during the DBRWP, mean IHSS total scores increased in the DBRWP, then returned to a similar level as was seen during the SDP13,19 by OLE W2. Changes in IHSS total scores from study baseline to end of the OLE were similar in subgroups defined by sex, age, baseline treatment, and LXB dosing regimen (all comparisons, P > .05). For each of the 3 IHSS components, the mean scores remained low during the OLE (Figure 3A–C). Among participants who had taken placebo during the DBRWP with available scores at OLE W2 (n = 47), mean (SD) IHSS component scores decreased slightly from end of the DBRWP to OLE W2: day/performance (18.9 [6.6] to 10.6 [6.5]; mean [SD] change: −8.5 [6.3]), night/inertia (9.8 [3.5] to 6.1 [3.2]; mean [SD] change: −3.7 [3.7]), and napping (4.1 [1.7] to 2.4 [1.9]; mean [SD] change: −1.6 [1.8]).
Figure 3. IHSS component scores throughout the study: day/performance (A), night/inertia (B), and napping (C).
Higher scores indicate greater idiopathic hypersomnia symptom severity. The blue-shaded portions represent the 2-week SDP, and the pink-shaded portions represent the 2-week DBRWP. Night/Inertia component includes items 1, 2, 3, 4, and 8. Day/Performance component includes items 5, 9, 10, 11, 12, 13, and 14. Napping component includes items 6 and 7.20 amITT population excludes participants taking high-sodium oxybate at study entry. Time points prior to the OLE include the total mITT population. DB = double-blind, IHSS = Idiopathic Hypersomnia Severity Scale, LXB = low-sodium oxybate, mITT = modified intent-to-treat, OLE = open-label extension period, OLT = open-label titration and optimization period, SDP = stable-dose period, W = week.
Change in symptoms in placebo group from the DBRWP to OLE W2
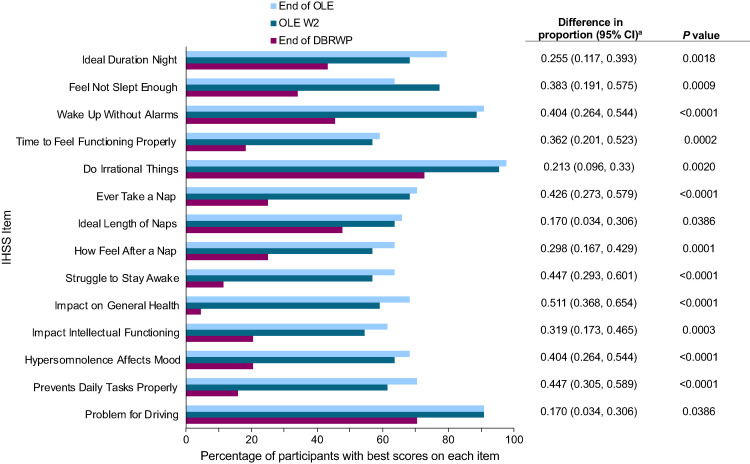
For participants who took placebo during the DBRWP, mean (SD) ESS scores at end of the DBRWP, OLE W2, and end of the OLE were 13.3 (4.1), 6.7 (4.5), and 5.5 (3.8), respectively, and mean (SD) IHSS scores were 28.7 (9.2), 16.6 (9.1), and 14.8 (8.5), respectively. For those participants who took placebo with available scores at OLE W2 and end of the OLE (n = 44), the proportion with the least impairment (ie, those who responded with “1” or “0”) on each IHSS item notably increased from end of the DBRWP to OLE W2 (Figure 4). These increases were observed for all 14 individual IHSS items upon return to LXB treatment for 2 weeks. Relatively minor changes occurred from OLE W2 to end of the OLE.
Figure 4. Increase in proportion of participants with the least impairment on each individual IHSS item from DBRWP to OLE W2 and end of OLE (placebo group).
Higher IHSS total scores indicate more severe or frequent idiopathic hypersomnia symptoms. Due to no adjustments for multiplicity, P values are nominal. Placebo group = mITT population, excluding participants taking high-sodium oxybate at study entry; percentages are based on 44 participants in the placebo group with IHSS scores available at the end of DBRWP, OLE W2, and OLE end. aDifference between the proportions at the end of the DBRWP and OLE W2. CI = confidence interval, DBRWP = double-blind randomized withdrawal period, IHSS = Idiopathic Hypersomnia Severity Scale, mITT = modified intent-to-treat, OLE = open-label extension period, W = week.
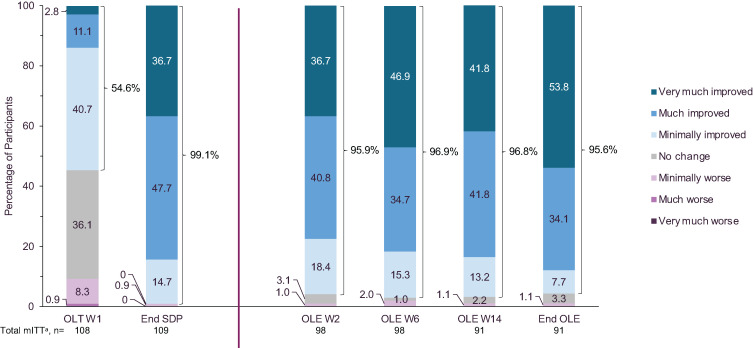
Participant-reported change in symptom severity
The percentage of participants who rated their symptoms as improved (minimally, much, or very much improved) on the PGIc remained stable throughout the OLE, following the increased percentage (from 54.6% to 99.1%, respectively) observed from OLT W1 to end of the SDP (Figure 5). PGIc ratings generally improved or remained stable during the OLE in all subgroups (Figure S1 (326KB, pdf) in the supplemental material). The proportions of participants who indicated improvement to any extent (ie, minimally, much, or very much improved) were different between some subgroups at the end of the OLE. A greater proportion of participants taking alerting agents at study entry indicated improvement compared with those who were treatment naive (53/53 [100%] and 34/38 [89.5%], respectively; P < .05). The same was true for participants taking LXB twice nightly compared with once nightly (73/73 [100%] and 13/17 [76.5], respectively; P < .001). There were no differences at any time point between subgroups defined by sex and age.
Figure 5. Change in PGIc ratings from baseline to OLT W1 and end of the SDP and during the OLE.
Categories with zero responses are not shown. amITT population excludes participants taking high-sodium oxybate at study entry. Time points prior to the OLE include the total mITT population. mITT = modified intent-to-treat, OLE = open-label extension period, OLT = open-label titration and optimization period, PGIc = Patient Global Impression of Change, SDP = stable-dose period, W = week.
Functioning and quality of life
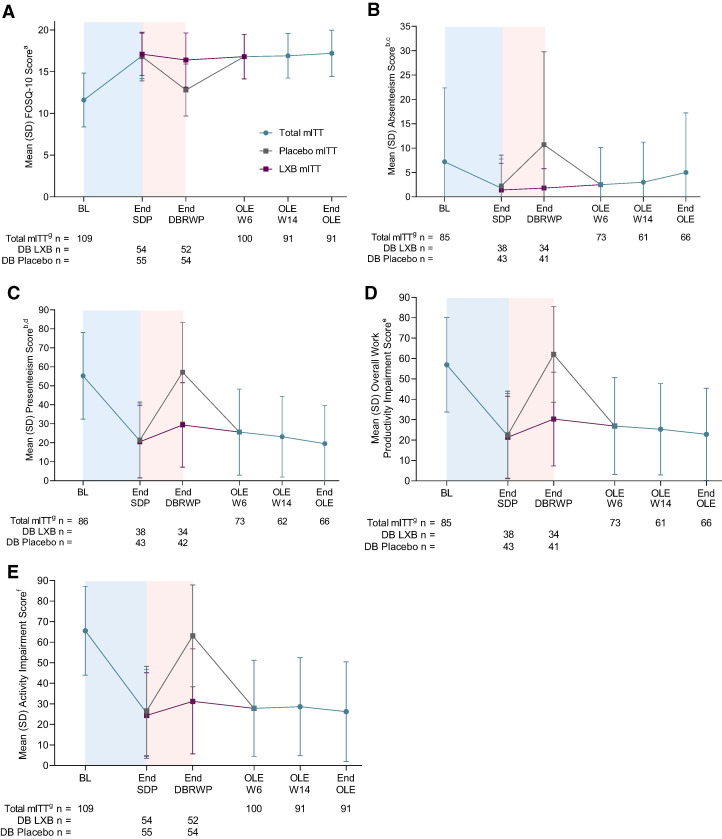
Mean (SD) FOSQ-10 scores remained stable throughout the OLE (OLE W6: 16.8 [2.7]; OLE W14: 16.9 [2.7]; end of the OLE: 17.2 [2.8]), consistent with the improved scores that were attained at the end of the SDP (17.1 [2.6]), and similar to a previously reported mean (SD) value from normal individuals who do not experience sleepiness-related impairment (17.8 [3.1]17; Figure 6A). On the WPAI:SHP, the improvements in absenteeism, presenteeism, overall work productivity, and overall activity impairment that had been observed at the end of the SDP were maintained during the OLE (Figure 6B).
Figure 6. FOSQ-10 (A), WPAI:SHP absenteeism (B), presenteeism (C), overall work productivity (D), and activity (E) scores throughout the study.
The blue-shaded portions represent the 10–14-week OLT and the 2-week SDP. The pink-shaded portions represent the 2-week DBRWP. aFOSQ-10 score is the mean of the non-missing 5 subscales multiplied by 5. Score range is 5–20 points, with higher scores indicating better functional status. WPAI:SHP score range is 0–100, with higher scores indicating greater impairment. bItems relating to work productivity were completed only by participants who were employed; the item relating to activity impairment was completed by all participants. cAbsenteeism was defined as percentage of work time missed because of idiopathic hypersomnia. dPresenteeism was defined as percentage of impairment while working because of idiopathic hypersomnia. eAbsenteeism plus presenteeism because of idiopathic hypersomnia. fRefers to overall daily activity, other than working. gmITT population excludes participants taking high-sodium oxybate at study entry. Time points prior to the OLE include the total mITT population. BL = baseline, DBRWP = double-blind randomized withdrawal period, FOSQ-10 = Functional Outcomes of Sleep Questionnaire, short version, LXB = low-sodium oxybate, mITT = modified intent-to-treat, OLE = open-label extension period, OLT = open-label titration and optimization period, SDP = stable-dose period, W = week, WPAI:SHP = Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
Safety
During the OLE, 21.7% (23/106) of participants reported only mild TEAEs; 25.5% (27/106) of participants reported TEAEs of, at most, moderate severity; and no participants reported severe TEAEs. Seventeen (28.8%) and 10 (17.5%) participants in the placebo group (n = 59) and LXB group (n = 57) during the DBRWP, respectively, reported at least 1 TEAE from end of the DBRWP to OLE W2, including decreased libido, nasopharyngitis, night sweats, and vomiting (each reported by 2 [1.7%]). Of 106 participants in the OLE, 50 (47.2%) reported at least 1 TEAE that began in the OLE. The incidence of those TEAEs decreased over the duration of the OLE (Table 2). TEAEs reported by ≥ 2 participants during at least one 4-week period included nasopharyngitis (7 [6.6%]); night sweats, upper respiratory tract infection, and vomiting (each reported by 5 [4.7%]); anxiety and hypertension (each reported by 4 [3.8%]); muscle spasms (3 [2.8%]); and enuresis and decreased libido (each reported by 2 [1.9%]) (Table 2). During the OLE, 48 (45.3%) participants reported a kind of TEAE that they had not personally reported during the main study, and 29 (27.4%) participants reported a TEAE that was not reported by any participant during the main study (Table S1 (326KB, pdf) in the supplemental material). Four serious TEAEs occurred during the OLE. One participant experienced pain syndrome due to nephrolithiasis, nephrolithiasis with planned hospitalization due to lasering nephrolithiasis, and pyelonephritis, all of mild to moderate severity, and not considered related to LXB treatment. The other participant experienced noncardiac chest pain that was of mild severity and also considered unrelated to LXB treatment. No hypertension TEAEs were considered related to LXB, based on the investigators’ assessments.
Table 2.
Newly occurring TEAEs by month during OLE (safety population).
| TEAEs | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | Month 7 | Total |
|---|---|---|---|---|---|---|---|---|
| (n = 106) | (n = 102) | (n = 101) | (n = 97) | (n = 97) | (n = 97) | (n = 62) | (n = 106) | |
| Any TEAE | 30 (28.3) | 18 (17.6) | 17 (16.8) | 10 (10.3) | 12 (12.4) | 12 (12.4) | 3 (4.8) | 50 (47.2) |
| TEAEs in ≥ 2 participants during at least one 4-week period | ||||||||
| Nasopharyngitis | 2 (1.9) | 1 (1.0) | 0 | 1 (1.0) | 1 (1.0) | 2 (2.1) | 0 | 7 (6.6) |
| Night sweats | 2 (1.9) | 0 | 2 (2.0) | 0 | 1 (1.0) | 0 | 0 | 5 (4.7) |
| Upper respiratory tract infection | 2 (1.9) | 0 | 1 (1.0) | 0 | 0 | 1 (1.0) | 1 (1.6) | 5 (4.7) |
| Vomiting | 2 (1.9) | 1 (1.0) | 1 (1.0) | 0 | 1 (1.0) | 0 | 0 | 5 (4.7) |
| Anxiety | 1 (0.9) | 3 (2.9) | 0 | 0 | 0 | 0 | 0 | 4 (3.8) |
| Hypertension | 1 (0.9) | 0 | 2 (2.0) | 0 | 0 | 1 (1.0) | 0 | 4 (3.8) |
| Muscle spasms | 0 | 2 (2.0) | 1 (1.0) | 0 | 0 | 0 | 0 | 3 (2.8) |
| Enuresis | 2 (1.9) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1.9) |
| Libido decreased | 2 (1.9) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1.9) |
Data are presented as n (%). TEAEs could have occurred in the main study, but only new monthly incidences of TEAEs during the OLE are reported in this table. OLE = open-label extension period, TEAE = treatment-emergent adverse event.
DISCUSSION
The efficacy and safety of LXB in people with idiopathic hypersomnia were established in a phase 3, placebo-controlled, double-blind, randomized withdrawal clinical trial, and the primary results have been published.13 Data from the 6-month OLE, reported here, demonstrated maintenance or continued improvement of LXB efficacy across multiple symptom and functional assessments, including the ESS, IHSS, PGIc, FOSQ-10, and WPAI:SHP. Importantly, there was no evidence that the beneficial effects of LXB waned with continued treatment over at least 6 months; rather, the effects generally appeared to be maintained over that period. Of the individuals who enrolled in the main study, a majority (68.8%) continued on to the OLE; during the OLE, most (89.6%) OLE participants continued to completion. These results support the long-term durability of LXB efficacy. The safety profile of LXB remained stable; the incidence of TEAEs decreased throughout the OLE, and no concerning new or unexpected safety signals were observed.
In response to continued open-label LXB treatment in the OLE, ESS scores further decreased from the levels seen during the main study, suggesting additional improvement in EDS. The decreases in IHSS total scores that occurred during the main study trended downward throughout the OLE. The study provided an opportunity for a more detailed analysis of the 3 individual IHSS component scores (daytime functioning, long sleep duration and sleep inertia, and napping),20 which may provide a more comprehensive clinical picture in addition to the total IHSS score, thus better informing treatment decisions. Sleep inertia, in particular, is more commonly associated with idiopathic hypersomnia than it is with narcolepsy or other hypersomnolence disorders, and it is often more severe in patients with idiopathic hypersomnia.1,2,14 Unlike the ESS, the IHSS has the unique ability to assess changes in this debilitating symptom, among others including long sleep duration and EDS.14 LXB efficacy was observed similarly in each of the 3 IHSS components (ie, daytime/performance, night/inertia, and napping) throughout the entire study, indicating the broad impact of LXB treatment across a variety of idiopathic hypersomnia symptoms. This finding may provide confidence to both patients and health care providers, as well as anticipatory guidance on what to expect regarding symptoms with LXB treatment. In addition to supporting LXB as a treatment option, these findings further support the IHSS as an important research tool. The proportion of participants who rated their symptoms as being “very much improved” on the PGIc steadily increased during the OLE. Importantly, these 3 measures (ESS, IHSS, and PGIc) showed similar efficacy when analyzed in participant subgroups divided by sex, age, baseline treatment, or LXB dosing regimen. These analyses, combined with similar subgroup efficacy analyses during the DBRWP (in participants who remained on LXB),13 demonstrate consistent efficacy despite variability in these parameters in people with idiopathic hypersomnia treated with LXB.
Improvements in daily functioning (FOSQ-10) and work productivity and overall activity levels (WPAI:SHP) in the main study were observed throughout the OLE. These findings have important implications for people with idiopathic hypersomnia, as significant impairments have been reported in their ability to perform daily activities and in their overall quality of life.6,21 Work absenteeism decreased from baseline to the end of the OLE (7.2–5.0%, respectively),13 but was still higher than what is observed in the general population (1.3% total lost worktime due to illness or injury [hours absent as a percentage of hours usually worked] among the total employed US population in 2021).22 Nonetheless, the decline in absenteeism rates observed with LXB treatment during the main study, staying relatively stable throughout the OLE, is reassuring.
To evaluate how quickly LXB efficacy could return in participants who reinitiated their treatment after withdrawing to placebo for 2 weeks in the DBRWP, their ESS and IHSS scores were compared from the DBRWP to OLE W2, as well as at end of the OLE. In these participants, ESS and IHSS total scores returned to levels similar to those seen prior to LXB withdrawal, within just 2 weeks after reinitiating LXB treatment, then were largely stable. This timely return in symptom reduction was reflected similarly across each of the 3 IHSS components (ie, daytime/performance, night/inertia, and napping). Even at the individual IHSS item level, the proportion of participants who indicated the least impairment (ie, the 2 best responses) on each item increased within 2 weeks. Collectively, these more granular analyses reinforce that starting LXB treatment again (after withdrawal) is associated with a rapid reinstatement of efficacy, which is likely to be reassuring to both patients and health care providers. These findings also demonstrate the sensitivity of the IHSS instrument to detect changes with treatment, even at the single-item level, on a rapid time scale.
A paucity of available data exists on treatments for idiopathic hypersomnia, especially over longer durations.23 Although several short-term studies have demonstrated benefits of modafinil over treatment periods ranging from 5 days to 3 weeks, none of them included a longer duration, OLE phase.24–27 Retrospective chart reviews have suggested a long-term benefit of mazindol, modafinil, and pitolisant in idiopathic hypersomnia.5,28,29 A retrospective chart review of 41 people with idiopathic hypersomnia treated with SXB for a mean of 15.8 months showed maintenance of benefit and a lack of treatment tolerance with long-term treatment.30 The present findings provide new information on the long-term benefits of LXB for people with idiopathic hypersomnia. Importantly, LXB can be taken concomitantly with stimulants or wake-promoting agents, as evidenced by approximately half of the participants in the present study. In fact, all (100%) participants who were taking stimulants or wake-promoting agents at study entry indicated improvement in their symptoms at the end of the OLE, suggesting a possible advantage of LXB over prior treatments. The majority of participants maintained their LXB dose and regimen, which they had previously established during the OLT, from the end of the DBRWP to the end of the OLE. The dosing/regimen stability suggests that the open-label treatment and titration period in the main study was of a sufficient duration (10–14 weeks) to achieve an optimal dose/regimen. Further, it is consistent with satisfactory long-term efficacy and tolerability.
During the OLE, the most common newly reported TEAEs (reported by ≥ 2 participants in any month, and not starting in the main study) were nasopharyngitis (n = 6), upper respiratory tract infection (n = 5), hypertension (n = 4), vomiting (n = 4), anxiety (n = 3), diarrhea (n = 3), and sinusitis (n = 3). This is generally consistent with TEAEs observed earlier in the trial,13 in long-term clinical trials of oxybate in adult and pediatric participants with narcolepsy,7,31,32 and in a postauthorization safety study of SXB in narcolepsy.11,33 The incidence of newly occurring TEAEs decreased over the duration of the OLE, from 28.3% in month 1 to 4.8% in month 7. This is similar to the rate of new TEAEs in the extension phase of the study of LXB in narcolepsy, where the incidence of TEAEs (headache, nasopharyngitis, dizziness, upper respiratory tract infection, and influenza) was highest in OLE month 2 and declined thereafter.34 Importantly, no severe TEAEs were reported, and only 2 participants experienced serious TEAEs.
Importantly, participants did not show clinically significant or new safety signals indicative of LXB withdrawal signs or symptoms. Furthermore, no participants in this study showed signs of LXB abuse after careful monitoring for specific signs of abuse. This is consistent with the low incidence of suspected abuse identified in individuals prescribed SXB (ie, there were 31 cases of suspected abuse of SXB identified among 17,037 patients through the Risk Evaluation and Mitigation Strategy Program from 2016 to 2017).35
One strength of this analysis is the inclusion of endpoints (in particular, the IHSS) that assessed multiple aspects of idiopathic hypersomnia symptoms and the functional consequences of the disorder. Previous randomized controlled studies of other idiopathic hypersomnia treatments have focused solely on daytime sleepiness, clinician-rated symptom severity, and/or functional impairment (driving performance).24–27 In addition, the design of this study makes the present findings relevant to real-world clinical practice. In addition to dose titration during the OLT period, LXB dose titration was permitted during the OLE for individualization of dosing to maximize efficacy and safety, and concurrent treatment with alerting agents was permitted, which is consistent with what is commonly observed in clinical practice.36 Limitations include that LXB treatment in the period reported here was open-label and the OLE population was enriched for response to and tolerance of LXB, as participants who previously experienced lack of efficacy or intolerable TEAEs may have discontinued earlier. Three of the 11 participants who discontinued during the OLE did so due to TEAEs, and 5 of the participants voluntarily withdrew for reasons other than TEAEs. In addition, to meet the study inclusion criteria, individuals taking SXB were required to have documented clinical benefit of SXB treatment (this included 5 of the 106 OLE participants of the safety population). However, such participants were excluded from the OLE efficacy analyses.
In conclusion, the efficacy and safety of LXB remained stable or improved over 6 months during the OLE, supporting long-term LXB treatment in adults with idiopathic hypersomnia. Multiple idiopathic hypersomnia symptoms showed improvements that were consistent across the participant population. These findings support the broad utility of LXB as a treatment for idiopathic hypersomnia and provide a more comprehensive picture of the impact of long-term LXB treatment in people with idiopathic hypersomnia.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was conducted at 50 specialist sleep centers in Belgium, Czech Republic, Finland, France, Poland, Spain, and the United States. This study was sponsored by Jazz Pharmaceuticals. Anne Marie Morse has received research/grant support and consultancy fees from Avadel, Harmony Biosciences, Jazz Pharmaceuticals, Takeda Pharmaceutical Co, Ltd, NLS Pharmaceutics, Alkermes, UCB Pharmaceuticals, and the National Institutes of Health (NIH). Yves Dauvilliers is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Avadel, Harmony Biosciences, Idorsia, Orexia, Takeda, Paladin, and Bioprojet. Isabelle Arnulf has participated in advisory boards for Idorsia (2020), Ono (2019) Pharma, and Roche Pharma (2018). Michael J. Thorpy has received research/grant support and consultancy fees from Axsome, Balance Therapeutics, Flamel/Avadel, Harmony Biosciences, Jazz Pharmaceuticals, Suven Life Sciences Ltd, Takeda Pharmaceutical Co, Ltd, NLS Pharmaceutics, XW Pharma, Idorsia Pharmaceuticals, and Eisai Pharmaceuticals. Nancy Foldvary-Schaefer has served on an advisory committee for Jazz Pharmaceuticals and participated in clinical trials supported by Jazz Pharmaceuticals, Suven Life Sciences, and Takeda Pharmaceuticals. Patricia Chandler, Abby Chen, and Luke Hickey are full-time employees of Jazz Pharmaceuticals who, in the course of this employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. Jed Black is a part-time employee of Jazz Pharmaceuticals and shareholder of Jazz Pharmaceuticals plc. Richard K. Bogan is a shareholder of Watermark Medical and Healthy Humming, LLC; serves on the Board of Directors for Watermark; is a medical consultant to Jazz Pharmaceuticals, Harmony Biosciences, Avadel Pharmaceuticals, Takeda, and Oventus; has conducted industry-funded research for Avadel, Axsome, Bresotec, Bayer, Idorsia, Suven, Jazz Pharmaceuticals, Balance, NLS Pharmaceutics, Vanda, Merck, Eisai, Philips, Fresca, Takeda, LivaNova, Roche, Sanofi, Sommetrics, and Noctrix; and is on speaker’s bureaus for Jazz Pharmaceuticals, Eisai, and Harmony.
ACKNOWLEDGMENTS
The authors thank the study participants, study investigators, and study staff for their contributions to this research. This study was sponsored by Jazz Pharmaceuticals. Under the direction of the authors, Karyn Liu, PhD, and Emily Bruggeman, PhD (employees of Peloton Advantage, LLC, an OPEN Health company), provided medical writing and editorial support. Jazz Pharmaceuticals provided funding to Peloton Advantage for medical writing and editorial support.
Data availability: All relevant data are provided within the manuscript and supporting files. Jazz Pharmaceuticals has established a process to review requests from qualified external researchers for data from Jazz Pharmaceuticals–sponsored clinical trials in a responsible manner that includes protecting patient privacy, assurance of data security and integrity, and furthering scientific and medical innovation. Additional details on Jazz Pharmaceuticals data sharing criteria and process for requesting access can be found at: https://www.jazzpharma.com/science/clinical-trial-data-sharing/.
ABBREVIATIONS
- DBRWP
double-blind randomized withdrawal period
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- FOSQ-10
Functional Outcomes of Sleep Questionnaire, short version
- IHSS
Idiopathic Hypersomnia Severity Scale
- ITT
intent-to-treat
- LXB
low-sodium oxybate
- OLE
open-label extension
- OLT
open-label titration and optimization period
- PGIc
Patient Global Impression of Change
- SDP
stable-dose period
- SXB
high-sodium oxybate
- TEAE
treatment-emergent adverse event
- W
week
- WPAI:SHP
Work Productivity and Activity Impairment Questionnaire: Specific Health Problem
REFERENCES
- 1. Trotti LM . Idiopathic hypersomnia . Sleep Med Clin. 2017. ; 12 ( 3 ): 331 – 344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dauvilliers Y , Bogan RK , Arnulf I , Scammell TE , St Louis EK , Thorpy MJ . Clinical considerations for the diagnosis of idiopathic hypersomnia . Sleep Med Rev. 2022. ; 66 : 101709 . [DOI] [PubMed] [Google Scholar]
- 3. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 4. Bassetti C , Aldrich MS . Idiopathic hypersomnia. A series of 42 patients . Brain. 1997. ; 120 ( Pt 8 ): 1423 – 1435 . [DOI] [PubMed] [Google Scholar]
- 5. Anderson KN , Pilsworth S , Sharples LD , Smith IE , Shneerson JM . Idiopathic hypersomnia: a study of 77 cases . Sleep. 2007. ; 30 ( 10 ): 1274 – 1281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wasling HB , Bornstein A , Wasling P . Quality of life and procrastination in post-H1N1 narcolepsy, sporadic narcolepsy and idiopathic hypersomnia, a Swedish cross-sectional study . Sleep Med. 2020. ; 76 : 104 – 112 . [DOI] [PubMed] [Google Scholar]
- 7. Bogan RK , Thorpy MJ , Dauvilliers Y , et al . Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; JZP-258) in a placebo-controlled, double-blind, randomized withdrawal study in adults with narcolepsy with cataplexy . Sleep. 2021. ; 44 ( 3 ): zsaa206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szarfman A , Kuchenberg T , Soreth J , Lajmanovich S . Declaring the sodium content of drug products . N Engl J Med. 1995. ; 333 ( 19 ): 1291 . [DOI] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration . Clinical review for Binosto, NDA 202344. 2012. . https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202344Orig1s000MedR.pdf . Accessed February 28, 2023.
- 10. US Food and Drug Administration . Quantitative labeling of sodium, potassium, and phosphorus for human over-the-counter and prescription drug products. Guidance for industry. 2022. . https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quantitative-labeling-sodium-potassium-and-phosphorus-human-over-counter-and-prescription-drug . Accessed October 11, 2022.
- 11. Jazz Pharmaceuticals, Inc . Xywav® (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII [prescribing information]. Palo Alto, CA: : Jazz Pharmaceuticals, Inc; ; 2023. . https://pp.jazzpharma.com/pi/xywav.en.USPI.pdf . Accessed July 13, 2023. [Google Scholar]
- 12. Jazz Pharmaceuticals . Jazz Pharmaceuticals announces U.S. FDA approval of Xywav® (calcium, magnesium, potassium, and sodium oxybates) oral solution for idiopathic hypersomnia in adults [press release]. 2021. . http://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-us-fda-approval-xywavr-calcium . Accessed February 14, 2023.
- 13. Dauvilliers Y , Arnulf I , Foldvary-Schaefer N , et al . Safety and efficacy of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomised withdrawal study . Lancet Neurol. 2022. ; 21 ( 1 ): 53 – 65 . [DOI] [PubMed] [Google Scholar]
- 14. Dauvilliers Y , Evangelista E , Barateau L , et al . Measurement of symptoms in idiopathic hypersomnia: the Idiopathic Hypersomnia Severity Scale . Neurology. 2019. ; 92 ( 15 ): e1754 – e1762 . [DOI] [PubMed] [Google Scholar]
- 15. American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd ed. Westchester, IL: : American Academy of Sleep Medicine; ; 2005. . [Google Scholar]
- 16. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 17. Chasens ER , Ratcliffe SJ , Weaver TE . Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire . Sleep. 2009. ; 32 ( 7 ): 915 – 919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly MC , Zbrozek AS , Dukes EM . The validity and reproducibility of a work productivity and activity impairment instrument . PharmacoEconomics. 1993. ; 4 ( 5 ): 353 – 365 . [DOI] [PubMed] [Google Scholar]
- 19. Thorpy MJ , Arnulf I , Foldvary-Schaefer N , et al . Efficacy and safety of lower-sodium oxybate in an open-label titration period of a phase 3 clinical study in adults with idiopathic hypersomnia . Nat Sci Sleep. 2022. ; 14 : 1901 – 1917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rassu AL , Evangelista E , Barateau L , et al . Idiopathic Hypersomnia Severity Scale to better quantify symptoms severity and their consequences in idiopathic hypersomnia . J Clin Sleep Med. 2022. ; 18 ( 2 ): 617 – 629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whalen M , Roy B , Steininger T , Dronamraju N , Enson D . Patient perspective on idiopathic hypersomnia: impact on quality of life and satisfaction with the diagnostic process and management [abstract]. Presented at: Annual Scientific Meeting of the Associated Professional Sleep Societies; June 4–8, 2022. ; Charlotte, NC.
- 22. US Bureau of Labor Statistics . Household data annual averages. 47. Absences from work of employed full-time wage and salary workers by occupation and industry. 2022. . https://www.bls.gov/cps/cpsaat47.htm . Accessed July 14, 2022.
- 23. Arnulf I , Thomas R , Roy A , Dauvilliers Y . Update on the treatment of idiopathic hypersomnia: progress, challenges, and expert opinion . Sleep Med Rev. 2023. ; 69 : 101766 . [DOI] [PubMed] [Google Scholar]
- 24. Inoue Y , Tabata T , Tsukimori N . Efficacy and safety of modafinil in patients with idiopathic hypersomnia without long sleep time: a multicenter, randomized, double-blind, placebo-controlled, parallel-group comparison study . Sleep Med. 2021. ; 80 : 315 – 321 . [DOI] [PubMed] [Google Scholar]
- 25. Mayer G , Benes H , Young P , Bitterlich M , Rodenbeck A . Modafinil in the treatment of idiopathic hypersomnia without long sleep time—a randomized, double-blind, placebo-controlled study . J Sleep Res. 2015. ; 24 ( 1 ): 74 – 81 . [DOI] [PubMed] [Google Scholar]
- 26. Philip P , Chaufton C , Taillard J , et al . Modafinil improves real driving performance in patients with hypersomnia: a randomized double-blind placebo-controlled crossover clinical trial . Sleep. 2014. ; 37 ( 3 ): 483 – 487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sagaspe P , Micoulaud-Franchi JA , Coste O , et al . Maintenance of Wakefulness Test, real and simulated driving in patients with narcolepsy/hypersomnia . Sleep Med. 2019. ; 55 : 1 – 5 . [DOI] [PubMed] [Google Scholar]
- 28. Nittur N , Konofal E , Dauvilliers Y , et al . Mazindol in narcolepsy and idiopathic and symptomatic hypersomnia refractory to stimulants: a long-term chart review . Sleep Med. 2013. ; 14 ( 1 ): 30 – 36 . [DOI] [PubMed] [Google Scholar]
- 29. Leu-Semenescu S , Nittur N , Golmard JL , Arnulf I . Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review . Sleep Med. 2014. ; 15 ( 6 ): 681 – 687 . [DOI] [PubMed] [Google Scholar]
- 30. Leu-Semenescu S , Louis P , Arnulf I . Benefits and risk of sodium oxybate in idiopathic hypersomnia versus narcolepsy type 1: a chart review . Sleep Med. 2016. ; 17 : 38 – 44 . [DOI] [PubMed] [Google Scholar]
- 31. US Xyrem Multicenter Study Group . A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy . Sleep. 2003. ; 26 ( 1 ): 31 – 35 . [PubMed] [Google Scholar]
- 32. Plazzi G , Ruoff C , Lecendreux M , et al . Treatment of paediatric narcolepsy with sodium oxybate: a double-blind, placebo-controlled, randomised-withdrawal multicentre study and open-label investigation . Lancet Child Adolesc Health. 2018. ; 2 ( 7 ): 483 – 494 . [DOI] [PubMed] [Google Scholar]
- 33. Mayer G , Plazzi G , Iranzo Á , et al . Long-term compliance, safety, and tolerability of sodium oxybate treatment in patients with narcolepsy type 1: a postauthorization, noninterventional surveillance study . Sleep. 2018. ; 41 ( 9 ): zsy128 . [DOI] [PubMed] [Google Scholar]
- 34. Bogan RK , Foldvary-Schaefer N , Skowronski R , Chen A , Thorpy MJ . Long-term safety and tolerability during a clinical trial and open-label extension of lower-sodium oxybate in participants with narcolepsy with cataplexy . CNS Drugs. 2023. ; 37 ( 4 ): 323 – 335 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strunc MJ , Black J , Lillaney P , et al . The Xyrem® (Sodium Oxybate) Risk Evaluation and Mitigation Strategy (REMS) Program in the USA: results from 2016 to 2017 . Drugs Real World Outcomes. 2021. ; 8 ( 1 ): 15 – 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trotti LM , Ong JC , Plante DT , Friederich Murray C , King R , Bliwise DL . Disease symptomatology and response to treatment in people with idiopathic hypersomnia: initial data from the Hypersomnia Foundation Registry . Sleep Med. 2020. ; 75 : 343 – 349 . [DOI] [PMC free article] [PubMed] [Google Scholar]