Abstract
Introduction
Psoriatic arthritis (PsA) affects around 150 000 people in the UK of whom around 50% require treatment with biologics. The most used biologics for PsA target tumour necrosis factor (TNF) or interleukin-17A (IL-17A). About 50% of patients respond to each, but it is not currently possible to predict response for individual patients, necessitating sequential treatment steps. A recent proof of concept study in PsA suggested that using peripheral immunophenotype to choose therapy could improve time to treatment response.
This study will test the hypothesis, within an open-label parallel-group biomarker-stratified multicentre randomised controlled trial, which the baseline proportion of CD4+T cells with an activated type 17 immunophenotype (Th17 levels) predicts response to IL-17A or TNF inhibitors in PsA. Additional analyses will identify if the model can be refined by combining additional clinical and immunophenotypic factors. Statistical modelling will be used to predict the likely effectiveness of these approaches compared with standard care.
Methods and analysis
Patients with PsA eligible to start their first biologic as part of standard care are recruited and baseline blood tests are taken for immunophenotyping. Participants are stratified equally by Th17 levels and randomised 1:1 to receive either TNF (adalimumab) or IL-17A (secukinumab) inhibitors. The primary analysis will establish the interaction between baseline immunophenotype and treatment on the primary outcome (achievement of minimal disease activity criteria at week 24). In secondary analysis, modelling will identify if this prediction model can be optimised further by incorporating clinical phenotypes and additional immunophenotyping techniques.
Ethics and dissemination
Ethical approval for the study was granted by the North West Preston Research Ethics Committee (ref 21/NW/0016). Dissemination will be via conference presentations and peer-reviewed publications, aiming to impact on treatment guidelines.
Trial registration number
ISRCTN17228602.
Keywords: Musculoskeletal disorders, RHEUMATOLOGY, Clinical Decision-Making, Clinical Trial
Strengths and limitations of this study.
The Optimising Psoriatic arthritis Therapy with Immunological Methods to Increase Standard Evaluation study is the first-powered randomised controlled trial investigating a precision medicine approach to biologic selection in psoriatic arthritis.
Broad eligibility criteria, in keeping with current UK treatment recommendations, increase the generalisability of the trial results to clinical practice.
Both participants and clinicians are blinded to the immunophenotyping data minimising bias in the analysis.
Detailed immunophenotyping using multiple laboratory approaches will maximise the chances of identifying key predictive markers for response.
Of note, immunophenotyping requires considerable cell processing and is not yet optimised for routine diagnostic use.
Introduction
Psoriatic arthritis (PsA) is an inflammatory arthritis that occurs in ~15% of people with psoriasis, affecting around 150 000 people in the UK.1 Two-thirds of people with PsA suffer joint damage with associated disability2 similar to levels reported for rheumatoid arthritis.3 PsA is associated with reduced life expectancy4 and average direct healthcare costs of £2400 per patient with indirect costs of >£8000 annually.5
The current treatment of PsA follows an empiric ‘step up’ ‘trial-and-error’ approach using different conventional disease-modifying antirheumatic drugs (DMARDs) followed by biologic DMARDs if patients do not respond.1 6 Approximately 50% of patients with PsA will require biologic therapy7 with four key mode of action drugs available. The most commonly used biologic treatments for PsA target one of two main immunological pathways: tumour necrosis factor (TNF) or interleukin (IL) 17. Arthritis response rates to both drugs are similar, with 60%–70% of patients achieving at least a partial response. In clinical practice, biologic therapies need to be used for a minimum of 12–16 weeks before response can be evaluated,1 6 with assessment of achievement of treatment target later.8 For many patients this means protracted administration of a therapy that may never work, in addition to financial and clinical National Health Service (NHS) costs.
Two head-to-head parallel-group randomised studies comparing TNF and IL-17A inhibitors in PsA have been performed showing no significant differences in peripheral arthritis outcomes.9 10 Currently, clinicians select therapies based on a limited clinical phenotype, such as differentiation in skin psoriasis, comorbidities, personal experience and cost. Despite similar responses at a group level, we know that some people who fail to respond to a first biologic will have a good response when they switch to a drug with a different mechanism of action,11 suggesting that disease immunopathogenesis varies between individuals. However, treatment in these studies was randomly allocated, with only one previous study in PsA with any precision medicine element.
This study in Japan evaluated the use of baseline CD4+T cell immunophenotype characteristics to inform selection of biologic therapy.12 They defined four groups based on predetermined cut-offs for high and low levels of Th1 and Th17 cells, based on quartiles in healthy controls. Sixty-four patients with patients starting biologic therapy were randomly divided into a standard care group (IL-12/23, IL-17A or TNF inhibitors) and a precision medicine group (n=26) in which the choice of therapy was based on the peripheral blood lymphocyte analysis. The precision medicine group had significantly higher rates of ACR20 response and low disease activity, although other measures, including psoriasis responses, were not significantly different. The study was not powered to compare the treatment groups and did not include a prespecified primary outcome. However, the results are promising, and the study urgently requires confirmation.
A more rational approach to treatment selection has the potential to make a substantial contribution to patient care by increasing the chance of identifying the biologically rational treatment for the patient. Thus, the primary aim of the Optimising Psoriatic arthritis Therapy with Immunological Methods to Increase Standard EvaluationOPTIMISE) study is to identify a peripheral immunophenotype that can predict response to biological therapy in PsA and facilitate a stratified approach to treatment.
Objectives
Our primary objective is to establish the interaction between baseline immunophenotype (proportion of CD4+T cells with an activated Th17 cell profile) and treatment (IL-17A or TNF inhibitor therapy) on the proportion of PsA patients achieving the minimal disease activity (MDA) criteria at week 24 (primary outcome).
Our secondary objectives will compare responses to both medications dependent on intracellular IL-17 levels and immune-subset transcriptomic signatures to see if additional immunological markers can predict response to either drug. Treatment response from a patient’s perspective is assessed through patient-reported outcome measures. We will also explore changes in the immunological markers with treatment and assess if these correlate with clinical response. These objectives are all summarised in table 1.
Table 1.
Primary, secondary and exploratory objectives for the OPTIMISE trial
| Objectives | Outcome measures | Timepoint(s) of evaluation of this outcome measure (if applicable) |
|
Primary objective To test whether the clinical response to TNF and IL-17A inhibitor therapy in participants with PsA differs according to the level of baseline activated Th17 cells. |
Clinical response as measured by the minimal disease activity (MDA) criteria | Immunophenotype data at baseline and clinical response at week 24. |
|
Secondary objectives To test whether the clinical response to TNF and IL-17A inhibitor therapy in participants with PsA differs according to intracellular IL-17 levels. |
Clinical response as measured by the MDA criteria | Immunophenotype data at baseline and clinical response at week 12/16 and week 24. |
| To understand if the activated Th17 surface and intracellular signature resolves after treatment with IL-17A blockade and how it is altered after TNF blockade. | Activated Th17 proportion and intracellular levels of IL-17 | Immunophenotype data at baseline and week 24. |
| To understand if changes in the activated Th17 surface and intracellular signature differ in treatment responders and non-responders. | Clinical response as measured by the MDA criteria. | Clinical disease pattern and Immunophenotype data at baseline and clinical response at week 12/16 and week 24. |
| To explore if the immune subset-specific transcriptomic signature can be used to predict response to IL-17A and TNF blocking therapies either alone or in combination with the activated surface and intracellular Th17 signatures. | Clinical response as measured by the MDA criteria. | Clinical disease pattern and Immunophenotype data at baseline and clinical response at week 12/16 and week 24. |
| To explore if any of the baseline immune signatures are associated with response in different PsA tissues | Clinical response in PsA tissues including joint counts, enthesitis, dactylitis, skin and nail disease scores and in overall disease as measured by the PsA disease activity score (PASDAS). | Immunophenotype data at baseline and clinical response at week 12/16 and 24. |
| To explore if any of the baseline immune signatures are associated with response and disease impact from the patients’ perspective | Response as measured by patient reported outcomes including PsAID, SF36 and WPAI | Immunophenotype data at baseline and clinical response at week 12/16 and 24. |
| To use the immune subset-specific transcriptomic signature to identify a limited number to of transcriptomic biomarkers that can be validated in whole blood. | Cell-specific transcriptomic data and whole blood transcriptomes | Immunophenotype data at baseline and week 24. |
| To use the immune subset-specific transcriptomic signature to define the pathways driving biologic-refractory disease. | Cell-specific transcriptomic data and whole blood transcriptomes | Immunophenotype data at baseline and week 24. |
| Exploratory objectives To use machine learning and predictive modelling to combine baseline clinical phenotypic markers such as disease duration and clinical expression of disease with additional immunophenotypic (intracellular CD4 Th17 frequency, CD8 Tc17 frequency, MAIT cell frequency, immune transcriptomic signature) factors to develop a predictive model for response to IL-17A and/or TNF inhibitor therapy in PsA. | Clinical response as measured by the MDA criteria. | Clinical disease pattern and Immunophenotype data at baseline and clinical response at week 24. |
| To test whether the clinical response to TNF and IL-17A inhibitor therapy in participants with PsA differs according to the level of baseline activated Th17 cells. | Clinical response as measured by the MDA criteria | Immunophenotype data at baseline and clinical response at week 12/16. |
| To explore if the change or absolute levels of activated Th17 surface and intracellular signature or the transcriptomics at week 4 can predict response to IL-17A and TNF blocking therapies | Clinical response as measured by the MDA criteria. | Immunophenotype data at baseline and 4 weeks and clinical response at week 12/16 and 24. |
SF-36 = short form 36
OPTIMISE, Optimising Psoriatic arthritis Therapy with Immunological Methods to Increase Standard Evaluation; PsA, psoriatic arthritis; TNF, target tumour necrosis factor; WPAI, work productivity and activity impairment.
Our exploratory objective is to use machine learning and predictive modelling to combine baseline clinical phenotypic markers such as disease duration and clinical expression of disease, with additional immunophenotypic (intracellular cytokine staining to determine IL-17A+CD4+ (Th17) or IL-17A+CD8+ (Tc17) frequencies, Mucosal-associated invariant T (MAIT) cell frequency, immune transcriptomic signature) factors to develop an optimal predictive model for individual responses to IL-17A and/or TNF inhibitor therapy in PsA.
Our exploratory mechanistic objectives are:
To understand if the activated Th17 surface and intracellular signature (and possibly also other IL-17 signatures) resolve after treatment with IL-17A inhibitors and how these are altered after TNF inhibitor therapy with additional focus on the polyfunctional cells producing multiple cytokines.
To understand whether changes in the activated Th17 surface and intracellular signature (and possibly other IL-17 signatures) differ in treatment responders and non-responders.
To explore if immune subset-specific transcriptomic signatures can be used to predict efficacy of IL-17A and TNF inhibitor therapies either alone or in combination with the activated surface and intracellular Th17 signatures.
To use the identified transcriptomic signature to identify a limited number of transcriptomic biomarkers that can be validated in whole blood.
To use the immune subset-specific transcriptomic signature to define the pathways driving biologic refractory disease.
To establish a biobank of samples at the end of this analysis to allow future investigation of novel scientific techniques and biomarkers within this population (with future separate funding).
Methods and analysis
Study design
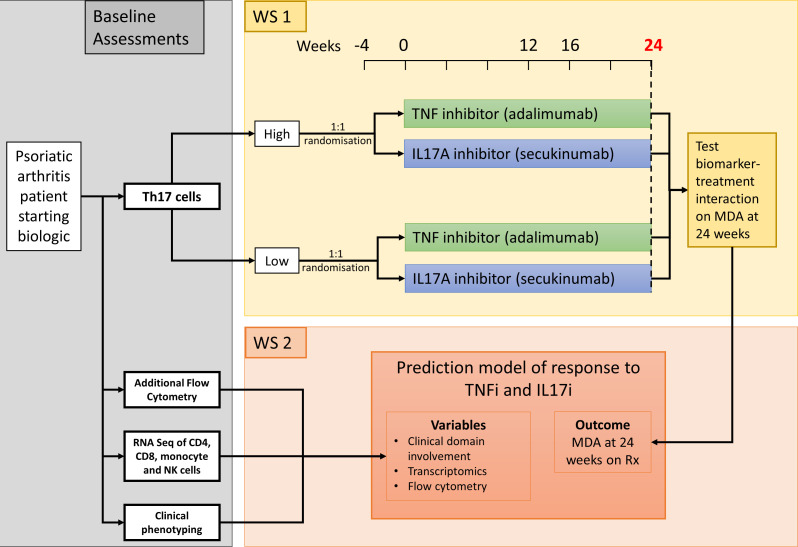
The OPTIMISE study is an open-label parallel-group biomarker-stratified multicentre randomised controlled trial of adults with PsA, where participants are randomised to either TNF or IL-17A inhibitors, testing whether this or other immunological markers can predict achievement of the MDA criteria after 24 weeks on therapy (figure 1). This paper describes V.7.0 (dated 24 May 2023) of the protocol. Changes in the protocol since V.1.0 include
Figure 1.
OPTIMISE study design. MDA, minimal disease activity; OPTIMISE, Optimising Psoriatic arthritis Therapy with Immunological Methods to Increase Standard Evaluation; TNF, target tumour necrosis factor.
Initial modification in response to research ethics committee review.
Addition of exclusion criteria for those unwilling to follow contraceptive advice.
Inclusion of eligibility for those who have failed one conventional DMARD but are eligible for treatment under local guidelines.
Changes to study recruitment dates and inclusion of patient identification centres.
Changes to sample size (as outlined below)
Selection of population
The population included are adults (≥18 years old) with PsA fulfilling the ClASsification of Psoriatic ARthritis (CASPAR) criteria who are due to start biological therapy for their PsA according to established UK eligibility criteria. This typically requires patients to have failed to respond to ≥2 conventional DMARDs and to have active disease demonstrated by ≥3 tender/swollen joints. Patients with previous exposure to biological therapies or those who have contraindications to either drug are excluded from participation. Full inclusion and exclusion criteria are shown in box 1.
Box 1. Inclusion and exclusion criteria for the Optimising Psoriatic arthritis Therapy with Immunological Methods to Increase Standard Evaluation trial.
Inclusion criteria
All participants should fulfil the following:
Participant is willing and able to give informed consent for participation in the study.
Man or woman, age 18 years or over.
Diagnosis of psoriatic arthritis (PsA) confirmed by the ClASsification of Psoriatic ARthritis (CASPAR) criteria.
Is eligible and planned to have biologic therapy for PsA using local guidelines or using National Institute of Health and Care Excellence (NICE)/Scottish Medicines Consortium (SMC) criteria (failure of ≥1 conventional DMARDs and ≥3 tender AND ≥3 swollen joints).
Exclusion criteria
The participant may not enter the study if ANY of the following apply:
-
Contraindications to either TNF inhibitor or secukinumab (determined by clinical team prior to recruitment):
History of previous demyelinating disease including multiple sclerosis
Heart failure (NYHA class 3 or 4)
Serious infections: active tuberculosis, chronic viral infections (including hepatitis B, C and HIV), recent serious bacterial infections.
Latent TB unless they have received appropriate antituberculous treatment as per local guidelines.
Active symptomatic inflammatory bowel disease.
History of cancer in the last 5 years, other than non-melanoma skin cell cancers cured by local resection or carcinoma in situ.
Hypersensitivity to active ingredient or excipients.
Current or previous treatment with biologic disease-modifying antirheumatic drugs (DMARDs) or targeted synthetic DMARDs.
Use of investigational therapies within 1 month month or 5 half-lives (whichever is longer) of baseline.
Women who are pregnant, lactating or planning pregnancy during the following 12 months or who are unwilling to follow standard of care contraceptive advice.
Received COVID-19 vaccination in the 2 weeks prior to screening visit.
Randomisation, blinding and allocation concealment
Prior to randomisation, we record the therapy that was planned by the physician if they had not been recruited to the trial.
Eligible and consented patients are randomised centrally by clinical trial unit staff using the bespoke computerised trial unit-specific randomisation system. Patients are randomised in a 1:1 allocation ratio to either TNF (adalimumab) or IL-17A (secukinumab) inhibitor. The randomisation uses a minimisation algorithm to ensure balanced allocation across the treatment groups, stratified by activated Th17 proportion (≤/> 1.58%), psoriasis severity (psoriasis area and severity index (PASI) < or ≥10) and study centre. The minimisation algorithm will include a probabilistic element and a small number of participants randomised by simple randomisation at the start of the trial to seed the algorithm to ensure the unpredictability of treatment allocation. There is no blinding of therapy allocation for patients or clinicians, so no allocation code or code-breaking procedure is required; however, the baseline immunophenotype data will be blinded from all participants and clinical study site personnel, while laboratory staff will be blinded to the allocated therapy. Unblinding should not be required during the study as it will not have clinical relevance to treatment decisions.
Interventions, patient follow-up, visits and trial procedures
Following consent, patients undergo a baseline clinical assessment and blood is taken for immunophenotyping. Fresh peripheral blood samples (50 mL) are couriered to one of the three laboratory hub sites (Oxford, Glasgow, London) for processing within 6 hours and are then cryopreserved for mechanistic cellular work (peripheral blood mononuclear cells) or whole-blood RNA sequencing). Our preliminary analysis shows that peripheral Th17 surface and intracellular signatures at 6 hours are comparable to freshly isolated samples. The measurement of the biomarker will be processed simultaneously with local processing of standard clinical safety screening for biological therapies (eg, hepatitis/TB screening), avoiding delay to patients’ treatment.
Analysis will be performed on cryopreserved samples rather than fresh samples to allow standardisation across centres, avoid delays to samples arriving late in the day and avoid issues with temporary unavailability of essential laboratory machinery such as flow cytometers. In the primary laboratory analysis, the samples will undergo 10 colour flow cytometry. In the first instance, activated Th17 frequencies will be identified based on CCR6+ and CXCR3− expression on CD3+CD4+CD8 T cells and coexpression of known T cell activation markers CD38 and HLA-DR, as described in the Miyagawa study.12 The proportion of activated Th17 cells will be included in the randomisation process to ensure equal stratification across the treatment arms.
The TNF inhibitor used in the study is adalimumab (any brand, including biosimilars) and is given at the usual licensed dose of 40 mg by subcutaneous injection every 2 weeks, with no loading doses. The IL-17A inhibitor used is secukinumab, brand name Cosentyx, and is given at the usual licensed dose, which varies based on the level of baseline skin psoriasis. The usual recommended dose as a first-line biologic in PsA is 150 mg by subcutaneous injection with initial dosing at weeks 0, 1, 2, 3 and 4 followed by a monthly maintenance dose. For patients with concomitant moderate to severe plaque psoriasis, the recommended dose is 300 mg by subcutaneous injection at the same timepoints. This study, therefore, follows routine practice and the current label by using the appropriate dose of secukinumab based on the baseline psoriasis disease activity, with the cut-off for moderate to severe psoriasis as ≥10% body surface area. Dose escalation from 150 mg to 300 mg in the case of a partial response to treatment as per the licence is permitted. Both drugs are provided from usual NHS stock and are self-administered by the patients following standard initial training, as per usual clinical practice.
The study involvement for each participant is 24 weeks plus the screening period (typically 4–8 weeks). Drug treatment is started at baseline and continued for the 24 weeks with study assessments at baseline, week 12 (for those on adalimumab) or week 16 (for those on secukinumab) and 24 weeks (for both) in keeping with current clinical practice and NICE guidance. Patients recruited at the hub sites are also asked to attend at week 4 for a research blood sample to be taken. After the 24-week study treatment period, participants who have responded well to treatment can continue on treatment following the end of the study period or switch to another treatment in line with usual NHS practice. Any patient discontinuing treatment for clinical reasons will be encouraged to attend for study visits and any treatment changes will be documented.
Sample size
This study has been powered to test for a biomarker–treatment interaction in response as defined by achievement of the MDA criteria at 24 weeks. Based on randomised controlled trial (RCT) and registry data for both drugs,13–15 we expect similar non-biomarker stratified MDA response rates in each treatment arm in the RCT and estimate the MDA response rate overall to be~50%.
Initially, the analysis planned to detect a biomarker-treatment relative interaction effect of 0.2, with >90% power and 5% type I error, using a difference in the MDA-response rate according to whether the proportion of activated Th17 cells is either high or low. This infers that we assume that the proportion of MDA responders (the trial primary outcome) is 60% and 40% for participants with low/high Th17 treated with TNF inhibitors, and 40% and 60% for participants with low/high Th17 are treated with IL-17A inhibitors This resulted in an original sample size of 424 participants.
However, this analysis would have converted the Th17 levels recorded in the trial into a dichotomous variable split around the median, creating two subgroups: ‘high Th17’ and ‘low Th17’. Applying such a dichotomy causes information loss and reduces available power. Therefore, during recruitment, an amendment was proposed and approved to use the proportion of activated Th17 cells in the analysis as a continuous outcome, while assuming the same relative interaction effect of 0.2, type I error rate of 0.05 and 90% power. This resulted in a reduction in the required sample size to 240 participants without a loss of power. This assumes a ‘main effect’ of treatment response (the difference in response between treatment arms distincts from the interaction effect) of 0.2, and no direct correlation between Th17 level and response after including the interaction effect.
Recruitment
Enrolment occurs within rheumatology outpatient clinics at participating UK hospital sites. Potential participants are approached by their clinical team after the decision has been made to start biologic therapy as part of standard clinical care and guidance. Written consent is obtained from potential trial participants by the principal investigator or a designated member of study staff using the approved consent form (see online supplemental file). With 17 sites, it is estimated that recruitment will complete in 36 months. The trial opened for recruitment in January 2022 and the estimated completion date is December 2024.
bmjopen-2023-078539supp001.pdf (62KB, pdf)
Outcomes
The primary outcome will be treatment response as measured by the proportion of patients achieving the MDA criteria16 at 24 weeks (see box 2).
Box 2. Minimal disease activity (MDA) criteria.
Patients are classified as being in MDA when they achieve any 5 or more of the following 7 criteria
Tender joint count ≤1.
Swollen joint count ≤1.
Psoriasis area and severity index ≤1.
Enthesitis score ≤1.
Patient global visual analogue scale of disease activity ≤20 mm.
Patient visual analogue scale of pain ≤15 mm.
Health assessment questionnaire score ≤0.5.
Individual secondary outcome measures covering all of the new 2016 Outcome Measures in Rheumatology Clinical Trials core and strongly recommended domains for PsA studies17 are collected at all timepoints, with the exception of radiographic damage, which is inappropriate in a short duration, active comparator study. The secondary outcome measures are listed in table 2. The electronic case report form system REDCap is being used to collect the data.
Table 2.
Secondary outcome measures for the OPTIMISE trial
| Musculoskeletal disease activity | Physician global visual analogue scale (VAS), 68 tender joint count (TJC) and 66 swollen joint count (SJC),19 Leeds20 and Spondyloarthritis Research Consortium of Canada (SPARCC)21 enthesitis indexes, dactylitis count22 |
| Psoriasis disease activity | PASI23 and nail disease VAS |
| Pain | Patient pain VAS19 |
| Global | Global disease activity VAS24 |
| Physical function | HAQ25 |
| Health-related quality of life (HRQoL), fatigue, emotional well-being | PsA impact of disease (PsAID)26 |
| Systemic inflammation | C reactive protein |
| Participation | Work productivity and activity impairment (WPAI),27 PSAID26 |
| Health economic evaluation | EuroQol (EQ‐5D-5L) and health resource utilisation |
| Health economic evaluation | EuroQol (EQ‐5D-5L) and health resource utilisation |
| Common adverse events | Common adverse events reported by patient related to the biologic DMARD. |
EuroQoL, European quality of life index; HAQ, Health Assessment Questionnaire; PASI, psoriasis area and severity score; PsAID, PsA impact of disease.
Statistical analysis plan
A detailed statistical analysis plan will be drafted early in the trial and will be finalised and preregistered prior to any primary outcome analysis. All analyses will be on an intention to treat basis, that is according to group a patient is randomised to, irrespective of compliance with treatment allocation. A per-protocol population will be defined, and the primary outcome is reanalysed on this population.
The primary outcome will be assessed via logistic regression adjusted for activated Th17 level as a continuous indicator, treatment and an interaction between the two; the stratification factors of study centre and psoriasis severity will also be included. A random effect will be included to account for any heterogeneity in the response due to recruitment centre, with the other variables being incorporated as fixed effects. The primary focus is on the interaction between biomarker and treatment; the p value for this interaction will be reported and considered significant if it falls below 0.05. Mean response rate by treatment and by the four groups defined by treatment and biomarker (high/low) will be reported along with 95% CIs.
It is assumed that there is no difference between randomised group difference in MDA response at 24 weeks. To test this, response rates for the randomised groups will be reported. An OR, and its 95% CI, will come from the same model as used in the primary analysis but without the treatment/biomarker interaction (Th17 biomarker will be included as continuous variable).
The secondary outcome of MDA at the 12 (adalimumab)/16 (secukinumab) week time point will be analysed using the same model as defined for the primary outcome but with MDA at the secondary time point as the response. All other secondary outcomes analysed as part of this trial are continuous and will be analysed using the same model but adjusting for the appropriate variables in each analysis. All continuous outcomes at 24 weeks will be analysed using a mixed effects linear regression model. The model will include study centre as a random effect, baseline PASI (continuous), Th17 proportions (continuous), baseline measures of the outcome being analysed and randomised treatment as fixed effects. A treatment by biomarker interaction will be included in the model to formally test the interaction between treatment and biomarker.
The appropriateness of the assumption of approximate normality of the residuals for the analysis models will be assessed graphically.
Missing data will be minimised by careful data management. Missing data will be described with reasons given where available. Missing data analysis will be performed on the primary analysis only. It is intended that analysis will be on complete cases, but the nature and pattern of missingness will be carefully considered and documented, in particular, as to whether the missing data can be treated as missing at random. If it is plausible that the data are missing not at random, a search for factors not included in the primary analysis model that explains missingness will be performed and if variables are found, multiple-imputation will be used, using the primary analysis model but including these variables. If no variables are identified, multiple imputation will not be performed.
Additional hypothesis generating analyses will be undertaken to investigate alternative potential models for predicting response to different classes of biologic. Analysis methods for exploratory, lab-based or machine learning outcomes will not be defined in this paper as these are not performed as part of the compilation of the final statistical report.
Monitoring
The study is managed by a trial management committee including the Chief Investigator (CI), laboratory lead and Oxford Clinical Trials Research Unit (OCTRU) staff. An independent trial steering committee and data safety and monitoring committee oversee the OPTIMISE study. They are independent of the study sponsor and full charters are available on request from OCTRU. OCTRU will audit the study once in its lifetime and also perform a detailed review prior to issuing green light in line with OCTRU standard operating procedures (SOPs). These audits are independent from the investigators but not independent from the Sponsor.
Patient and public involvement
The lack of data informing the choice of biologics is frustrating for clinicians and for patients who want to know in advance which therapy would be best for them. This was reflected in the recent PsA James Lind Priority Setting Partnership where the question ‘What is the best strategy for managing patients with psoriatic arthritis including non-drug and drug treatments?’ was ranked highest in the top 10 unmet needs.18 Patient research partners from the British PsA Consortium assisted with the design of the study, including research question, timing of follow-up visits (to minimise burden for participants) and selection of outcome measures. Four patient partners living with PsA sit on the trial steering committee overseeing the trial throughout and helping with dissemination of the future results.
Ethics and dissemination
The OPTIMISE trial is being conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Approval from the Health Research Authority and the North West—Preston Research Ethics Committee with reference 21/NW/00016. Collection of personal data is minimised within the study, with identifiable data being held securely in order to maintain confidentiality before, during and after the trial.
The deliverables from this project will include peer-reviewed publications describing the clinical and mechanistic results of the study and a predictive panel that could predict response to IL-17 and/or TNF inhibitor therapy in PsA. This panel will be used to develop a more feasible and scalable companion diagnostic for clinical practice. This could be tested in further large-scale studies in the next step towards routine implementation of precision medicine in PsA. Health economic data from our study will assist in the planning of future cost-effectiveness trials. A biobank repository of remaining biological samples will allow future mechanistic and precision medicine biomarker work with additional funding. Data may be shared with other research groups on reasonable request following the completion of the primary analysis.
Discussion
The OPTIMISE study is the first powered randomised, controlled trial investigating a precision medicine approach to biologic selection in PsA. Although treatment is open label, blinding to the immunophenotyping data will minimise any bias in the study. This work has the potential to increase the likelihood of a significant response and good outcome and reducing delay and risks of potentially ineffective therapies. The study has been designed with broad eligibility criteria to increase generalisability to clinical practice and reflect patients currently treated with these drugs in the UK. Detailed immunophenotyping will maximise the chance of identifying key predictive markers of response. Although immunophenotyping requires considerable cell processing and this would not be feasible in the same fashion within clinical laboratories, we believe that these markers can be further developed into practical tests that could be used in hospitals for routine clinical use. Further confirmatory studies outlined above would be required, there is great potential for this work to impact on UK NICE guidance for the use of biologics in PsA in the UK, particularly if this approach demonstrates health economic benefits. This would provide clear efficiency savings to both patients and the NHS as 3–6-month courses of ineffective therapies would be avoided.
Supplementary Material
Acknowledgments
The OPTIMISE study team acknowledge the expert statistical input of Associate Professor Susan Dutton in the development of the study proposal and the OCTRU programming team for support with the preparation and maintenance of the study database.
Footnotes
Twitter: @StefanSiebert1, @drlauracoates
Contributors: HA-M, PB, CG, BWK, IBM, DR, SS, LST and LC are responsible for the conception of the study. AO, HA-M, LB, MB, PB, AF, CG, BWK, SL, IBM, DR, SS, LST, ATV, NY and LC designed the protocol. AO and LCC wrote the original draft. All authors revised the draft.
Funding: The OPTIMISE study is funded by the National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation (EME) grant number NIHR129023. The research is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We acknowledge the support of the National Institute for Health Research Clinical Research Network (NIHR CRN).
Disclaimer: The study is sponsored by the Research Governance, Ethics and Assurance Team, University of Oxford (rgea.sponsor@admin.ox.ac.uk). The study is manged by the Oxford Clinical Trials Research Unit (OCTRU), at the University of Oxford.
Competing interests: The institutions of all co-authors received funding from the NIHR-MRC-EME programme (NIHR129023) for conducting this research. HA-M is a full-time employee of AstraZeneca and former employee of UCB. He own shares in UCB, AZ and GSK. He has previously received unrestricted research funding from UCB. He has received consulting and speaker fees from UCB, Novartis, Pfizer and Abbvie. PB has received research support from Regeneron, Novartis and GSK. CG has received grants/contracts from AstraZeneca, BMS, Eli Lilly, Galvani, GSK, Istesso, Janssen, MedAnnex, MiroBio, Revolo, UCB; Consulting fees from AstraZeneca, BMS, Galvani, MedAnnex, Medincell; Payment/honoraria from Abbvie, BMS, UCB. BWK has received research support from Eli Lilly and Novartis and consultation fees/speaker fees from Abbvie, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer and UCB. IBM has received research support, consultation fees and honoraria from Abbvie, Amgen, BMS, Causeway Therapeutics, Cabaletta, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sanofi, UCB, Evelo, Compugen, AstraZeneca, Moonlake. He has stock or stock options in Evolo, Cabaletta, Compugen, Causewauy Therapeutics and Dextera. He is a board members/trustee of NHS GGC, Evolo and Versus Arthritis. DR Consultant to OMASS therapeutics Ltd. Scientific advisory board of Avicenna Biosciences Inc. SS has received institutional grants/research support from AbbVie, Amgen, Eli Lilly, GSK, Janssen and UCB; and has received speaker/consultancy fees from AbbVie, Astra Zeneca, Eli Lilly, Janssen and UCB. LST has previously received research support from Sanofi, UCB, GSK and Novartis, outside of the work described here. LC has received grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB; worked as a paid consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, Janssen, Moonlake, Novartis, Pfizer and UCB; and has been paid as a speaker for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Medac, Novartis, Pfizer and UCB. The remainder of authors declare no conflicts of interest.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.National Institute for Health and Care Excellence . Psoriatic arthritis - etanercept, infliximab and adalimumab; 2010.
- 2.Gladman DD, Stafford-Brady F, Chang CH, et al. Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol 1990;17:809–12. [PubMed] [Google Scholar]
- 3.Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001;28:1842–6. [PubMed] [Google Scholar]
- 4.Gladman DD, Farewell VT, Wong K, et al. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis & Rheumatism 1998;41:1103–10. [DOI] [PubMed] [Google Scholar]
- 5.Huscher D, Merkesdal S, Thiele K, et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis 2006;65:1175–83. 10.1136/ard.2005.046367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence . Certolizumab pegol and secukinumab for treating active psoriatic arthritis after inadequate response to DMARDs; 2017. [DOI] [PMC free article] [PubMed]
- 7.Gorlier C, Orbai A-M, Puyraimond-Zemmour D, et al. Comparing patient-perceived and physician-perceived remission and low disease activity in psoriatic arthritis: an analysis of 410 patients from 14 countries. Ann Rheum Dis 2019;78:201–8. 10.1136/annrheumdis-2018-214140 [DOI] [PubMed] [Google Scholar]
- 8.Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral Spondyloarthritis, especially Psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. 10.1136/annrheumdis-2017-211734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus Adalimumab for treatment of active Psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet 2020;395:1496–505. 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 10.Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of Ixekizumab and Adalimumab in biological-naive patients with active Psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. 10.1136/annrheumdis-2019-215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagerli KM, Kearsley-Fleet L, Watson KD, et al. Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis data from the British society for rheumatology Biologics register. RMD Open 2018;4:e000596. 10.1136/rmdopen-2017-000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyagawa I, Nakayamada S, Nakano K, et al. Precision medicine using different biological DMARDs based on characteristic phenotypes of peripheral T helper cells in psoriatic arthritis. Rheumatology (Oxford) 2019;58:336–44. 10.1093/rheumatology/key069 [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ, Heckaman M, Kary S, et al. Application and modifications of minimal disease activity measures for patients with psoriatic arthritis treated with adalimumab: subanalyses of ADEPT. J Rheumatol 2013;40:647–52. 10.3899/jrheum.120970 [DOI] [PubMed] [Google Scholar]
- 14.Coates LC, Mease PJ, Gossec L, et al. Minimal disease activity among active psoriatic arthritis patients treated with Secukinumab: 2-year results from a multicenter randomized, double-blind, parallel-group, placebo-controlled phase-III study. Arthritis Care Res (Hoboken) 2018;70:1529–35. 10.1002/acr.23537 [DOI] [PubMed] [Google Scholar]
- 15.Perrotta FM, Marchesoni A, Lubrano E. Minimal disease activity and remission in psoriatic arthritis patients treated with anti-TNF-alpha drugs. J Rheumatol 2016;43:350–5. 10.3899/jrheum.150805 [DOI] [PubMed] [Google Scholar]
- 16.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. 10.1136/ard.2008.102053 [DOI] [PubMed] [Google Scholar]
- 17.Orbai A-M, de Wit M, Mease P, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis 2017;76:673–80. 10.1136/annrheumdis-2016-210242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hailey L, Kinsella S, Bundy C, et al. The top 10 research priorities in psoriatic arthritis: a James LIND alliance priority setting partnership. Rheumatology (Oxford) 2022;61. 10.1093/rheumatology/keac132.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 2007;34:1167–70. [PubMed] [Google Scholar]
- 20.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. 10.1002/art.23568 [DOI] [PubMed] [Google Scholar]
- 21.Maksymowych WP, Mallon C, Morrow S, et al. Development and validation of the spondyloarthritis research consortium of Canada (SPARCC) enthesitis index. Ann Rheum Dis 2009;68:948–53. 10.1136/ard.2007.084244 [DOI] [PubMed] [Google Scholar]
- 22.Healy PJ, Helliwell PS. Measuring dactylitis in clinical trials: which is the best instrument to use J Rheumatol 2007;34:1302–6. [PubMed] [Google Scholar]
- 23.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978;157:238–44. 10.1159/000250839 [DOI] [PubMed] [Google Scholar]
- 24.Cauli A. Patient and physician perception of disease in Psoriatic arthritis (PSA). A multicentre GRAPPA and OMERACT study [abstract]. Arthritis & Rheumatism 2007;56:610. [Google Scholar]
- 25.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 26.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the psoriatic arthritis impact of disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Bansback N, Boonen A, et al. Validity of the work productivity and activity impairment questionnaire--general health version in patients with rheumatoid arthritis. Arthritis Res Ther 2010;12:R177. 10.1186/ar3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078539supp001.pdf (62KB, pdf)