Abstract
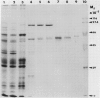
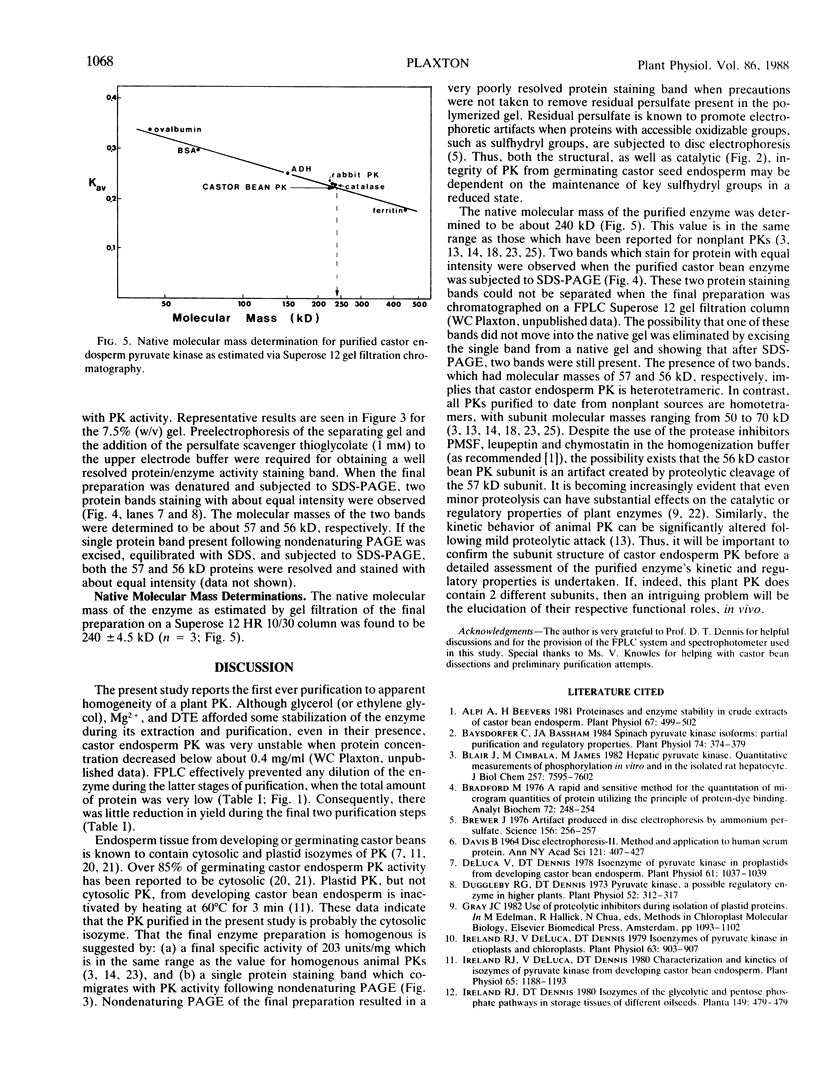
Cytosolic pyruvate kinase from endosperm of germinating castor beans (Ricinus communis L.; cv Hale) has been purified 3100-fold to apparent homogeneity and a final specific activity of 203 micromole pyruvate produced/minute per milligram protein. Purification steps included: heat treatment, polyethylene glycol fractionation, Q-Sepharose, ADP-agarose, Mono-Q and Phenyl Superose chromatography. Nondenaturing polyacrylamide gel electrophoresis of the final sample resulted in a single protein staining band which co-migrated with pyruvate kinase activity. Two protein staining bands of 57 and 56 kilodaltons were observed following SDS polyacrylamide gel electrophoresis of the final preparation. The native molecular mass was found to be about 240 kilodaltons. This enzyme appears to be a tetramer composed of two different subunits. The presence of dithioerythritol (2 millimolar) was required for optimal activity of the purified enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpi A., Beevers H. Proteinases and enzyme stability in crude extracts of castor bean endosperm. Plant Physiol. 1981 Mar;67(3):499–502. doi: 10.1104/pp.67.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. B., Cimbala M. A., James M. E. Hepatic pyruvate kinase. Quantitative measurements of phosphorylation in vitro and in the isolated rat hepatocyte. J Biol Chem. 1982 Jul 10;257(13):7595–7602. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brewer J. M. Artifact produced in disc electrophoresis by ammonium persulfate. Science. 1967 Apr 14;156(3772):256–257. doi: 10.1126/science.156.3772.256. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Luca V., Dennis D. T. Isoenzyme of pyruvate kinase in proplastids from developing castor bean endosperm. Plant Physiol. 1978 Jun;61(6):1037–1039. doi: 10.1104/pp.61.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Pyruvate kinase, a possible regulatory enzyme in higher plants. Plant Physiol. 1973 Oct;52(4):312–317. doi: 10.1104/pp.52.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. J., De Luca V., Dennis D. T. Characterization and kinetics of isoenzymes of pyruvate kinase from developing castor bean endosperm. Plant Physiol. 1980 Jun;65(6):1188–1193. doi: 10.1104/pp.65.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. J., Deluca V., Dennis D. T. Isoenzymes of pyruvate kinase in etioplasts and chloroplasts. Plant Physiol. 1979 May;63(5):903–907. doi: 10.1104/pp.63.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A., Marie J., Garreau H., Sprengers E. D. The genetic system of the L-type pyruvate kinase forms in man. Subunit structure, interrelation and kinetic characteristics of the pyruvate kinase enzymes from erythrocytes and liver. Biochim Biophys Acta. 1978 Mar 14;523(1):59–74. doi: 10.1016/0005-2744(78)90009-8. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Subcellular distribution of gluconeogenetic enzymes in germinating castor bean endosperm. Plant Physiol. 1979 Jul;64(1):31–37. doi: 10.1104/pp.64.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Preiss J. Purification and Properties of Nonproteolytic Degraded ADPglucose Pyrophosphorylase from Maize Endosperm. Plant Physiol. 1987 Jan;83(1):105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Storey K. B. Purification and properties of aerobic and anoxic forms of pyruvate kinase from red muscle tissue of the channelled whelk, Busycotypus canaliculatum. Eur J Biochem. 1984 Sep 3;143(2):257–265. doi: 10.1111/j.1432-1033.1984.tb08367.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson J. D., Turner J. F. Pyruvate kinase of higher plants. Biochim Biophys Acta. 1973 Nov 2;329(1):128–139. doi: 10.1016/0304-4165(73)90015-9. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Mort J. S., Sanwal B. D. The control of pyruvate kinase of Escherichia coli. Binding of substrate and allosteric effectors to the enzyme activated by fructose 1,6-bisphosphate. Biochemistry. 1976 Jan 27;15(2):277–282. doi: 10.1021/bi00647a006. [DOI] [PubMed] [Google Scholar]