This comparative effectiveness study investigates the comparative effectiveness of levetiracetam and lamotrigine as initial antiseizure medication in female patients of childbearing age with idiopathic generalized epilepsy.
Key Points
Question
What is the comparative effectiveness of levetiracetam and lamotrigine as initial antiseizure medication in female patients of childbearing age with idiopathic generalized epilepsy (IGE)?
Findings
In this comparative effectiveness study including 543 female patients with IGE, levetiracetam was more effective than lamotrigine in juvenile myoclonic epilepsy (JME), whereas no differences were found in absence epilepsy and epilepsy with generalized tonic-clonic seizures alone.
Meaning
Study results suggest using levetiracetam as initial alternative monotherapy in female patients with JME, whereas a similar effectiveness between levetiracetam and lamotrigine was observed in other IGE syndromes.
Abstract
Importance
After the recent limitations to prescribing valproate, many studies have highlighted the challenging management of female patients of reproductive age with idiopathic generalized epilepsy (IGE). However, no study, to the authors’ knowledge, has addressed the comparative effectiveness of alternative antiseizure medications (ASMs) in these patients.
Objective
To compare the effectiveness and safety of levetiracetam and lamotrigine as initial monotherapy in female patients of childbearing age with IGE.
Design, Setting, and Participants
This was a multicenter, retrospective, comparative effectiveness cohort study analyzing data from patients followed up from 1994 to 2022. Patients were recruited from 22 primary, secondary, and tertiary adult and child epilepsy centers from 4 countries. Eligible patients were female individuals of childbearing age, diagnosed with IGE according to International League Against Epilepsy (2022) criteria and who initiated levetiracetam or lamotrigine as initial monotherapy. Patients were excluded due to insufficient follow-up after ASM prescription.
Exposures
Levetiracetam or lamotrigine as initial monotherapy.
Main Outcomes and Measures
Inverse probability of treatment weighting (IPTW)–adjusted Cox proportional hazards regression was performed to compare treatment failure (TF) among patients who received levetiracetam or lamotrigine as initial monotherapy.
Results
A total of 543 patients were included in the study, with a median (IQR) age at ASM prescription of 17 (15-21) years and a median (IQR) follow-up of 60 (24-108) months. Of the study population, 312 patients (57.5%) were prescribed levetiracetam, and 231 (42.5%) were prescribed lamotrigine. An IPTW-adjusted Cox model showed that levetiracetam was associated with a reduced risk of treatment failure after adjustment for all baseline variables (IPTW-adjusted hazard ratio [HR], 0.77; 95% CI, 0.59-0.99; P = .04). However, after stratification according to different IGE syndromes, the higher effectiveness of levetiracetam was confirmed only in patients with juvenile myoclonic epilepsy (JME; IPTW-adjusted HR, 0.47; 95% CI, 0.32-0.68; P < .001), whereas no significant differences were found in other syndromes. Patients treated with levetiracetam experienced adverse effects more frequently compared with those treated with lamotrigine (88 of 312 [28.2%] vs 42 of 231 [18.1%]), whereas the 2 ASMs had similar retention rates during follow-up (IPTW-adjusted HR, 0.91; 95% CI, 0.65-1.23; P = .60).
Conclusions and Relevance
Results of this comparative effectiveness research study suggest the use of levetiracetam as initial alternative monotherapy in female patients with JME. Further studies are needed to identify the most effective ASM alternative in other IGE syndromes.
Introduction
Idiopathic generalized epilepsy (IGE) represents a common form of epilepsy, accounting for almost one-fifth of patients attending children and adult epilepsy units.1 Four distinct syndromes have been recognized within the IGE group, namely childhood absence epilepsy (CAE), juvenile absence epilepsy (JAE), juvenile myoclonic epilepsy (JME), and idiopathic generalized epilepsy with generalized tonic-clonic seizures alone (GTCA).2 These 4 syndromes share a strong genetic background, overlapping electroencephalography features, and a favorable response to appropriately selected antiseizure medications (ASMs).3,4
Valproate (VPA) has traditionally been considered the most effective ASM in IGE, based on its effectiveness in all generalized seizure types.5 However, regulatory agencies advised against its use in female patients of childbearing potential due to the increased risk of major congenital malformations and neurodevelopmental disorders in offspring exposed to VPA in utero.6,7,8 Nevertheless, multiple reports showed the possible increased chance of uncontrolled seizures in female patients with IGE who are not prescribed VPA and highlighted the challenging treatment of these patients.9,10,11,12 Despite the relevance of this topic and the female preponderance observed in IGE,13 no study, to our knowledge, has specifically investigated the most effective ASM alternatives to VPA in a target group of female individuals of childbearing age, and available evidence is mainly based on monocentric studies and case series.14,15,16
Among available ASM alternatives, levetiracetam and lamotrigine have been consistently found as the least teratogenic drugs among those effective for generalized seizure types.7,17 Thus, the 2022 National Institute for Health and Care Excellence (NICE) guidelines recommend levetiracetam or lamotrigine as first-line monotherapies in female patients of childbearing potential with new-onset generalized tonic-clonic seizures (GTCSs); ethosuximide, levetiracetam, or lamotrigine in those with new-onset absence seizures; and levetiracetam in patients with new-onset myoclonic seizures, keeping in mind the reported possible worsening of this seizure type with lamotrigine.18
In this multicenter, retrospective study, we aimed to compare the effectiveness and the safety of levetiracetam and lamotrigine as first-line ASMs in a cohort of female patients of childbearing age with IGE, defined according to the International League Against Epilepsy (ILAE) diagnostic criteria.
Methods
Study Participants
This multicenter, retrospective, comparative effectiveness research study was conducted according to the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guidelines.19,20 A local ethics committee approved the study, and informed consent was signed by all participants.
The study cohort was identified from a population of patients attending 22 primary, secondary, and tertiary adult and child epilepsy centers setting from January 1, 1994, to January 31, 2022. Inclusion criteria were as follows: (1) female sex, (2) diagnosis of IGE according to ILAE criteria,2 (3) prescription of either levetiracetam or lamotrigine as first-line ASM, (4) childbearing age (age between 10 and 50 years) at the moment of levetiracetam or lamotrigine prescription, and (5) follow-up duration of a minimum of 12 months after ASM prescription unless treatment failure occurred earlier.
The diagnosis of a specific IGE syndrome, namely CAE, JAE, JME, and GTCA, was established following the current ILAE criteria.2 According to these criteria, the occurrence of myoclonic seizures was considered mandatory for the diagnosis of JME.
Baseline clinical characteristics including demographic data, age at first seizure, family history of epilepsy in first- and second-degree relatives, history of febrile seizures, psychiatric comorbid conditions, mild intellectual disability, type of seizures experienced before the start of ASMs (namely absences, myoclonic seizures, and GTCSs), catamenial worsening of seizures, number of GTCSs before start ASMs (dichotomized as ≥3 or <3), presence of electroencephalography photosensitivity at baseline, and epilepsy syndromes were recorded in each patient. The type and dosage of first-prescribed ASM and the age at first ASM prescription were also noted. No data pertaining to race and ethnicity were collected as part of this study. It is important to note that the majority of the patient cohort consisted of individuals identifying as White. However, for the precise objectives of the study, this demographic information was deemed to be nonessential and, therefore, not incorporated into the analysis.
Outcome Measures
The primary outcome of this study was the time from ASM prescription to treatment failure (TF), defined as either ASM discontinuation in favor of another ASM due to ineffectiveness or adverse effects or the adding of a second ASM due to ineffectiveness.
As secondary outcomes, we considered the following: (1) time to TF due to ineffectiveness only, (2) time to ASM withdrawal considering either adverse effects or ineffectiveness (hereinafter referred as ASM retention), and (3) time to ASM withdrawal due to adverse effects only. Seizure freedom rate at 12 months of follow-up was also evaluated, considering as seizure-free only those patients who achieved remission on their first monotherapy (ie, without a previous treatment failure). Tolerability and safety assessment included the occurrence of adverse effects that were considered related to the treatment with levetiracetam and lamotrigine by the treating physicians.
Statistical Analysis
Baseline clinical characteristics were computed using nonimputed data. Missing data were handled with multiple imputations with chained equations, imputing both outcome and predictors.21,22 The rate of missing data for all variables is shown in the eFigure in Supplement 1.
We conducted a propensity score analysis with inverse probability of treatment weighting (IPTW) to account for the conditional probability of treatment selection. The IPTW was calculated by taking the reciprocal of the probability of receiving either levetiracetam or lamotrigine, which was estimated through logistic regression using the following covariates: age at ASM prescription, epilepsy syndrome (ie, absence epilepsy, JME, GTCA), family history of epilepsy, history of febrile seizures, mild intellectual disability, psychiatric comorbidities, previous status epilepticus, catamenial worsening of seizures, and photosensitivity. These covariates were selected based on their association with the primary outcome (ie, TF) and exposure, as recommended.23 Age was flexibly modeled using a restricted cubic spline with knots at percentiles (ie, 1st, 25th, 50th, 75th, and 99th percentile) of the age distribution. Standardized mean differences were used to evaluate the balance of baseline characteristics between the 2 treatment groups.
We then used an IPTW-adjusted Cox proportional hazards model to assess the differences in time to TF between the 2 treatment groups. The time of entry was the date of ASM prescription, and the time of end point was the date of TF or the last follow-up visit, truncated at 10 years of follow-up.
We repeated the same IPTW-adjusted Cox model with secondary outcome measures, using time to TF due to ineffectiveness only in the first model, ASM retention in the second model, and ASM withdrawal due to adverse effects only in the third model. We then conducted exploratory analyses of IGE subsyndromes, exploring treatment effects through IPTW-adjusted Cox regression analysis using TF due to ineffectiveness or adverse effects and ASM retention as the dependent variables and the same covariates except for epilepsy syndrome for IPTW balancing. Unadjusted and adjusted hazard ratios (HRs) with 95% CIs were presented for primary and secondary outcomes.
As sensitivity analyses, we conducted the following: (1) an IPTW-adjusted binary logistic regression analysis using seizure freedom at 12 months as the dependent variable and (2) a multivariable Cox regression model analysis using all the covariates included for IPTW balancing.
Finally, the proportion of patients experiencing adverse effects in the 2 treatment groups was compared through Fisher exact test. Statistical analysis was performed using R, version 3.5.1 (R Project for Statistical Computing).
Results
Baseline Clinical Characteristics
Between January 1, 1994, to January 31, 2022, 566 female patients of childbearing age diagnosed with IGE were treated with levetiracetam or lamotrigine as initial monotherapy in 22 epilepsy centers and met the inclusion criteria. Among these patients, 23 (4.1%) were excluded because of insufficient follow-up after ASM prescription. Therefore, the final study population included 543 patients with a median (IQR) age at ASM prescription of 17 (15-21) years and a median (IQR) follow-up duration of 60 (24-108) months. Among them, 312 patients (57.5%) were prescribed levetiracetam, and 231 (42.5%) were prescribed lamotrigine. A total of 109 patients (20.1%) were diagnosed with absence epilepsy (2 with CAE; 107 with JAE), 259 (47.7%) with JME, and 175 (32.2%) had a diagnosis of GTCA. A family history of epilepsy was found in 179 patients (33.1%), whereas mild intellectual disability was observed in 20 individuals (3.7%). Comparison of baseline characteristics in both treatment groups before and after IPTW balancing are described in Table 1.
Table 1. Patient Clinical Characteristics According to Treatment.
| Variable | LEV (n = 312) |
LTG (n = 231) |
P value | IPTW-adjusted, P value |
|---|---|---|---|---|
| Age at ASM prescription, median (IQR), y | 17 (14-20) | 17 (15-22) | .10 | .96 |
| History of febrile seizures, No. (%) | 30 (9.6) | 17 (7.4) | .40 | .97 |
| Family history of epilepsy in a 1st- or 2nd-degree relative, No. (%) | 99 (31.7) | 80 (35.1) | .40 | .99 |
| Psychiatric comorbidities, No. (%) | 37 (11.9) | 39 (16.9) | .10 | .99 |
| Mild intellectual disability, No. (%) | 10 (3.2) | 10 (4.3) | .50 | .95 |
| Epilepsy syndrome | ||||
| Absence epilepsy, No. (%) | 48 (15.4) | 61 (26.4) | <.001 | .99 |
| Juvenile myoclonic epilepsy, No. (%) | 162 (51.9) | 97 (42) | ||
| Epilepsy with GTCS alone, No. (%) | 102 (32.7) | 73 (31.6) | ||
| Previous status epilepticus, No. (%) | 15 (5.1) | 4 (1.8) | .06 | .96 |
| Catamenial worsening of seizures, No. (%) | 49 (15.9) | 36 (15.8) | >.99 | .99 |
| History of photosensitivity, No. (%) | 99 (32.1) | 63 (27.8) | .30 | .99 |
Abbreviations: ASM, antiseizure medication; GTCS, generalized tonic-clonic seizures; IPTW, inverse probability of treatment weighting; LEV, levetiracetam; LTG, lamotrigine.
Treatment Data and Seizure Outcome
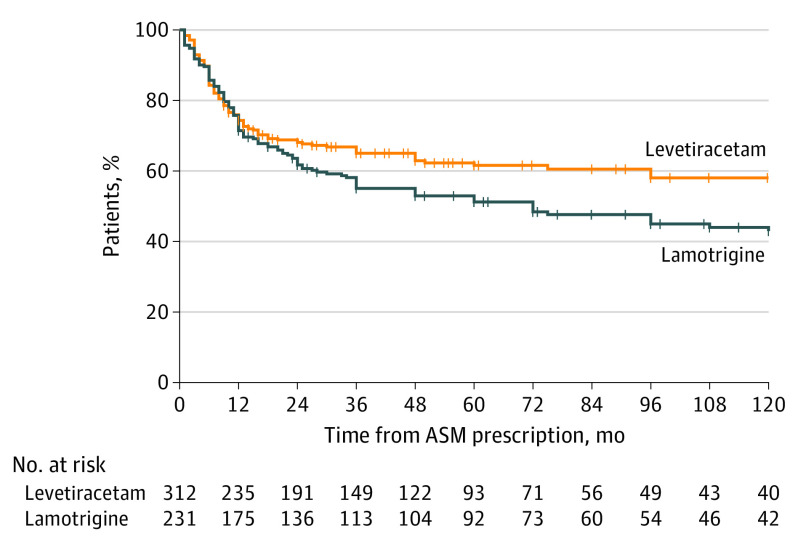
The mean initial maintenance dose was 219 mg (95% CI, 199-239 mg) for lamotrigine and 1197 mg (95% CI, 1149-1246 mg) for levetiracetam. During follow-up, there were 238 TF events in 114 participants in the levetiracetam group and in 117 participants in the lamotrigine group (unadjusted HR, 0.74; 95% CI, 0.57-0.96; P = .02) (Figure 1). Multivariable Cox model confirmed that levetiracetam was associated with a reduced risk of TF after adjustment for all baseline variables compared with lamotrigine (IPTW-adjusted HR, 0.77; 95% CI, 0.59-0.99; P = .04).
Figure 1. Treatment Failure for Any Reason.
Survival curves for treatment failure due to ineffectiveness and adverse effects. ASM indicates antiseizure medication.
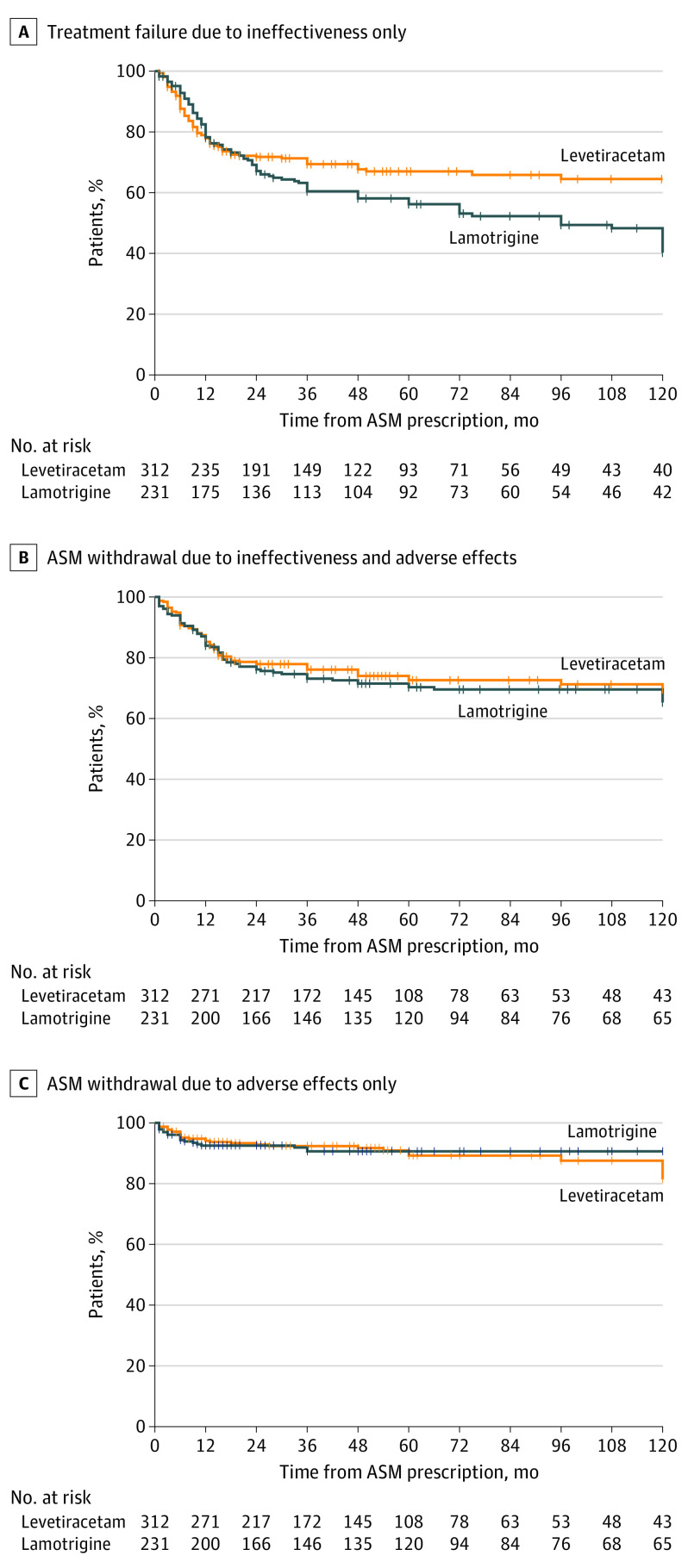
When considering secondary outcomes, TF due to ineffectiveness only occurred in 95 patients in the levetiracetam group and in 104 patients in the lamotrigine group (unadjusted HR, 0.71; 95% CI, 0.53-0.93; P = .01) (Figure 2A); the significant effect of levetiracetam (IPTW-adjusted HR, 0.73; 95% CI, 0.56-0.97; P = .03) was confirmed after adjusting for all baseline variables. No significant differences were found in terms of either ASM retention (IPTW-adjusted HR, 0.91; 95% CI, 0.65-1.23; P = .60) (Figure 2B) or ASM withdrawal due to adverse effects only (IPTW-adjusted HR, 1.15; 95% CI, 0.64-2.09; P = .60) (Figure 2C).
Figure 2. Treatment Failure for Ineffectiveness Only and Antiseizure Medication (ASM) Retention.
A, Survival curves for treatment failure due to ineffectiveness only. B, Survival curves for ASM withdrawal due to both ineffectiveness and adverse effects. C, Survival curves for ASM withdrawal due to adverse effects only.
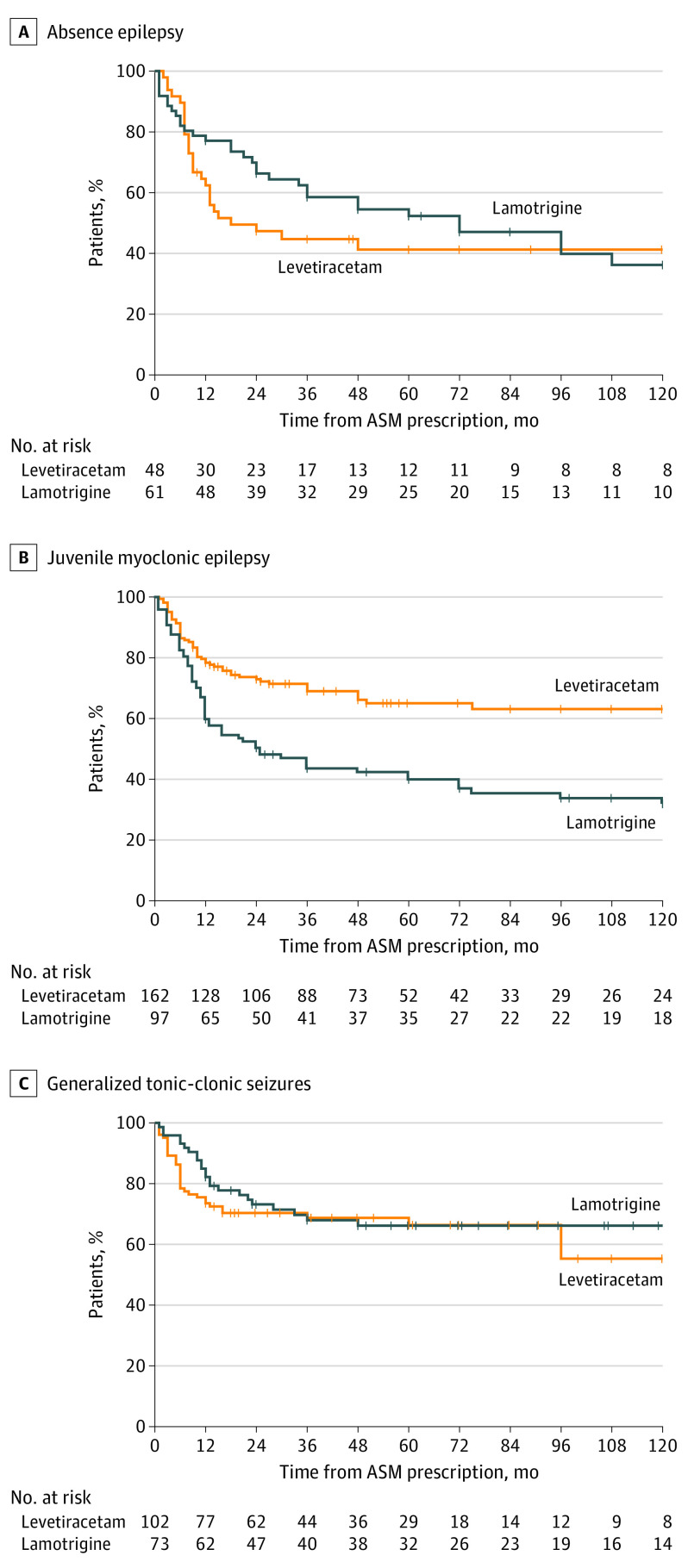
After stratification according to different IGE subsyndromes, the higher effectiveness of levetiracetam was confirmed only in patients with JME (IPTW-adjusted HR, 0.47; 95% CI, 0.32-0.68; P < .001), whereas no significant differences were found in absence epilepsy (IPTW-adjusted HR, 1.17; 95% CI, 0.69-1.99; P = .60) and GTCA (IPTW-adjusted HR, 1.02; 95% CI, 0.58-1.77; P = .90) (Figure 3). Additionally, levetiracetam was associated with a higher ASM retention compared with lamotrigine among patients with JME (IPTW-adjusted HR, 0.57; 95% CI, 0.37-0.89; P = .01), whereas no significant differences were found in absence epilepsy (IPTW-adjusted HR, 1.76; 95% CI, 0.88-3.52; P = .10) and GTCA (IPTW-adjusted HR, 1.22; 95% CI, 0.54-2.76; P = .60).
Figure 3. Treatment Failure Across Different Idiopathic Generalized Epilepsy Syndromes.
Shown are the survival curves for absence epilepsy (A), juvenile myoclonic epilepsy (B), and epilepsy with generalized tonic-clonic seizures alone (C).
Seizure freedom at 12 months occurred in 208 of 519 patients (40.1%) in whom information on seizure outcome was available at this time point. In the sensitivity analysis, levetiracetam use was associated with a higher likelihood of seizure freedom at 12 months both in univariable analysis (unadjusted odd ratio [OR], 2.06; 95% CI, 1.38-3.08; P < .001) and after adjusting for baseline confounders (IPTW-adjusted OR, 1.68; 95% CI, 1.15-2.44; P = .006). In the additional sensitivity analysis, multivariable Cox proportional hazards model confirmed a significant reduced risk of TF among patients associated with use of levetiracetam compared with lamotrigine (adjusted HR, 0.75; 95% CI, 0.58-0.98; P = .04). In addition, a worsening of myoclonic seizures was reported by treating clinicians more commonly among patients treated with lamotrigine compared with those treated with levetiracetam (4 of 97 [3.3%]; 95% CI, 0%-2.2% vs 1 of 162 [0.6%]; 95% CI, 1.3%-6.8%; P = .03).
Among patients experiencing TF, an add-on ASM regimen was used in 112 patients (48.5%; 95% CI, 41.9-55.1), whereas substitution monotherapy was used in 119 patients (51.5%; 95% CI, 44.9-58.1). A total of 47 patients (41.2%; 95% CI, 32.1-50.8) in the levetiracetam subgroup used an add-on regimen compared with 65 patients (55.6%; 95% CI, 46.1-64.7) in the lamotrigine subgroup (P = .02).
Adverse Effects
Information about adverse effects after ASM treatment were available in 466 of 543 patients (85.8%). Patients treated with levetiracetam experienced adverse effects more frequently compared with those treated with lamotrigine (88 of 312 [28.2%]; 95% CI, 22.8-34.2 vs 42 of 231 [18.1%]; 95% CI, 13.1-23.9; P = .01), whereas the 2 ASMs had similar retention rates during follow-up (IPTW-adjusted HR, 0.91; 95% CI, 0.65-1.23; P = .60). In this study, approximately 6.4% of patients (13 of 204) taking lamotrigine experienced skin rashes and other dermatologic adverse effects. A detailed comparison of adverse effects subtypes based on treatment group is summarized in Table 2.
Table 2. Adverse Effects Comparison According to Treatment.
| Variable | LEV (n = 262) |
LTG (n = 204) |
P value |
|---|---|---|---|
| Any adverse effect, No. (%) | 74 (28.2) | 37 (18.1) | .01a |
| Adverse effect subtype | |||
| Behavioral, No. (%) | 42 (16) | 2 (1) | <.001a |
| Drowsiness | 32 (12.2) | 6 (2.9) | <.001a |
| Gastrointestinal | 5 (1.9) | 0 | .07 |
| Dizziness/tremor | 8 (3.1) | 8 (3.9) | .60 |
| Hematological | 2 (0.8) | 2 (1) | >.99 |
| Dermatological | 1 (0.4) | 13 (6.4) | <.001a |
Abbreviations: LEV, levetiracetam; LTG, lamotrigine.
Discussion
In this comparative effectiveness research study, we aimed to address a crucial knowledge gap by exploring the efficacy and safety of levetiracetam and lamotrigine as a first-line ASM in female patients with IGE, a group who has faced limited availability of suitable treatments due to the teratogenic concerns associated with VPA. Our results suggest the use of levetiracetam as initial alternative monotherapy in female patients with JME, as shown by the analysis of TF and ASM retention, whereas no significant differences were found in other IGE syndromes.
Previous studies have highlighted the challenge of treating female patients with IGE, due to the growing restrictions on the use of VPA in patients during childbearing age.24,25,26 In the randomized clinical studies comparing Standard and New Antiepileptic Drugs (SANAD), VPA has been repeatedly found as the most effective ASM in the treatment of generalized epilepsy, when compared with levetiracetam, lamotrigine, and topiramate.27,28 However, due to the lack of studies focusing on the identification of the most effective VPA alternatives, great uncertainties still exist regarding the best ASM to prescribe as initial monotherapy in these patients. A previous open-label, randomized clinical trial compared the efficacy of levetiracetam and lamotrigine as initial monotherapy in patients with both focal and generalized epilepsy.29 The authors did not find any difference between the 2 treatment groups, although the low number of patients with generalized epilepsy who were recruited (143 of 409) and the short observation period (26 weeks) may have underpowered the comparison in this study. In addition, the few randomized clinical studies exploring the efficacy of ASMs in patients with generalized seizures have used heterogenous criteria to define generalized epilepsies,30 with almost one-half of patients in the SANAD II study classified as having undetermined generalized epilepsies.28 In the era of personalized epilepsy treatment, it is unclear whether the results of these studies could be generalized to well-defined IGE syndromes.31,32
Our study included a large number of patients with IGE defined according to the recently published ILAE criteria,2 indicating higher effectiveness of levetiracetam compared with lamotrigine during the long-term observation period. TF, both due to ineffectiveness and adverse effects, was found to be significantly lower in the levetiracetam group when adjusted for all confounding factors, and this finding was confirmed by the sensitivity analysis of seizure freedom at 12 months of follow-up. However, this result appeared to be mainly driven by the remarkable superiority of levetiracetam observed among female patients with JME. Indeed, when stratifying for different IGE syndromes, we found a superiority of levetiracetam both in terms of effectiveness and retention only in patients with JME, whereas no differences were observed in other IGE subsyndromes. In light of these results, clinicians should particularly take into account patients’ comorbidities in the choice of the first-line ASM in IGE syndromes other than JME.
In our study, only a minority of patients was diagnosed with CAE due to the study design including patients starting the first ASM during the childbearing years, and almost all patients with absence epilepsy had a diagnosis of JAE. Ethosuximide has been found to show a comparable efficacy with VPA in patients with absence seizures only and should be still considered as a feasible first-line monotherapy in patients with CAE.33 Conversely, lamotrigine and levetiracetam have been suggested as possible initial monotherapies by the NICE guidelines and by the ILAE in patients with JAE, considering their high probability of experiencing GTCS along with absences.2,18 In addition, it is worth underscoring that levetiracetam and lamotrigine have been repeatedly found as the safest ASMs to be prescribed during pregnancy,7,14 whereas teratogenic issues have been raised with ethosuximide, and its effects on offspring have not been sufficiently investigated.34,35 For this reason, we avoided including ethosuximide in our study, and we purposefully focused on the comparative effectiveness of levetiracetam and lamotrigine. Our findings suggest a noninferiority of levetiracetam compared with lamotrigine in absence epilepsies, especially in JAE; in this regard, however, the subgroup of absence epilepsies was possibly underpowered to detect meaningful differences, and a slight trend toward a higher effectiveness and retention of lamotrigine was observed.
When considering the safety analysis, both ASMs were found to be well tolerated, with similar retention rates during the long-term follow-up in the entire cohort. A higher number of adverse effects was observed in the levetiracetam group, especially in terms of behavioral adverse effects and drowsiness, confirming previous literature findings.36,37 Accordingly, our data suggest that the use of lamotrigine may be preferable in absence epilepsy and GTCA in the presence of psychiatric comorbidities, based on the comparable effectiveness of lamotrigine and levetiracetam in these specific syndromes and their tolerability profile. In this study, approximately 6.4% of patients taking lamotrigine experienced skin rashes and other dermatologic adverse effects, which are known to be particularly challenging due to their potential evolution toward Stevens-Johnson syndrome.38 In addition, a small but relatively higher number of patients taking lamotrigine showed worsening of myoclonic seizures during follow-up (3.3% of patients), confirming the potentially detrimental effect of sodium-channel blockers on this seizure type and further supporting the use of levetiracetam as initial alternative monotherapy in patients with JME.39,40,41
Strengths and Limitations
The main strength of our study was the selective inclusion of female patients of childbearing potential with IGE, who have been facing limited access to effective treatment options. The multicenter design, including primary, secondary, and tertiary care settings, and the definition of IGE syndromes according to the recently published ILAE criteria represent other strengths of the study.
There are also several limitations to the present study. First, the retrospective design implies recall bias, selection bias, and analysis bias, including a possible tendency of clinicians to prescribe levetiracetam in patients with a higher severity of myoclonus based on previous literature findings.39,40,41 Second, the heterogenous distribution of different IGE subsyndromes in our cohort may have underpowered the analysis stratified per epilepsy syndrome. Finally, relying on the records of clinical visits instead of standardized questionnaires for reporting adverse effects may have underestimated their true prevalence.
Conclusions
In conclusion, by specifically focusing on female patients of childbearing age with IGE, this comparative effectiveness research study provided useful information for the challenging treatment of this special population. Our data suggest the use of levetiracetam as initial alternative monotherapy in patients with JME, whereas further studies are warranted to investigate the comparative effectiveness and safety of VPA alternatives in IGE syndromes other than JME in female patients of childbearing age.
eFigure. Missingness of Variables and Combinations of Missingness Using the Intersecting Sets Visualization Method
Nonauthor Collaborators. Women With Epilepsy Treatment Options and Research (WETOR) Study Group
Data Sharing Statement.
References
- 1.Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46(suppl 9):10-14. doi: 10.1111/j.1528-1167.2005.00309.x [DOI] [PubMed] [Google Scholar]
- 2.Hirsch E, French J, Scheffer IE, et al. ILAE definition of the idiopathic generalized epilepsy syndromes: position statement by the ILAE Task Force on nosology and definitions. Epilepsia. 2022;63(6):1475-1499. doi: 10.1111/epi.17236 [DOI] [PubMed] [Google Scholar]
- 3.Vorderwülbecke BJ, Wandschneider B, Weber Y, Holtkamp M. Genetic generalized epilepsies in adults—challenging assumptions and dogmas. Nat Rev Neurol. 2022;18(2):71-83. doi: 10.1038/s41582-021-00583-9 [DOI] [PubMed] [Google Scholar]
- 4.Peljto AL, Barker-Cummings C, Vasoli VM, et al. Familial risk of epilepsy: a population-based study. Brain. 2014;137(Pt 3):795-805. doi: 10.1093/brain/awt368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolson A, Appleton RE, Chadwick DW, Smith DF. The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry. 2004;75(1):75-79. [PMC free article] [PubMed] [Google Scholar]
- 6.Bjørk MH, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672-681. doi: 10.1001/jamaneurol.2022.1269 [DOI] [PubMed] [Google Scholar]
- 7.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530-538. doi: 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 8.Daugaard CA, Pedersen L, Sun Y, Dreier JW, Christensen J. Association of prenatal exposure to valproate and other antiepileptic drugs with intellectual disability and delayed childhood milestones. JAMA Netw Open. 2020;3(11):e2025570. doi: 10.1001/jamanetworkopen.2020.25570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerulli Irelli E, Morano A, Cocchi E, et al. Doing without valproate in women of childbearing potential with idiopathic generalized epilepsy: implications on seizure outcome. Epilepsia. 2020;61(1):107-114. doi: 10.1111/epi.16407 [DOI] [PubMed] [Google Scholar]
- 10.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Withdrawal of valproic acid treatment during pregnancy and seizure outcome: observations from EURAP. Epilepsia. 2016;57(8):e173-e177. doi: 10.1111/epi.13437 [DOI] [PubMed] [Google Scholar]
- 11.Cerulli Irelli E, Cocchi E, Morano A, et al. Valproate impact and sex-dependent seizure remission in patients with idiopathic generalized epilepsy. J Neurol Sci. 2020;415:116940. doi: 10.1016/j.jns.2020.116940 [DOI] [PubMed] [Google Scholar]
- 12.Vajda FJE, O’Brien TJ, Graham JE, Hitchcock AA, Lander CM, Eadie MJ. Pregnancy after valproate withdrawal-Fetal malformations and seizure control. Epilepsia. 2020;61(5):944-950. doi: 10.1111/epi.16505 [DOI] [PubMed] [Google Scholar]
- 13.Gesche J, Christensen J, Hjalgrim H, Rubboli G, Beier CP. Epidemiology and outcome of idiopathic generalized epilepsy in adults. Eur J Neurol. 2020;27(4):676-684. doi: 10.1111/ene.14142 [DOI] [PubMed] [Google Scholar]
- 14.Kiiski R, Basnyat P, Raitanen J, Rainesalo S, Peltola J, Mäkinen J. Treatment outcomes in women with idiopathic generalized epilepsy. Acta Neurol Scand. 2022;145(4):423-433. doi: 10.1111/ane.13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesche J, Hjalgrim H, Rubboli G, Beier CP. Patterns and prognostic markers for treatment response in generalized epilepsies. Neurology. 2020;95(18):e2519-e2528. doi: 10.1212/WNL.0000000000010644 [DOI] [PubMed] [Google Scholar]
- 16.Mostacci B, Ranzato F, Giuliano L, et al. Alternatives to valproate in girls and women of childbearing potential with idiopathic generalized epilepsies: state of the art and guidance for the clinician proposed by the Epilepsy and Gender Commission of the Italian League Against Epilepsy (LICE). Seizure. 2021;85:26-38. doi: 10.1016/j.seizure.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Díaz S, Smith CR, Shen A, et al. ; North American AED Pregnancy Registry; North American AED Pregnancy Registry . Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692-1699. doi: 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 18.NICE: National Institute for Health and Care Excellence . 2022; Epilepsies in children, young people, and adults. Accessed March 22, 2023. https://www.nice.org.uk/guidance/ng217/resources/epilepsies-in-children-young-people-and-adults-pdf-66143780239813 [PubMed]
- 19.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report–Part I. Value Health. 2009;12(8):1044-1052. doi: 10.1111/j.1524-4733.2009.00600.x [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092-1101. doi: 10.1016/j.jclinepi.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 22.Stevelink R, Al-Toma D, Jansen FE, et al. Individualised prediction of drug resistance and seizure recurrence after medication withdrawal in people with juvenile myoclonic epilepsy: a systematic review and individual participant data meta-analysis. EClinicalMedicine. 2022;53:101732. doi: 10.1016/j.eclinm.2022.101732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021;15(1):14-20. doi: 10.1093/ckj/sfab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliano L, Mainieri G, Aguglia U, et al. Long-term prognosis of juvenile myoclonic epilepsy: a systematic review searching for sex differences. Seizure. 2021;86:41-48. doi: 10.1016/j.seizure.2021.01.005 [DOI] [PubMed] [Google Scholar]
- 25.Harden CL, Meador KJ, Pennell PB, et al. ; American Academy of Neurology; American Epilepsy Society . Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): II. teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1237-1246. doi: 10.1111/j.1528-1167.2009.02129.x [DOI] [PubMed] [Google Scholar]
- 26.Tomson T, Marson A, Boon P, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015;56(7):1006-1019. doi: 10.1111/epi.13021 [DOI] [PubMed] [Google Scholar]
- 27.Marson AG, Al-Kharusi AM, Alwaidh M, et al. ; SANAD Study group . The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1016-1026. doi: 10.1016/S0140-6736(07)60461-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marson A, Burnside G, Appleton R, et al. ; SANAD II collaborators . The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, noninferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397(10282):1375-1386. doi: 10.1016/S0140-6736(21)00246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenow F, Schade-Brittinger C, Burchardi N, et al. ; LaLiMo Study Group . The LaLiMo Trial: lamotrigine compared with levetiracetam in the initial 26 weeks of monotherapy for focal and generalised epilepsy—an open-label, prospective, randomised controlled multicenter study. J Neurol Neurosurg Psychiatry. 2012;83(11):1093-1098. doi: 10.1136/jnnp-2011-301999 [DOI] [PubMed] [Google Scholar]
- 30.French JA, Krauss GL, Wechsler RT, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology. 2015;85(11):950-957. doi: 10.1212/WNL.0000000000001930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panayiotopoulos CP. Evidence-based epileptology, randomized controlled trials, and SANAD: a critical clinical view. Epilepsia. 2007;48(7):1268-1274. doi: 10.1111/j.1528-1167.2007.01172.x [DOI] [PubMed] [Google Scholar]
- 32.Cross H, Ferrie C, Lascelles K, Livingston J, Mewasingh L. Old vs new antiepileptic drugs: the SANAD study. Lancet. 2007;370(9584):314-316. doi: 10.1016/S0140-6736(07)61151-9 [DOI] [PubMed] [Google Scholar]
- 33.Glauser TA, Cnaan A, Shinnar S, et al. ; Childhood Absence Epilepsy Study Group . Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362(9):790-799. doi: 10.1056/NEJMoa0902014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. doi: 10.1186/s12916-017-0845-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nucera B, Brigo F, Trinka E, Kalss G. Treatment and care of women with epilepsy before, during, and after pregnancy: a practical guide. Ther Adv Neurol Disord. 2022;15:17562864221101687. doi: 10.1177/17562864221101687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Josephson CB, Engbers JDT, Jette N, et al. Prediction tools for psychiatric adverse effects after levetiracetam prescription. JAMA Neurol. 2019;76(4):440-446. doi: 10.1001/jamaneurol.2018.4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell C, McCormack M, Patel S, et al. ; EpiPGX Consortium . A pharmacogenomic assessment of psychiatric adverse drug reactions to levetiracetam. Epilepsia. 2022;63(6):1563-1570. doi: 10.1111/epi.17228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch LJ, Weintraub DB, Buchsbaum R, et al. Predictors of lamotrigine-associated rash. Epilepsia. 2006;47(2):318-322. doi: 10.1111/j.1528-1167.2006.00423.x [DOI] [PubMed] [Google Scholar]
- 39.Genton P, Gelisse P, Thomas P, Dravet C. Do carbamazepine and phenytoin aggravate juvenile myoclonic epilepsy? Neurology. 2000;55(8):1106-1109. doi: 10.1212/WNL.55.8.1106 [DOI] [PubMed] [Google Scholar]
- 40.Thomas P, Valton L, Genton P. Absence and myoclonic status epilepticus precipitated by antiepileptic drugs in idiopathic generalized epilepsy. Brain. 2006;129(Pt 5):1281-1292. doi: 10.1093/brain/awl047 [DOI] [PubMed] [Google Scholar]
- 41.Gesche J, Hjalgrim H, Rubboli G, Beier CP. Risk factors of paradoxical reactions to antiseizure medication in genetic generalized epilepsy. Epilepsy Res. 2021;170:106547. doi: 10.1016/j.eplepsyres.2020.106547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Missingness of Variables and Combinations of Missingness Using the Intersecting Sets Visualization Method
Nonauthor Collaborators. Women With Epilepsy Treatment Options and Research (WETOR) Study Group
Data Sharing Statement.