Abstract
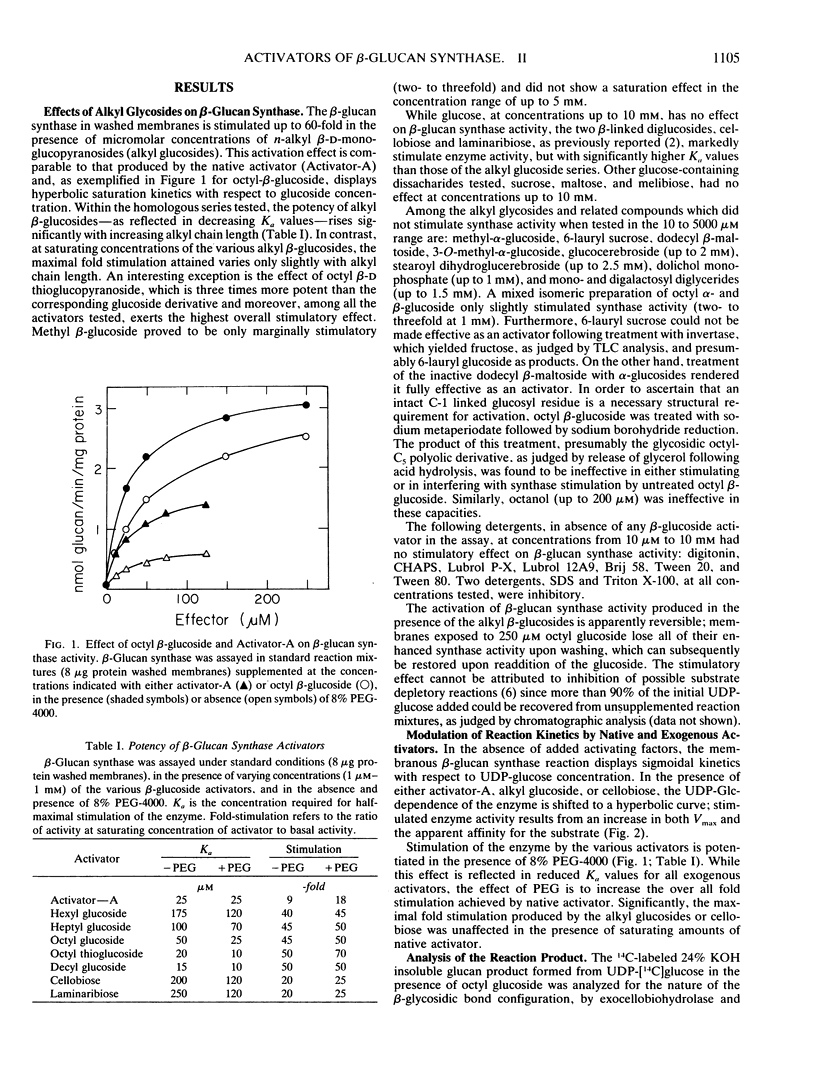
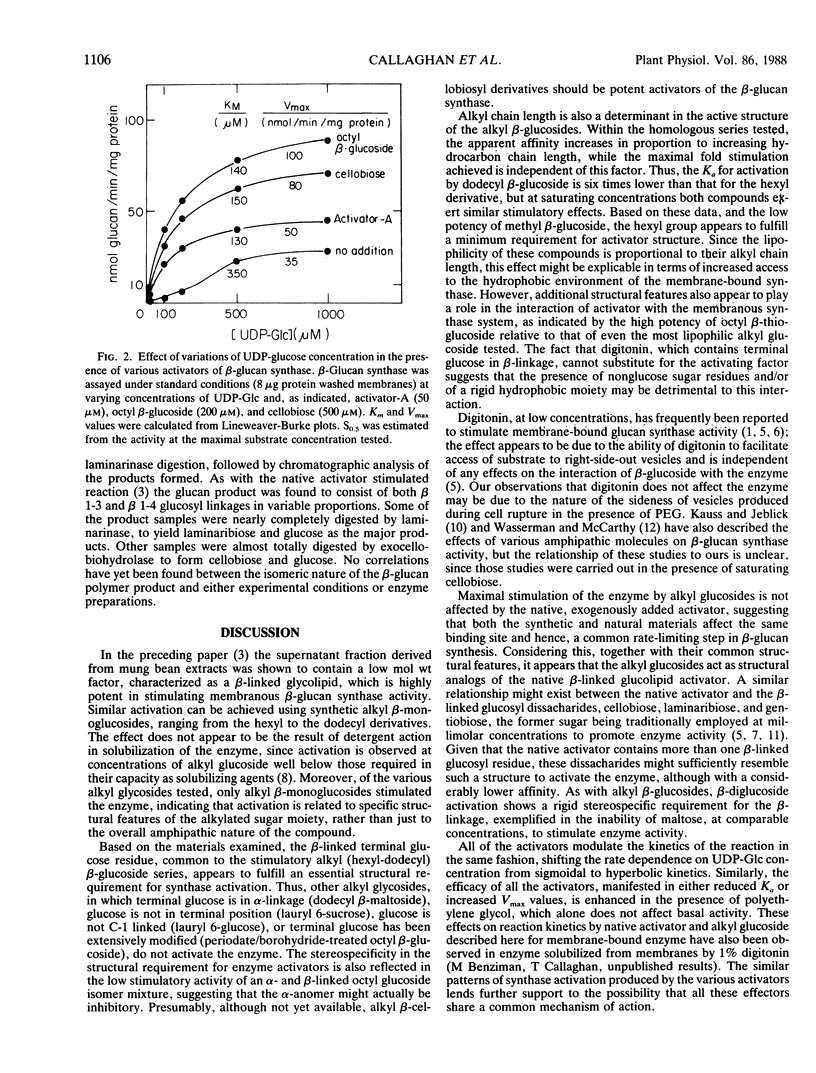
n-Alkyl (C6-C12) β-d-monoglucopyranosides have been found to be highly potent activators of mung bean β-glucan synthase in vitro, increasing the Vmax of the enzyme as much as 60-fold and with Ka values as low as 10 micromolar. Activation is highly specific for the β-linked terminal glucose residue; other alkyl glycosides such as, octyl-α-glucoside, dodecyl β-maltoside, 6-lauryl sucrose, 6-lauryl glucose, which lack this structure, are ineffective as activators. Based on the similarities in their structure and effects on β-glucan synthesis under a variety of conditions, it is proposed that the alkyl β-glucosides are structural analogs of the native glucolipid activator of β-glucan synthase isolated from mung bean extracts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Cohen R., Benziman M., Delmer D. Solubilization of the UDP-glucose:1,4-beta-D-glucan 4-beta-D-glucosyltransferase (cellulose synthase) from Acetobacter xylinum. A comparison of regulatory properties with those of the membrane-bound form of the enzyme. J Biol Chem. 1983 Apr 10;258(7):4419–4423. [PubMed] [Google Scholar]
- Callaghan T., Ross P., Weinberger-Ohana P., Garden G., Benziman M. beta-Glucoside Activators of Mung Bean UDP-Glucose: beta-Glucan Synthase : I. Identification of an Endogenous beta-Linked Glucolipid Activator. Plant Physiol. 1988 Apr;86(4):1099–1103. doi: 10.1104/pp.86.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Read S. M., Bussell J., Thelen M., Lin F. C., Brown R. M., Delmer D. P. UDP-Glucose: (1-->3)-beta-Glucan Synthases from Mung Bean and Cotton: Differential Effects of Ca and Mg on Enzyme Properties and on Macromolecular Structure of the Glucan Product. Plant Physiol. 1987 Apr;83(4):1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Factors Influencing beta-Glucan Synthesis by Particulate Enzymes from Suspension-Cultured Lolium multiflorum Endosperm Cells. Plant Physiol. 1982 Mar;69(3):632–636. doi: 10.1104/pp.69.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Solubilization of beta-glucan synthases from the membranes of cultured ryegrass endosperm cells. Biochem J. 1982 Jun 1;203(3):629–636. doi: 10.1042/bj2030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L. M., Chrambach A. Solubilization of functional membrane proteins. Methods Enzymol. 1984;104:305–318. doi: 10.1016/s0076-6879(84)04097-0. [DOI] [PubMed] [Google Scholar]
- Jacob S. R., Northcote D. H. In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in determining the type of linkage formed. J Cell Sci Suppl. 1985;2:1–11. doi: 10.1242/jcs.1985.supplement_2.1. [DOI] [PubMed] [Google Scholar]
- Kauss H., Jeblick W. Influence of Free Fatty Acids, Lysophosphatidylcholine, Platelet-Activating Factor, Acylcarnitine, and Echinocandin B on 1,3-beta-d-Glucan Synthase and Callose Synthesis. Plant Physiol. 1986 Jan;80(1):7–13. doi: 10.1104/pp.80.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D. L., Lucas W. J. (1-->3)-beta-d-Glucan Synthase from Sugar Beet : I. Isolation and Solubilization. Plant Physiol. 1986 May;81(1):171–176. doi: 10.1104/pp.81.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman B. P., McCarthy K. J. Regulation of Plasma Membrane beta-Glucan Synthase from Red Beet Root by Phospholipids : Reactivation of Triton X-100 Extracted Glucan Synthase by Phospholipids. Plant Physiol. 1986 Oct;82(2):396–400. doi: 10.1104/pp.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]



