Abstract
Background:
Intention-to-treat analyses do not address adherence. Per protocol analyses treat nonadherence as a protocol deviation and assess if the intervention is effective if followed.
Objective:
To determine the rate of early preterm birth (EPTB, <34 weeks gestation) and preterm birth (PTB, <37 weeks gestation) in participants who adhered to a randomly assigned docosahexaenoic acid (DHA) dose of 1000 mg/day.
Study Design:
Eleven hundred women with a singleton pregnancy were enrolled before 20-weeks’ gestation, provided a capsule with 200 mg/day DHA and randomly assigned to two additional capsules containing a placebo or 800 mg of DHA. In the Bayesian Adaptive Design, new randomization schedules were determined at prespecified intervals. In each randomization, the group with the most EPTB was assigned fewer participants than the other group. Adherence was defined a priori as a postpartum red blood cell phospholipid DHA (RBC-PL-DHA) ≥5.5%.and post hoc as ≥8.0% RBC-PL-DHA, the latter after examination of postpartum RBC-PL-DHA. Bayesian mixture models were fitted for gestational age and dichotomized for EPTB and PTB as a function of baseline RBC-PL-DHA and dose-adherence. Bayesian hierarchical models were also fitted for EPTB by dose adherence and quartiles of baseline RBC-PL-DHA.
Results:
Adherence to the high dose using both RBC-PL-DHA cut points resulted in less EPTB compared to 200 mg [Bayesian posterior probability (pp)=0.93 and 0.92, respectively]. For participants in the two lowest quartiles of baseline DHA status, adherence to the higher dose resulted in lower EPTB (≥5.5% RBC-PL-DHA, quartiles 1 and 2, pp=0.95 and 0.96; ≥8% RBC-PL-DHA, quartiles 1 and 2, pp=0.94 and 0.95). Using the Bayesian model, EPTB was reduced by 65%, from 3.45% to 1.2%, using both cut points. Adherence also reduced PTB before 35, 36 and 37 weeks using both cut points (pp≥0.95). In general, performance of the nonadherent subgroup mirrored that of participants assigned to 200 mg.
Conclusion:
Adherence to high dose DHA reduced EPTB and PTB. The largest effect of adherence on reducing EPTB was observed in women with low baseline DHA levels. ClinicalTrials.gov (NCT02626299).
Keywords: Pregnancy, DHA, preterm birth, clinical trial adherence, red blood cell phospholipid DHA
Introduction
A Cochrane Review published in November 2018 found strong evidence that omega-3 fatty acid supplementation during pregnancy reduced early preterm birth (EPTB, <34 weeks) by 42% and preterm birth (PTB, <37 weeks) by 11% (1). The results were based on 9 randomized clinical trials that included more than 5000 pregnant individuals, however, no trial in the analysis was designed to evaluate EPTB as a primary aim. The review did not identify a dose of omega-3 fatty acids that reduced EPTB and PTB, although the conclusion was largely informed by trials that provided greater than 500 mg per day of docosahexaenoic acid (DHA) (1).
Two large trials published after the Cochrane Review examined the effect of omega-3 fatty acids on EPTB as a primary outcome. The “Omega-3 to Reduce the Incidence of Preterm Birth (ORIP)” trial, conducted in Australia with over 5000 participants, did not find a benefit of omega-3 supplementation (800 mg DHA and 100 mg eicosapentaenoic acid/day) in reducing EPTB (2). A secondary analysis of that trial, however, found that women beginning the study with low baseline omega-3 fatty acid status had a significant reduction in EPTB (3). We conducted the” Assessment of DHA on Reducing Early Preterm Birth (ADORE)” trial in the US, a trial that provided all 1100 participants with a labeled supplement of 200 mg DHA and randomized them to 2 additional capsules with either 0 or 800 mg DHA. Analyzed by intention-to-treat, the Bayesian posterior probability (pp) that the higher dose was superior in reducing EPTB was 0.81, however, analogous to the ORIP trial, participants who began the trial with low baseline DHA status were those who benefited with lower EPTB (pp=0.93) (4). The higher dose also reduced the incidence of PTB overall (pp=0.95).
Intention-to-treat analyses are conservative because they do not address adherence or cross-over. As it is well known that not all participants in intervention trials adhere to protocol, per protocol analyses are conducted to assess if the intervention is more effective if followed. The primary aim of this study was to conduct a modified per protocol analysis to determine how adherence to high dose DHA supplementation in the ADORE trial influenced EPTB and PTB.
Materials and Methods
Study Design
Participants were recruited and enrolled in prenatal clinics at the University of Kansas Medical Center (KUMC), University of Cincinnati (UC) and The Ohio State University (OSU) and their affiliated prenatal clinics between June 8, 2016 and March 13, 2020. All participants were provided a daily capsule with 200 mg DHA. Depending on randomization, they were provided two more capsules daily that contained either a mixture of corn and soybean oil without DHA or 800 mg of an algal source of DHA (Life’s DHA™-S oil, DSM Nutritional Products LLC, Switzerland). There were several reasons we did not conduct the ADORE trial as a placebo-controlled trial. First, a DHA supplement or seafood intake is recommended for pregnancy by various scientific groups to provide at least 200 mg DHA/day (5–9). Second, we observed in a secondary analysis of an earlier clinical trial that consuming even 200 mg of an assigned dose of 600 mg reduced EPTB and PTB compared to placebo (10). Third, participants who were consuming a supplement containing 200 mg or more DHA at baseline agreed to stop their voluntary supplement and begin their assigned supplement immediately.
Assignment to dose in the ADORE trial was under a response adaptive randomization design that began after the first 300 participants enrolled and were randomized equally to each dose. Thereafter, a new randomization schedule was created every 13 weeks, which assigned more participants to the group with the lower rate of EPTB. The process was repeated 10 times during the study. All members of the study team and participants were blinded to assignment except two individuals responsible for conducting the interim analysis and adding the new allocation tables. Neither had contact with participants. Capsules were dispensed in opaque bottles at the first study visit and, thereafter, mailed to participants by the Investigational Pharmacy at the University of Cincinnati and used bottles returned to the Pharmacy each month. Details of the primary trial design are published (11).
Participants
At enrollment, participants were ≥18 years of age, between 12- and 20-weeks’ gestation and able to speak and read either English or Spanish. Women were excluded with multi-fetal gestations, <18 years of age, <12 and >20-weeks’ gestation, unwilling to discontinue a daily prenatal DHA supplement of 200 mg or more, or allergic to any component of DHA or vegetable oil. The exclusion criteria were limited by the US Food and Drug Administration (Investigational New Drug or IND #129,482) to ensure the trial did not exclude women with clinical conditions that increased their risk of preterm birth. A total of 1100 women (1000 mg, n=576; 200 mg, n=524) gave written consent for the trial in either English or Spanish. Of these, 36 participants in the 1000 mg group and 32 in the 200 mg group were lost to follow-up or had voluntary or involuntary withdrawal. Delivery data were available for 540 and 492 participants in the 1000 mg and 200 mg groups, respectively. All were included in the analyses. The CONSORT diagram is published (4).
The trial profile, additional details of randomization and masking and population demographics are published (4). In brief, the group was diverse in race and ethnicity (22% Black, 22% non-Black Hispanic, 50% non-Hispanic white), educationally (ranging from less than high school (14.5%) to a doctorate (8%), and by family income (<$15,000, 21% to >$150,000, 11%). Seventy percent had a prior pregnancy, and, of those, 18.4% ended in PTB (18.5% and 18.4%, 200 mg and 1000 mg DHA dose, respectively) and 7% in EPTB (7.4% and 6.6%, 200 mg and 1000 mg DHA dose, respectively). Participants with prior preterm birth who qualified for progesterone and cerclage under the American Society of Obstetrics and Gynecology guidelines received these treatments to prevent preterm birth.
Blood collection and analysis
Maternal blood samples were collected at baseline and during the antenatal hospitalization or the morning following birth. Details of the blood collection, processing and storage of blood samples are published (4). The method of RBC-PL-DHA analysis (weight percent of total fatty acids) is published (11) except integration of fatty acid methyl ester peaks used Agilent 6890N GC with ChemStation OpenLab C.01.09 software. Blood samples obtained at OSU and UC were separated into red blood cells and plasma, aliquots prepared, frozen and shipped to KUMC on dry ice approximately monthly. Samples from all 3 sites were bar coded and stored at −80° C until analysis.
Adherence
Clinical trials are required to be analyzed as planned, therefore, we consider an RBC-PL-DHA of 5.5% as a cut point for adherence in our analysis as planned (11) even though it is clear from an examination of Figure 1 that few participants had a postpartum RBC-PL-DHA ≤5.5%. In an earlier trial before the ready availability of DHA supplements for prenatal use, we observed baseline levels of 4.3% in women who consumed little dietary DHA and did not use supplements (12). Women in this cohort had a mean RBC-PL-DHA of 6.4% before randomization, reflecting the fact that 47% were already consuming a DHA supplement in some amount (4). Therefore, we also analyzed for rates of EPTB and PTB using a cut point of 8% RBC-PL-DHA that was determined by visual inspection of the bimodal distribution of postpartum RBC-PL-DHA in participants assigned to high dose DHA, and we assumed that participants with an RBC-PL-DHA <8% were less adherent to high dose supplementation that those ≥8% (see Figure 1).
Figure 1.
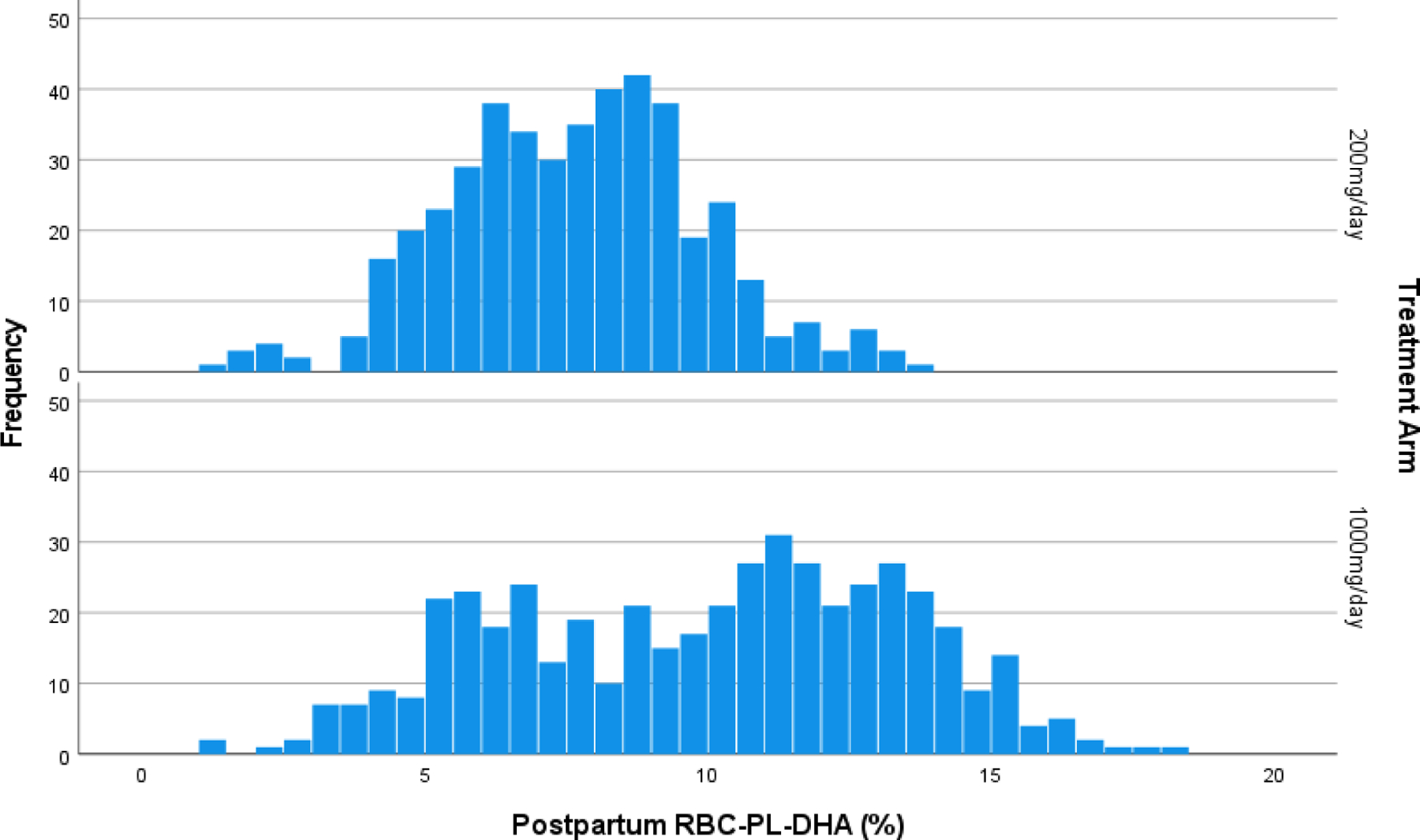
Histogram of postpartum red blood cell phospholipid DHA (RBC-PL-DHA) (% of total fatty acids) by assigned dose.
Ethics
The University of Kansas Medical Center granted approval under a central IRB with reliance by the other institutions (STUDY00003455). Participants provided written consent for study. The trial was registered (ClinicalTrials.gov: NCT02626299) on December 8, 2015.
Statistical analyses
For the purposes of our analysis, we made the assumption that all participants would consume the labeled supplement of 200 mg DHA daily; therefore, this may be considered a modified per protocol analysis in that we looked only at adherence among participants assigned to the high dose of DHA and not at adherence to the labeled capsule. We also focused on the high dose group in this secondary analysis, because this is the group who benefited with a lower risk of EPTB in the intention-to-treat analysis (4). Maternal RBC-PL-DHA <5.5% and <8% of total fatty acids at the time of birth were analyzed independently as indicators of non-adherence to the higher dose of DHA. We used a mixture of three normal distributions to model gestational age at birth as a continuous time-to-event value (13). We utilized the continuous data to dichotomize observed EPTB by dose and adherence. We also fitted Bayesian hierarchical models (14) by dose and adherence as a function of quartiles of baseline DHA status at enrollment following the earlier finding that low baseline status predicted benefit of the higher dose for reducing EPTB (4). Data of participants who withdrew or were lost to follow-up were treated as missing and multiple imputation was performed within the Bayesian model. We utilized OpenBUGS version 3.2.3 rev 1012 for all Bayesian analyses. All analyses were fitted using 10,000 burnin draws of Markov chain Monte Carlo, followed by 40,000 draws for inference. Both the study protocol and statistical analysis plan are accessible at https://r2d2.kumc.edu/ADORE/index.jsp. Posterior probability (pp) represents the probability the EPTB and PTB rates of the group adherent to the high dose (1000+ mg) were lower than the nonadherent (1000- mg) group or the group given only 200 mg. The 95% credible interval represents the EPTB and PTB rate intervals having 0.95 probability given the trial data.
Results
Table 1 and Table 2 shows the characteristics of participants in the three comparison groups: those assigned to 200 mg/d, those assigned to high dose who were adherent (1000 +) and those assigned to high dose DHA who were nonadherent (1000 -) using the 5.5% and 8% cut points for adherence, respectively. The primary efficacy outcome (EPTB) for both cut points for adherence is shown in Table 3 and the results by quartile of baseline RBC-PL-DHA in Table 4 (5.5% cut point) and Table 5 (8.0% cut point). The adherent group is designated as 1000+ mg and the nonadherent group as 1000- mg. Using the 5.5% cut point, 27% of participants were nonadherent while using the 8% cut point, 40.9% were nonadherent. Both the protocol-designated cut point of 5.5% RBC-PL-DHA and the 8% cut point showed 1000+ mg was superior to 200 mg in reducing EPTB (pp=0.93 and 0.92, respectively) (Table 3). Participants in the two lowest quartiles for RBC-PL-DHA at baseline and who achieved a delivery RBC-PL-DHA ≥5.5% on the higher dose had a lower rate of EPTB compared to the group assigned to 200 mg (pp=0.92 and 0.97 for quartile 1 and 2, respectively) (Table 4). In participants with low baseline DHA status, who achieved a delivery RBC-PL-DHA ≥8%, the 1000+ group also had a lower rate of EPTB compared to the group assigned to 200 mg (pp=0.92 and 0.96 for quartile 1 and 2, respectively) (Table 5).
Table 1.
Definitions of adherence for the 1000 mg group (1000- mg/day, RBC-PL-DHA <5.5%a; 1000+ mg (RBC-PL-DHA ≥5.5%b). The 5.5% cut point for adherence was defined from our earlier trial (a priori).
| Baseline Characteristics | 200 mg/day N=492/1032 | 1000- mg/daya N=124/1032 | 1000+ mg/dayb N=416/1032 |
|---|---|---|---|
| Age at enrollment, yr | 30.1 ± 5.6 | 27.6 ± 5.6 | 31.3 ± 5.5 |
| Maternal Education, n (%) | |||
| Less than high school graduate | 65 (13.2) | 21 (16.9) | 60 (14.4) |
| HS graduate or GED | 100 (20.3) | 49 (39.5) | 59 (14.2) |
| Some college or tech school | 91 (18.5) | 32 (25.8) | 73 (17.6) |
| Bachelor’s Degree Obtained | 128 (26.0) | 13 (10.5) | 98 (23.6) |
| Master’s Degree Obtained | 73 (14.8) | 3 (2.4) | 81 (19.5) |
| Doctorate | 35 (7.1) | 6 (4.8) | 45 (10.8) |
| Family Income, n (%) | |||
| Less than $15,000 | 101 (20.5) | 46 (37.1) | 58 (13.9) |
| $15,000 - $24,999 | 55 (11.2) | 24 (19.4) | 47 (11.3) |
| $25,000 - $49,999 | 83 (16.9) | 20 (16.1) | 67 (16.1) |
| $50,000 - $99,999 | 85 (17.3) | 21 (16.9) | 91 (21.9) |
| $100,000 - $149,999 | 96 (19.5) | 6 (4.8) | 85 (20.4) |
| At least $150,000 | 59 (12.0) | 5 (4.0) | 54 (13.0) |
| Unknown | 13 (2.6) | 2 (1.6) | 14 (3.4) |
| DHA-FFQ Consumed DHA,1 mg/day, mean ± SD | 89.3 ± 72.0 | 69.3 ± 60.6 | 86.7 ± 81.4 |
| N=490 3 | N=124 | N=416 | |
| DHA-FFQ Supplement DHA,2 mg/day, mean ± SD | 67.0 ± 98.1 | 33.3 ± 70.4 | 72.5 ± 109.1 |
| N=490 5 | N=124 | N=414 5 | |
| Enrollment RBC-PL-DHA %,4 mean ± SD | 6.5 ± 1.8 | 5.4 ± 1.3 | 6.6 ± 1.8 |
| N=476 | N=103 | N=374 | |
| DHA from Study Capsules,6 mg/day, mean ± SD | 84.3 ± 41.0 | 270.5 ± 214.8 | 419.0 ± 173.0 |
Total DHA consumed in diet, captured by Q1-Q6 on DHA Food Frequency Questionnaire (DHA-FFQ) at enrollment.
Total DHA-containing dietary supplements and function foods, captured by on DHA-FFQ at enrollment.
Coordinators were unable to calculate for two participants.
Red blood cell phospholipid DHA % of total fatty acids.
Four participants were missing enrollment blood samples.
Total DHA consumed per day while pregnant from provided study capsules based on pharmacy capsule count (200 mg/day or 1000 mg/day).
Table 2.
Definitions of adherence for the 1000 mg group (1000- mg/day, RBC-PL-DHA <8.0%a; 1000+ mg/day (RBC-PL-DHA ≥8.0%b). The 8.0% cut point for adherence was determined by inspection of the histogram shown later in Figure 1.
| Baseline Characteristics | 200 mg/day N=492/1032 | 1000- mg/daya N=221/1032 | 1000+ mg/dayb N=319/1032 |
|---|---|---|---|
| Age at enrollment, yr | 30.1 ± 5.6 | 28.5 ± 5.7 | 31.8 ± 5.1 |
| Maternal Education, n (%) | |||
| Less than high school graduate | 65 (13.2) | 46 (20.8) | 35 (11.0) |
| HS graduate or GED | 100 (20.3) | 75 (33.9) | 33 (10.3) |
| Some college or tech school | 91 (18.5) | 61 (27.6) | 44 (13.8) |
| Bachelor’s Degree Obtained | 128 (26.0) | 23 (10.4) | 88 (28.6) |
| Master’s Degree Obtained | 73 (14.8) | 8 (3.6) | 76 (23.8) |
| Doctorate | 35 (7.1) | 8 (3.6) | 43 (13.5) |
| Family Income, n (%) | |||
| Less than $15,000 | 101 (20.5) | 74 (33.5) | 30 (9.4) |
| $15,000 - $24,999 | 55 (11.2) | 39 (17.7) | 32 (10.0) |
| $25,000 - $49,999 | 83 (16.9) | 47 (21.3) | 40 (12.5) |
| $50,000 - $99,999 | 85 (17.3) | 31 (14.0) | 81 (25.4) |
| $100,000 - $149,999 | 96 (19.5) | 14 (6.3) | 77 (24.1) |
| At least $150,000 | 59 (12.0) | 8 (3.6) | 51 (16.0) |
| Unknown | 13 (2.6) | 8 (3.6) | 8 (2.5) |
| DHA-FFQ Consumed DHA,1 mg/day, mean ± SD | 89.3 ± 72.0 | 82.8 ± 97.8 | 82.7 ± 59.5 |
| N=490 3 | N=221 | N=319 | |
| DHA-FFQ Supplement DHA,2 mg/day, mean ± SD | 67.0 ± 98.1 | 35.5 ± 72.1 | 82.8 ± 115.7 |
| N=490 5 | N=220 | N=318 5 | |
| Enrollment RBC-PL-DHA %,4 mean ± SD | 6.5 ± 1.8 | 5.5 ± 1.4 | 6.9 ± 1.8 |
| N=476 | N=151 | N=326 | |
| DHA from Study Capsules,6 mg/day, mean ± SD | 84.3 ± 41.0 | 278.6 ± 218.0 | 453.1 ± 138.6 |
Total DHA consumed in diet, captured by Q1-Q6 on DHA Food Frequency Questionnaire (DHA-FFQ) at enrollment.
Total DHA-containing dietary supplements and function foods, captured by DHA-FFQ at enrollment.
Coordinators were unable to calculate for two participants.
Red blood cell phospholipid DHA % of total fatty acids.
Four participants were missing enrollment blood samples.
Total DHA consumed per day while pregnant from provided study capsules based on pharmacy capsule count (200 mg/day or 1000 mg/day).
Table 3.
Primary efficacy outcomes with adherence based on RBC-PL-DHA. a Uses a model that dichotomizes the continuous variable via a continuous mixture of three normal distributions. Different definitions of adherence for the 1000 mg group (1000+ mg, RBC-PL-DHA ≥ 5.5%b or ≥ 8%c; 1000- mg (RBC-PL-DHA <5.5%b or <8%c). The 5.5% cut point for adherence was defined from our earlier trial (a priori) and the 8% cut point was determined by inspection of the histogram in Figure 1.
| Observed early preterm births <34 weeks, n/N (%) | Posterior mean % of EPTB (95% Bayesian credible interval) | Bayesian posterior probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200mg N=492 |
1000- mg N=124/221 |
1000+ mg N=416/319 |
200mg N=492 |
1000- mg N=124/221 |
1000+ mg N=416/319 |
1000+ better than 200 | 1000+ better than 1000- | 1000- better than 200 | |
| Group | |||||||||
| Early preterm birth (<34 wk)a,b | 12/492 (2.4) | 4/124 (3.2) | 5/416 (1.2) | 2.8 (1.5,4.6) | 2.8 (.9, 6.2) | 1.4 (.4, 3.0) | 0.93 | 0.85 | 0.51 |
| Early preterm birth (<34 wk)a,c | 12/492 (2.4) | 5/221 (2.3) | 4/319 (1.3) | 2.8 (1.5,4.6) | 2.2 (.7, 4.5) | 1.4 (.3, 3.2) | 0.92 | 0.75 | 0.70 |
Table 4.
Observed and Bayesian posterior means, 95% credible intervals and posterior probabilities of a dose effect for early preterm birth rate by quartile baseline DHA status and adherence (postpartum RBC-PL-DHA ≥5.5%) with the high dose. Posterior means and Bayesian 95% credible intervals and posterior probabilities of a dose effect.
| Observed early preterm birth <34 weeks, n/N (%) | Posterior mean% of EPTB (95% Bayesian credible interval)a | Bayesian posterior probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200mg | 1000- mg | 1000+ mg | 200mg | 1000- mg | 1000+ mg | 1000+ mg better than 200 mg | 1000+ mg better 1000- mg | 1000- mg better than 200 mg | |
| DHA Enrollment (%) | |||||||||
| Quartile 1 (<5.1] | 5/112 (4.5) | 3/63 (4.8) | 2/79 (2.5) | 3.4 (1.2,8.0) | 3.1 (1.1,7.7) | 1.3 (0.4,3.6) | 0.92 | 0.91 | 0.51 |
| Quartile 2 (5.1–6.2] | 6/132 (4.6) | 0/29 (0.0) | 0/97 (0.0) | 3.5 (1.5,7.7) | 2.7 (0.0,6.4) | 1.1 (0.0,2.4) | 0.97 | 0.85 | 0.63 |
| Quartile 3 (6.2–7.4] | 1/110 (0.9) | 1/22 (4.5) | 2/116 (1.7) | 1.3 (0.0,3.8) | 3.0 (0.6,8.3) | 1.3 (0.4,2.8) | 0.50 | 0.89 | 0.16 |
| Quartile 4 (>7.4) | 0/136 (0.0) | 0/10 (0.0) | 1/122 (0.8) | 0.6 (0.0,3.3) | 2.9 (0.0,7.2) | 1.1 (0.2, 2.6) | 0.34 | 0.86 | 0.12 |
Bayesian hierarchical model was fitted using the approach in Berry and Berry,8 except mu~N(−3.1,10^2). This approach avoids false discoveries in subgroups (quartiles). We burned-in 10,000 draws and used 40,000 draws for inference. High adherence, 1000+ mg, postpartum RBC-PL-DHA ≥ 5.5%; low adherence, 1000- mg, postpartum RBC-PL-DHA <5.5%.
Table 5.
Observed and Bayesian posterior means, 95% credible intervals and posterior probabilities of a dose effect for early preterm birth rate by quartile baseline DHA status and adherence (postpartum RBC-PL-DHA ≥8%) with the high dose.
| Observed early preterm births <34 weeks, n/N (%) | Posterior mean % of EPTB (95% Bayesian credible interval)a | Bayesian posterior probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 mg | 1000- mg | 1000+ mg | 200mg | 1000- mg | 1000+ mg | 1000+ mg better than 200mg | 1000+ mg better 1000- mg | 1000- mg better than 200mg | |
| DHA Enrollment (%) | |||||||||
| Quartile 1 (<5.1%] | 5/112 (4.5) | 4/96 (4.2) | 1/46 (2.2) | 3.4 (1.2,7.9) | 2.5 (1.0,6.7) | 1.3 (0.3,3.5) | 0.92 | 0.82 | 0.67 |
| Quartile 2 (5.1–6.2%] | 6/132 (4.6) | 0/51 (0.0) | 0/75 (0.0) | 3.5 (1.5,7.7) | 1.6 (0.0,4.4) | 1.1 (0.3,2.6) | 0.96 | 0.64 | 0.85 |
| Quartile 3 (6.2–7.4%] | 1/110 (0.9) | 1/52 (1.9) | 2/86 (2.3) | 1.3 (0.1,3.8) | 1.9 (0.2,5.2) | 1.3 (0.3,3.4) | 0.48 | 0.66 | 0.32 |
| Quartile 4 (>7.4%)] | 0/136 (0.0) | 0/21 (0.0) | 1/111 (0.9) | 0.6 (0.0,3.3) | 1.7 (0.0,5.0) | 1.2 (0.2,2.8) | 0.34 | 0.65 | 0.24 |
Bayesian hierarchical model was fitted using the approach in Berry and Berry,8 except mu~N(−3.1,10^2). This approach avoids false discoveries in subgroups (quartiles). We burned-in 10,000 draws. and used 40,000 draws for inference. High adherence, 1000+ mg, postpartum RBC-PL-DHA ≥ 8.0%; low adherence, 1000- mg, postpartum RBC-PL-DHA <8.0%.
The nonadherent group (1000- mg) with baseline DHA status in the two lowest quartiles had a similar rate of EPTB to the 200 mg group (pp=0.51–0.63) using the 5.5% cut point. The nonadherent group was moderately superior to 200 mg (lower rate of EPTB) using the 8% cut point (0.67–0.85). In contrast, the nonadherent group was moderately inferior to the 200 mg group (had a higher probability of EPTB) for participants who began the study in the two highest quartiles using the 5.5% cut point. Overall, the results suggest the nonadherent group is moderately superior to 200 mg for participants who began the study in the two lowest quartiles of DHA status and moderately inferior for participants who began in the two highest quartiles.
Tables 6–8 include results of the analysis for PTB, a secondary outcome of the ADORE trial. With both cut points the 1000+ mg group has lower PTB than either the 200 mg group or the 1000- mg group (pp=1.00) (Table 6), however, the 200 mg group had a lower rate of PTB than the 1000- mg group (pp=0.96/0.97) (Table 6). When adherence is evaluated by quartile of baseline DHA status, the superiority of the 1000+ mg group for reducing PTB compared to the 200 mg group (pp=0.83–0.91) and the 1000- mg group (pp=0.95–1.00) is very high across all 4 quartiles using the 5.5% cut point (Table 7). Using the 8% cut point 1000+ mg was superior to 200 mg (pp=0.90–1.00) and 1000 mg- (pp=0.87–1.00) (Table 8).
Table 6.
Secondary efficacy outcomes with adherence based on RBC-PL-DHA. a Uses a model that dichotomizes the continuous variable via a continuous mixture of three normal distributions. Different definitions of adherence for the 1000 mg group (1000+ mg, RBC-PL-DHA ≥ 5.5%b or ≥ 8%c; 1000- mg (RBC-PL-DHA <5.5%b or <8%c). The 5.5% cut point for adherence was defined from our earlier trial (a priori) and the 8% cut point was determined by inspection of the histogram in Figure 1.
| Observed preterm births <37 weeks, n/N (%) | Posterior mean % of PTB (95% Bayesian credible interval) | Bayesian posterior probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200mg N=492 |
1000- mg N=124/221 |
1000+ mg N=416/319 |
200mg N=492 |
1000- mg N=124/221 |
1000+ mg N=416/319 |
1000+ better than 200 | 1000+ better than 1000- | 1000- better than 200 | |
| Group | |||||||||
| preterm birth (< 37 wk)a,b | 54/492 (11.0) | 18/124 (14.5) | 26/416 (6.3) | 13.1 (10.7,15.6) | 17.7 (13.0, 22.9) | 8.7 (6.6, 11.2) | 1.00 | 1.00 | 0.04 |
| preterm birth (< 37 wk)a,c | 54/492 (11.0) | 28/221 (22.6) | 16/319 (3.9) | 13.1 (10.7,15.6) | 17.1 (13.6, 20.9) | 6.9 (4.8, 9.6) | 1.00 | 1.00 | 0.03 |
Table 8.
Observed and Bayesian posterior means, 95% credible intervals and posterior probabilities of a dose effect for preterm birth rate by quartile baseline DHA status and adherence (postpartum RBC-PL-DHA ≥8.0%) with the high dose. Posterior means and Bayesian 95% credible intervals and posterior probabilities of a dose effect.
| Observed preterm births <37 weeks, n/N (%) | Posterior mean% of PTB (95% Bayesian credible interval)a | Bayesian posterior probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200mg | 1000- mg | 1000+ mg | 200mg | 1000- mg | 1000+ mg | 1000+ mg better than 200 mg | 1000+ mg better 1000- mg | 1000- mg better than 200 mg | |
| DHA Enrollment (%) | |||||||||
| Quartile 1 (<5.1] | 18/112 (16.1) | 15/96 (15.6) | 4/46 (8.7) | 12.7 (8.6,19.9) | 13.5 (8.9,19.8) | 5.3 (3.0,9.8) | 0.99 | 0.99 | 0.39 |
| Quartile 2 (5.1–6.2] | 17/132 (12.9) | 8/51 (15.7) | 2/75 (2.7) | 11.4 (8.2,16.8) | 13.3 (8.2,20.5) | 4.7 (2.1,7.4) | 1.00 | 1.00 | 0.31 |
| Quartile 3 (6.2–7.4] | 12/110 (10.9) | 5/52 (9.6) | 4/86 (4.7) | 10.8 (7.0,15.4) | 11.9 (5.6,17.0) | 4.9 (2.7,7.9) | 0.99 | 0.97 | 0.36 |
| Quartile 4 (>7.4) | 7/136 (5.2) | 0/21 (0.0) | 6/111 (5.4) | 8.8 (3.7,12.8) | 10.8 (0.6,16.7) | 5.0 (2.9, 8.1) | 0.90 | 0.87 | 0.28 |
Bayesian hierarchical model was fitted using the approach in Berry and Berry,8 except mu~N(−3.1,10^2). This approach avoids false discoveries in subgroups (quartiles). We burned-in 10,000 draws and used 40,000 draws for inference. High adherence, 1000+ mg, postpartum RBC-PL-DHA ≥ 8.0%; low adherence, 1000- mg, postpartum RBC-PL-DHA <8.0%.
Table 7.
Observed and Bayesian posterior means, 95% credible intervals and posterior probabilities of a dose effect for preterm birth rate by quartile baseline DHA status and adherence (postpartum RBC-PL-DHA ≥5.5%) with the high dose. Posterior means and Bayesian 95% credible intervals and posterior probabilities of a dose effect.
| Observed preterm births <37 weeks, n/N (%) | Posterior mean% of PTB (95% Bayesian credible interval)a | Bayesian posterior probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200mg | 1000- mg | 1000+ mg | 200mg | 1000- mg | 1000+ mg | 1000+ mg better than 200 mg | 1000+ mg better 1000- mg | 1000- mg better than 200 mg | |
| DHA Enrollment (%) | |||||||||
| Quartile 1 (<5.1] | 18/112(16.1) | 9/63 (14.3) | 10/79 (12.7) | 12.7 (8.6,19.9) | 14.7 (9.1,20.8) | 7.6 (4.1,14.9) | 0.91 | 0.95 | 0.29 |
| Quartile 2 (5.1–6.2] | 17/132 (12.9) | 6/29 (20.7) | 4/97 (4.1) | 11.4 (8.2,16.8) | 15.4 (9.9,24.1) | 5.8 (2.6,8.8) | 0.99 | 1.00 | 0.17 |
| Quartile 3 (6.2–7.4] | 12/110 (10.9) | 3/22 (13.6) | 6/116 (5.2) | 10.8 (7.0,15.4) | 14.6 (7.8,21.6) | 6.0 (3.2,9.0) | 0.97 | 0.99 | 0.16 |
| Quartile 4 (>7.4) | 7/136 (5.2) | 0/10 (0.0) | 6/122 (5.0) | 8.8 (3.7,12.8) | 14.0 (3.3,20.6) | 5.9 (3.1, 8.9) | 0.83 | 0.95 | 0.12 |
Bayesian hierarchical model was fitted using the approach in Berry and Berry,8 except mu~N(−3.1,10^2). This approach avoids false discoveries in subgroups (quartiles). We burned-in 10,000 draws and used 40,000 draws for inference. High adherence, 1000+ mg, postpartum RBC-PL-DHA ≥ 5.5%; low adherence, 1000- mg, postpartum RBC-PL-DHA <5.5%.
The time to event analyses for the 1000+ mg, 1000- mg and 200 mg groups in Figure 2 shows the cumulative births in each category at 33-, 34-, 35-, 36- and 37-weeks’ gestation using the 5.5% cut point. It can be observed that the adherent group had a lower rate of PTB than the 200 mg group at gestational weeks 34, 35, 36 and 37 (pp=0.93, 0.95, 0.98 and 0.99, respectively). The adherent group also had a lower rate of PTB compared to the nonadherent group, at these respective weeks (pp=0.85, 0.92, 0.99 and 0.99). The nonadherent group had a higher rate of PTB than the 200 mg group at 37 weeks gestation (pp=0.97) but was similar at 34-, 35- and 36-weeks’ gestation.
Figure 2.
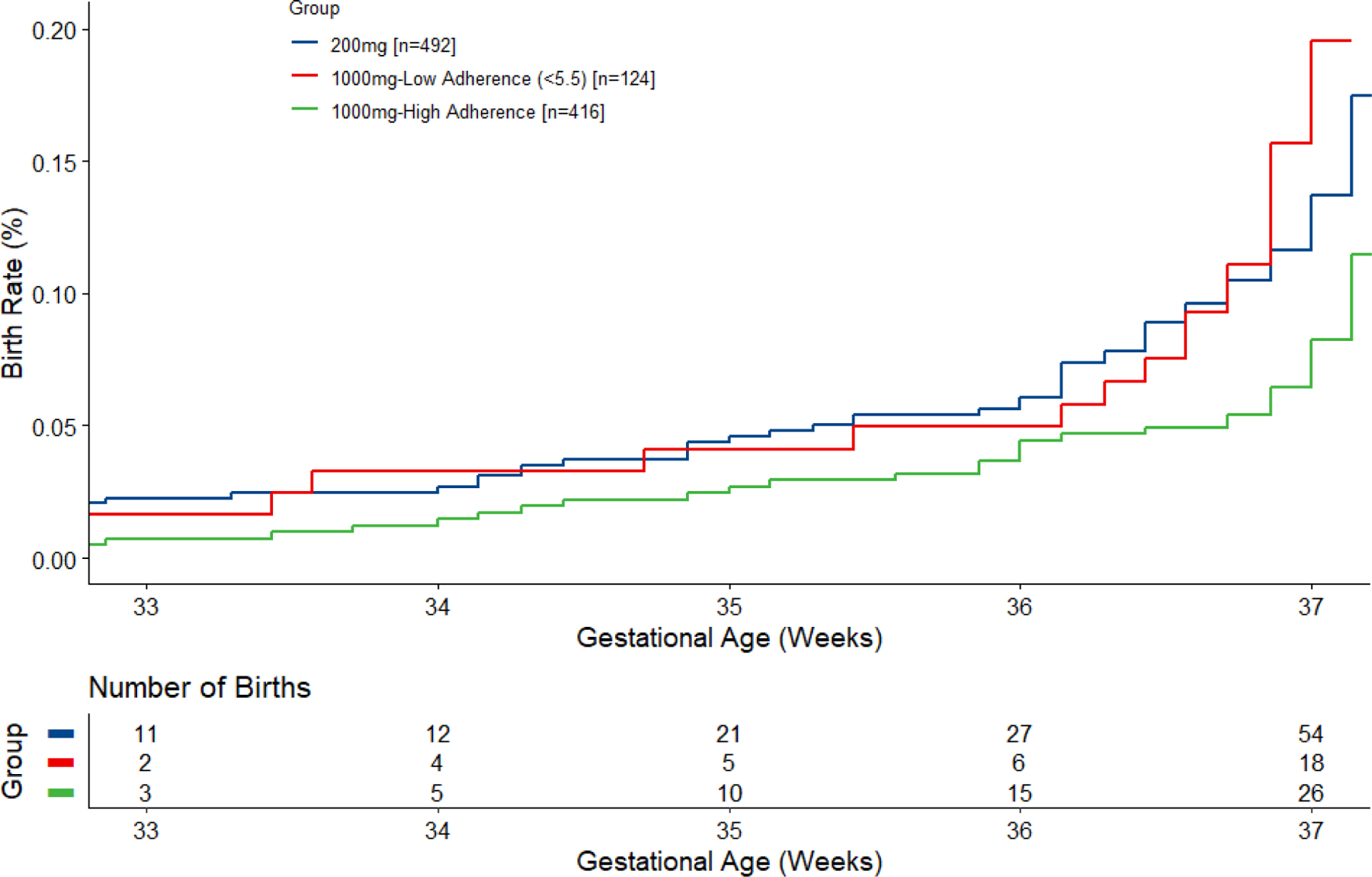
Time-to-event analysis of birth before 37 weeks by adherence with 1000 mg assigned dose using planned definition of adherence (red blood cell phospholipid or RBC-PL-DHA ≥5.5%). The number of births at each gestational age is cumulative. At 34, 35, 36 and 37 weeks, Bayesian posterior probabilities (pp) that the adherent group (1000+ mg) was superior to the 200 mg group were pp=0.93, 0.95, 0.98 and 0.99, respectively. The adherent group was also superior to the nonadherent group (1000- mg), respectively, 0.85, 0.92, 0.99 and 0.99, for these weeks. The non-adherent group (1000- mg) had a higher rate of PTB than the 200 mg group at 37 weeks gestation (pp=0.97) but was similar at 34-, 35- and 36-weeks’ gestation.
The time to event analysis for the 8% cut point in Figure 3 shows the cumulative births in each category at 33, 34-, 35-, 36- and 37-weeks’ gestation. The adherent group had a lower rate of PTB than the 200 mg group at 34-, 35-, 36- and 37-weeks’ gestation (pp=0.92, 0.95, 0.99 and 0.99, respectively). The adherent group also had a lower rate of PTB compared to the nonadherent group at these respective weeks (pp=0.75, 0.89, 0.99 and 0.99). The nonadherent group had a higher rate of PTB than the 200 mg group at 37 weeks gestation (pp=0.97) but was similar at 34-, 35- and 36-weeks’ gestation.
Figure 3.
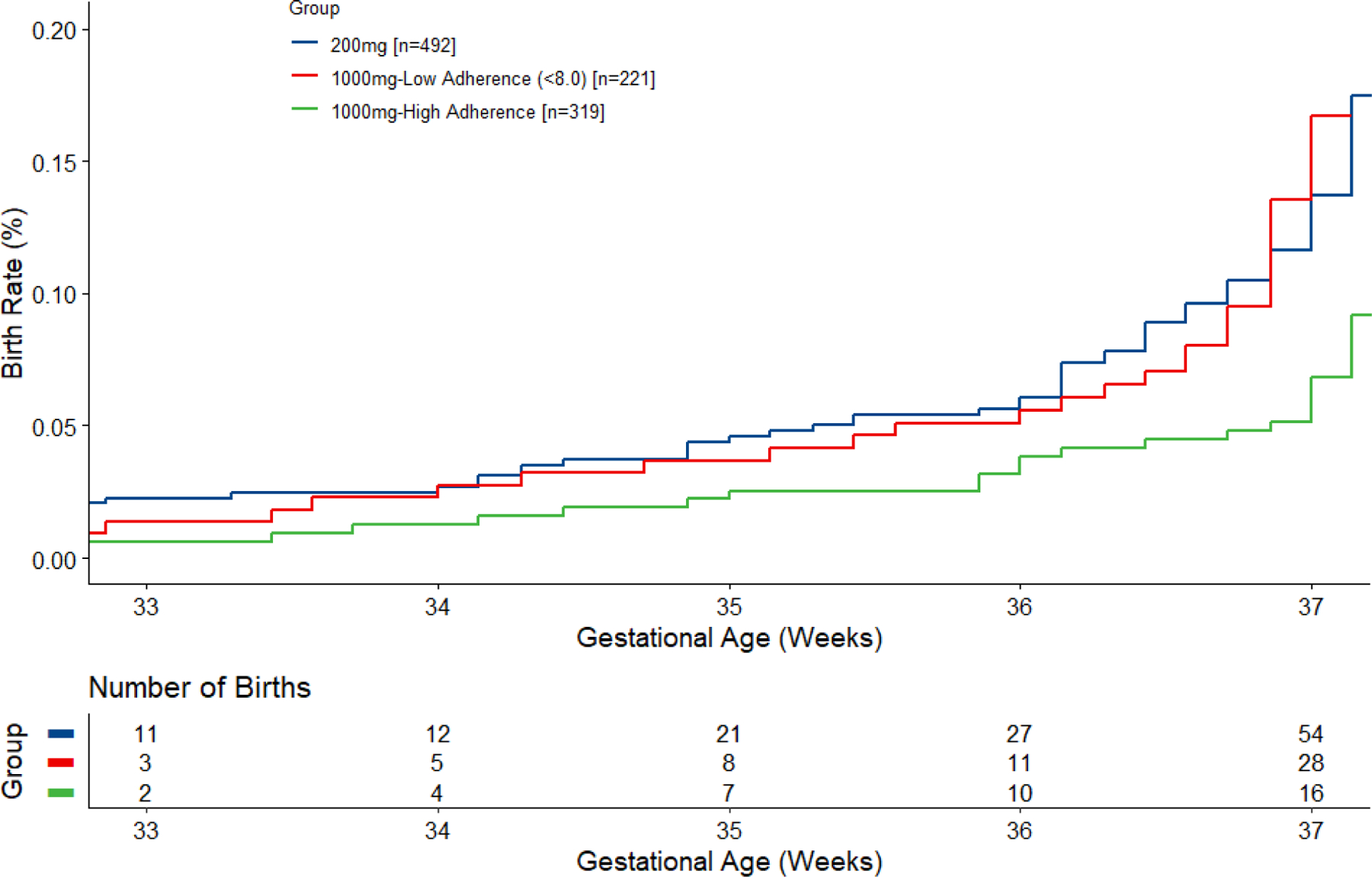
Time-to-event analysis of birth before 37 weeks by adherence with 1000 mg assigned dose using observed ≥8% (cut point in bimodal distribution of red blood cell phospholipid or RBC-PL-DHA) to define high adherence. The number of births at each gestational age is cumulative. At 34, 35, 36 and 37 weeks, Bayesian posterior probabilities (pp) that the adherent group (1000+ mg) was superior to the 200 mg group were 0.92, 0.95, 0.99 and 0.99. The adherent group was also superior to the nonadherent group (1000- mg) are 0.75, 0.89, 0.99 and 0.99, respectively, at these gestational weeks. The non-adherent group (1000- mg) had a higher rate of PTB than the 200 mg group at 37 weeks gestation (pp=0.97) but was similar at 34-, 35- and 36-weeks’ gestation.
Discussion
Principle findings
We conducted a modified per protocol analysis of the ADORE trial to determine if assignment to high-dose DHA was more effective compared to 200 mg/day in reducing EPTB and PTB among those who adhered to high dose DHA supplementation Despite the decrease in statistical power due to smaller group size, the risk of EPTB was reduced with adherence using both cut points compared to the published intention-to-treat analysis (pp=0.92 and 0.93 vs 0.81). The probability that adherence reduced late preterm birth, those occurring between 34- and 36.9-weeks’ gestation, was even greater (pp≥0.95).
Forty-seven percent of the ADORE cohort began the study in the two lowest quartiles of DHA status. Regardless of the cut point chosen for adherence, EPTB and PTB were reduced with adherence to the high dose in participants who began the study with RBC-PL-DHA in the two lowest quartiles. While the intention-to-treat analysis of the ADORE trial found that participants with low baseline status provided the higher DHA dose had an observed rate of EPTB half of those given 200 mg DHA (2.0 vs 4.1%) (4), participants who adhered to the assigned high dose DHA had an observed rate of EPTB that was reduced by 75% (1.14% vs 4.51%) using the 5.5% cut point and by 82% (0.8% vs 4.51% ) using the 8% cut point. Results suggest larger reductions in EPTB and PTB are possible with improving adherence.
Participants with a baseline RBC-PL-DHA in the top two quartiles at baseline (>6.2% RBC-PL-DHA) had the lowest rate of EPTB and PTB and did not benefit with lower rates of EPTB from receiving a supplement larger than 200 mg DHA. This finding is analogous to the intention-to-treat findings of both ORIP (3) and ADORE (4), which showed that women in the higher two quartiles of DHA status at baseline had a very low EPTB even assigned to placebo (3) or 200 mg DHA (4). The reasons for differences in baseline status are related to differences in dietary and supplement intake of DHA at enrollment that are shown in Table 1, but that exist within groups as well. We did not have a washout period for women who were taking a supplement prior to enrollment as it can take months for DHA status to decline. What is apparent from both recent large clinical trials is that women who have good DHA status early in pregnancy have the lowest rates of EPTB and PTB. We did not anticipate this would occur and the implications are that women who begin supplementation early might not require high dose supplementation to reduce EPTB. Results of both recent trials (3, 4) suggest that it is important for women to improve their DHA status at some yet to be determined time before mid-pregnancy, when participants in both trials were enrolled. In contrast, women with lower DHA status early in pregnancy benefit from high dose supplementation, especially if adherent, with lower rates of EPTB and PTB.
We also compared the nonadherent group (1000-) to the adherent group (1000+) and the 200 mg group. Overall, using both cut points, the 1000- group performed worse than the 1000+ group and better than the 200 mg group for reducing EPTB rates. This may be explained by the fact that even nonadherent participants consumed more DHA from study capsules than those who were assigned to 200 mg. This is apparent in Figure 1, which suggests that both groups were variably adherent to dose. It is interesting that women who achieved a RBC-PL-DHA ≥8% had the lowest rates of EPTB and PTB.
The adherent group, and to a lesser extent the 200 mg group, had lower rates of PTB than the nonadherent group. The fact that the 200 mg group had a lower PTB rate than the nonadherent group cannot be explained by differences in DHA intake during the study. Lower education and income are conflated with lower baseline DHA intake and DHA status (RBC-PL-DHA) in the nonadherent group compared to the 200 mg group. Preterm birth is known to have a complex etiology and this result suggests that other factors in addition to study DHA intake influenced PTB rate.
Results in the context of past studies
The 2018 Cochrane Review clearly demonstrates the importance of omega-3 supplementation in pregnancy for reducing both EPTB and PTB (2). Unlike the trials included in the Cochrane Review, this trial and ORIP were conducted after prenatal supplements containing DHA were marketed to pregnant women. Results of both recent trials suggest that women already consuming a DHA supplement or with already high DHA status are unlikely to benefit further from high dose DHA supplementation. Nearly half of the participants in ADORE were consuming a DHA supplement at the time they enrolled in the trial, and the mean baseline RBC-PL-DHA for the trial was 6.4% (4) compared to 4.3% in a trial we conducted between 2006 and 2010 (12). As we showed in the primary report of this trial (4), and confirm here, participants with a baseline RBC-PL-DHA this high had a very low rate of EPTB and did not benefit from assignment to 1000 mg DHA. It can be difficult to compare DHA status among trials, because the various trials have used RBC-PL-DHA, RBC-DHA and blood spot DHA to determine DHA status. Harris and Jackson (15) harmonized DHA levels among trials to RBC-DHA% through interlaboratory comparison conducted with the sites of both recent trials (3,4). They reported an RBC-DHA of 4.5% in ORIP participants at enrollment and 5.1% in ADORE, indicating women in ADORE began the trial with somewhat higher DHA status. In ORIP, RBC-DHA increased only to 5.1% on the 800 mg/day dose of DHA while RBC-DHA increased to 7.7% in ADORE participants assigned to 1000 mg/day.
Limitations
While the characteristics of participants assigned to 200 mg and 1000 mg in ADORE were similar (4), it is clear from Table 1 and Table 2 that the 3 groups compared differ in education, income and baseline DHA intake and status in addition to study DHA intake. As we have discussed above, the possible influence of these baseline differences or characteristics associated with them cannot be ruled out as contributing factors in our results, particularly those related to PTB.
Clinical significance.
ADORE compared 1000 mg DHA to a standard supplement dose of 200 mg DHA, an amount recommended by several authoritative groups (7–11). It is likely that the effect of DHA supplementation on EPTB and PTB rates would have been even more pronounced had this been a placebo-controlled trial. We showed previously that participants who consumed even 200 mg of an assigned 600 mg DHA had a lower rate of EPTB (2.4%) compared to placebo (3.7%) while those who adhered completely to the assigned dose of 600 mg DHA had an EPTB rate of 1.2% (5). Using 5.5% as the cut point for adherence, determined from that earlier trial, the rate of EPTB in the experimental group provided 200 mg is 2.4% while the rate in those who adhered to the higher dose of 1000 mg is 1.2%.
Nearly half of the participants in ADORE were taking a prenatal supplement containing DHA at baseline so beginning a lower dose DHA supplement very early in pregnancy may be sufficient to reduce EPTB and PTB without the need for high dose DHA supplementation. The good news is that the current recommendations for 200 mg DHA/day from supplements or seafood intake (7–11) appear to be sufficient to optimally reduced EPTB provided DHA status is improved before mid-pregnancy. On the other hand, the higher dose did reduce late preterm birth between 34 and 37 weeks (4) and may be desirable for some pregnancies as late preterm birth is more common than EPTB and is the most common cause of morbidity and mortality in developing countries (16).
Assessing baseline DHA intake is a pragmatic way to identify women who should consume more than 200 mg/day after mid-pregnancy to reduce EPTB and PTB. Participants in ADORE and another NICHD-funded trial we conducted in Kansas City, the Prenatal Autonomic Neurodevelopmental Assessment or PANDA trial, completed a validated 7-question Food Frequency Questionnaire (17) at baseline to estimate average daily DHA intake. A secondary analysis of the 1310 participants in ADORE and PANDA showed that participants consuming <150 mg DHA/day from diet and supplements at baseline had lower rates of EPTB (pp=0.99) and PTB (pp=0.97) if they were assigned to 800 or 1000 mg DHA/day instead of 200 mg/day (18). The Dietary Guidelines for America advise pregnant women to consume at least 8 ounces and up to 12 ounces of a variety of seafood per week (https://www.dietaryguidelines.gov/resources/2020-2025). The American College of Obstetricians and Gynecologists recommends eating at least two servings of fish or shellfish per week before and during pregnancy and during lactation (https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy). If pregnant women were to follow the advice of these recommendations, they would achieve an average DHA of at least 200 mg/day. Supplements should be considered for women who do not consume these amounts of seafood.
Acknowledgement
We thank the women who participated in this trial during their pregnancies and the staff who recruited and supported participants in this trial at KUMC, UC an OSU.
Funding statement
This work was supported by grants from The Eunice Kennedy Shriver National Institute of Health Child Health and Human Development (NICHD) (R01HD083292) and the National Institute of Health Office of Dietary Supplements (R01HD083292-03S1). The NICHD had no role in study design, data collection, data analysis, data interpretation, or writing of this article. Life’s DHA™-S oil, DSM Nutritional Products LLC, Switzerland donated the investigational capsules for both trials but had no role in study design, data collection, data analysis, data interpretation, or writing of this article.
Abbreviations
- ADORE
Assessment of DHA On Reducing Early Preterm Birth
- DHA
docosahexaenoic acid
- EPTB
early preterm birth
- DHA
docosahexaenoic acid
- KUMC
University of Kansas Medical Center
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- ORIP
Omega-3 to Reduce the Incidence of Preterm Birth
- OSU
The Ohio State University
- PANDA
Prenatal Autonomic Neurodevelopmental Assessment
- PTB
preterm birth
- RBC-PL
red blood cell phospholipids
- UC
University of Cincinnati
- US
United States
Footnotes
Conflict of interest
SEC has received honorariums for presentations about DHA in infancy and pregnancy. SEC, BJG and CJV were PIs of R01HD083292, CJV was an employee of RB Nutrition, which produces infant formulas and supplements with DHA at the time the study was conducted, however, RB was not involved in the study execution or analysis. She conducted this study through her role as an Adjunct Professor at The University of Cincinnati. The other authors have no competing interests.
References
- 1.Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev 2018;11(11): Cd003402. doi: 10.1002/14651858.CD003402.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makrides M, Best K, Yelland L, McPhee A, Zhou S, Quinlivan J, Dodd J, Atkinson E, et al. A randomized trial of prenatal n-3 fatty acid supplementation and preterm delivery. N Engl J Med 2019;381(11):1035–45. doi: 10.1056/NEJMoa1816832. [DOI] [PubMed] [Google Scholar]
- 3.Simmonds LA, Sullivan TR, Skubisz M, Middleton PF, Best KP, Yelland LN, et al. Omega-3 fatty acid supplementation in pregnancy – baseline omega-3 status and early preterm birth: exploratory analysis of a randomised controlled trial. BJOG Int J Obstet Gynaecol 2020;127(8):975–81. doi: 10.1111/1471-0528.16168 [DOI] [PubMed] [Google Scholar]
- 4.Carlson SE, Gajewski BJ, Valentine CJ, Kerling EH, Weinger CP, Cackovic M, Buhimschi CS, Rogers LK, Sands SA, Brown AR, Mudaranthakam DP, Crawford SA, DeFranco EA. Higher dose docosahexaenoic acid supplementation during pregnancy and early preterm birth: A randomised, double-blind, adaptive-design superiority trial. EClinMed 2021; 36:100905 doi: 10.1016/j.eclinm.2021.100905. eCollection 2021 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations. Fats and fatty acids in human nutrition: Report of an expert consultation. FAO Food and Nutrition paper 91. Rome 2010. Available at http://www.fao.org/docrep/013/i1953e/i1953e00.pdf [PubMed]
- 6.Koletzko B, Cetin I, Brenna JT for the Perinatal Lipid Intake Working Group. Consensus statement-Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007;98:873–7. [DOI] [PubMed] [Google Scholar]
- 7.March of Dimes. Omega-3 fatty acids during pregnancy. March of Dimes Web Site, 2009. Available at http://www.marchofdimes.com/pnhec/159_55030.asp.
- 8.Dietary Guidelines for Americans. Available at https://www.dietaryguidelines.gov/resources/2020-2025.
- 9.American College of Obstetricians and Gynecologists. Available at https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy.
- 10.Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr 2013;97(4):808–15. doi: 10.3945/ajcn.112.050021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson SE, Gajewski BJ, Alhayek S, Colombo J, Kerling EH, Gustafson KM. Dose-response relationship between docosahexaenoic acid (DHA) intake and lower rates of early preterm birth, low birth weight and very low birth weight. Prostagalandins Leukot Essent Fatty Acids 2018; 138:1–5. doi: 10.1016/j.plea.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson SE, Gajewski BJ, Valentine CJ, Rogers LK, Weiner CP, DeFranco EA, Buhimschi CS. Assessment of DHA on reducing early preterm birth: the ADORE randomized controlled trial protocol. BMC Pregnancy Childbirth 2017;17(1):62. doi: 10.1186/s12884-017-1244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski BJ, Reese CS, Colombo J and Carlson SE. Commensurate priors on a finite mixture model for incorporating repository data in clinical trials. Stat Biopharm Res 2016; 8(2):151–60. doi: 10.1080/19466315.2015.1133453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry SM, Berry DA. Accounting for multiplicities in assessing drug safety: a three-level hierarchical mixture model. Biometrics 2004; 69(2):418–25. doi: 10.1111/j.0006-341X.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson KH, Harris WS. Harmonizing blood DHA levels in pregnancy studies: An interlaboratory investigation. Prostaglandins Leukotrienes and Essential Fatty Acids 2022; 179:102417.doi: 10.1016/j.plefa.2022.102417. [DOI] [PubMed] [Google Scholar]
- 16.Sefidkar R, Zayeri F, Kazemi E, Salehi M, Dehnad A, Hafizi M. A trend study of preterm infant mortality rate in developed and developing countries over 1990 to 2017. Iran J Public health 2021; 50:369–75. Doi: 10.18502/ijph.v50i2.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford SA, Christifano DN, Kerling EH, Gajewski BJ, Valentine CJ, Gustafson KM, Mathis NB, Camargo JT, Gibbs HD, Sullivan DK, Sands SA, Carlson SE. Validation of an abbreviated food frequency questionnaire for estimating DHA intake of pregnant women in the United States. Prostaglandins Leukot Essent Fatty Acids 2022. Feb;177:102398, doi: 10.1016/j.plefa.2022.102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christifano DN, Crawford SA, Lee G, Brown AR, Camargo JT, Kerling EH, Gajewski BJ, Valentine CJ, Gustafson KM, DeFranco EA, Carlson SE. Docosahexaenoic acid (DHA) intake estimated from a 7-question survey identifies pregnancies most likely to benefit from high-dose DHA supplementation (Clin Nutr ESPEN, 10.1016/j.clnesp.2022.12.004). [DOI] [PMC free article] [PubMed] [Google Scholar]


