Abstract
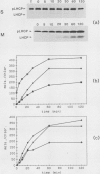
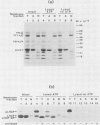
The apoprotein of the light-harvesting chlorophyll a/b protein (LHCP) is a major integral thylakoid membrane protein that is normally complexed with chlorophyll and xanthophylls and serves as the antenna complex of photosystem II. LHCP is encoded in the nucleus and synthesized in the cytosol as a higher molecular weight precursor that is subsequently imported into chloroplasts and assembled into thylakoids. In a previous study it was established that the LHCP precursor can integrate into isolated thylakoid membranes. The present study demonstrates that under conditions designed to preserve thylakoid structure, the inserted LHCP precursor is processed to mature size, assembled into the LHC II chlorophyll-protein complex, and localized to the appressed thylakoid membranes. Under these conditions, light can partially replace exogenous ATP in the membrane integration process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Cashmore A. R. Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding polypeptide. Proc Natl Acad Sci U S A. 1984 May;81(10):2960–2964. doi: 10.1073/pnas.81.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis P. R., Harel E., Kohorn B. D., Tobin E. M., Thornber J. P. Assembly of the precursor and processed light-harvesting chlorophyll a/b protein of Lemna into the light-harvesting complex II of barley etiochloroplasts. J Cell Biol. 1986 Mar;102(3):982–988. doi: 10.1083/jcb.102.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986 Nov 5;261(31):14804–14810. [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Darr S. C., Arntzen C. J. Reconstitution of the Light Harvesting Chlorophyll a/b Pigment-Protein Complex into Developing Chloroplast Membranes Using a Dialyzable Detergent. Plant Physiol. 1986 Apr;80(4):931–937. doi: 10.1104/pp.80.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Kohorn B. D., Thornber J. P., Tobin E. M. A chlorophyll a/b-protein encoded by a gene containing an intron with characteristics of a transposable element. J Mol Appl Genet. 1985;3(1):45–61. [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Tobin E. M. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986 Jan;5(1):9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K. J., Bell E., McIntosh L. Nuclear mutation leads to an accelerated turnover of chloroplast-encoded 48 kd and 34.5 kd polypeptides in thylakoids lacking photosystem II. EMBO J. 1985 Jul;4(7):1645–1653. doi: 10.1002/j.1460-2075.1985.tb03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Q., Jagendorf A. T. Neutral peptidases in the stroma of pea chloroplasts. Plant Physiol. 1986 Jun;81(2):603–608. doi: 10.1104/pp.81.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. B., Keller B. J., Hoober J. K. In Vitro Processing of Precursors of Thylakoid Membrane Proteins of Chlamydomonas reinhardtii y-1. Plant Physiol. 1985 Sep;79(1):108–113. doi: 10.1104/pp.79.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami E., Watanabe A. Thylakoid membranes: the translational site of chloroplast DNA-regulated thylakoid polypeptides. Arch Biochem Biophys. 1984 Dec;235(2):562–570. doi: 10.1016/0003-9861(84)90230-3. [DOI] [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]