Abstract
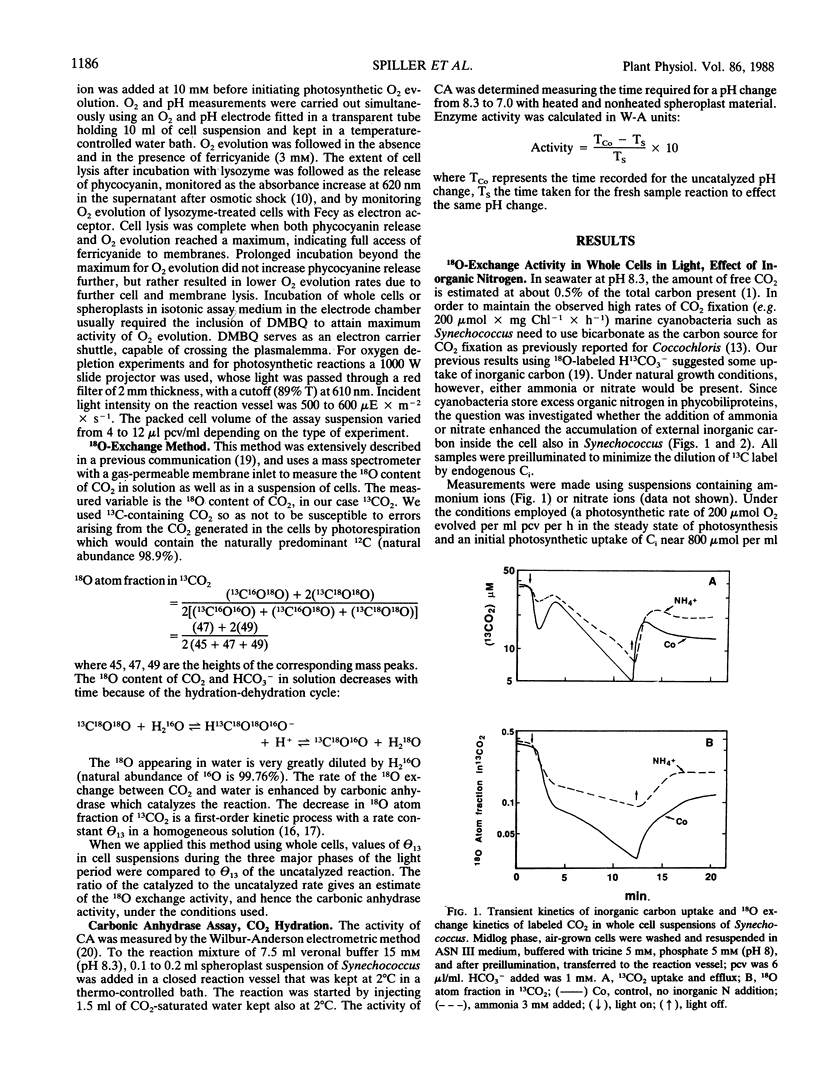
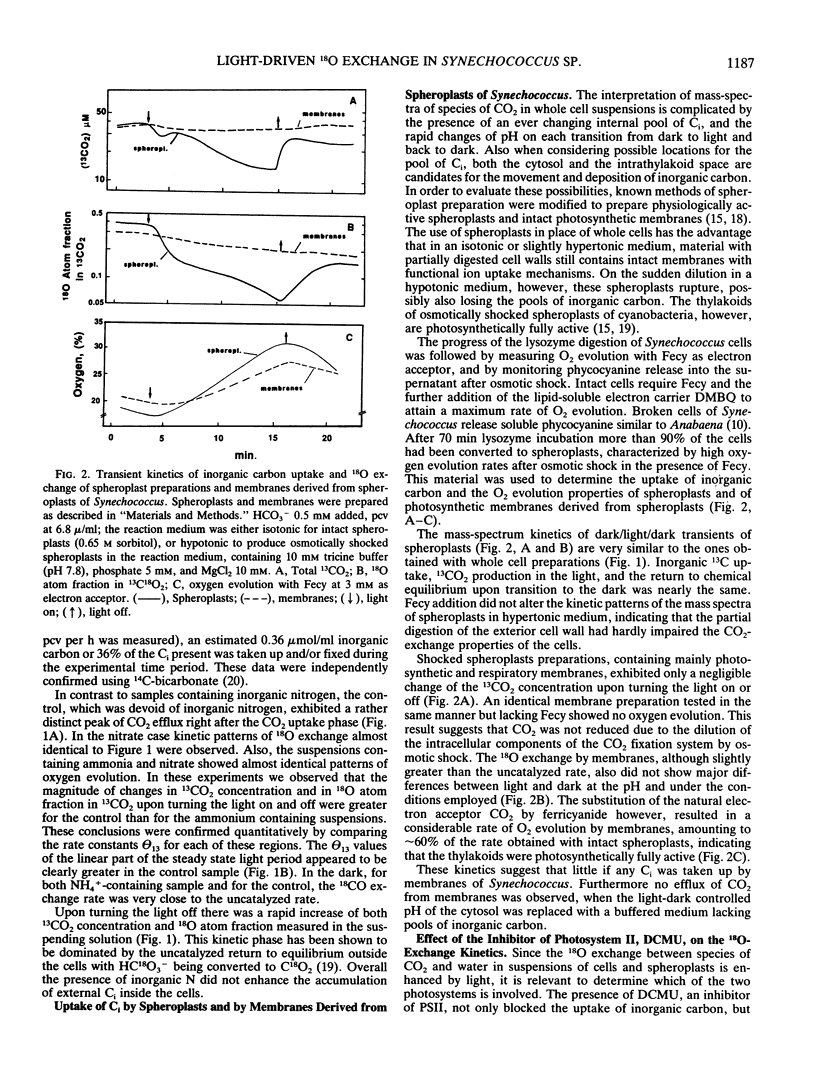
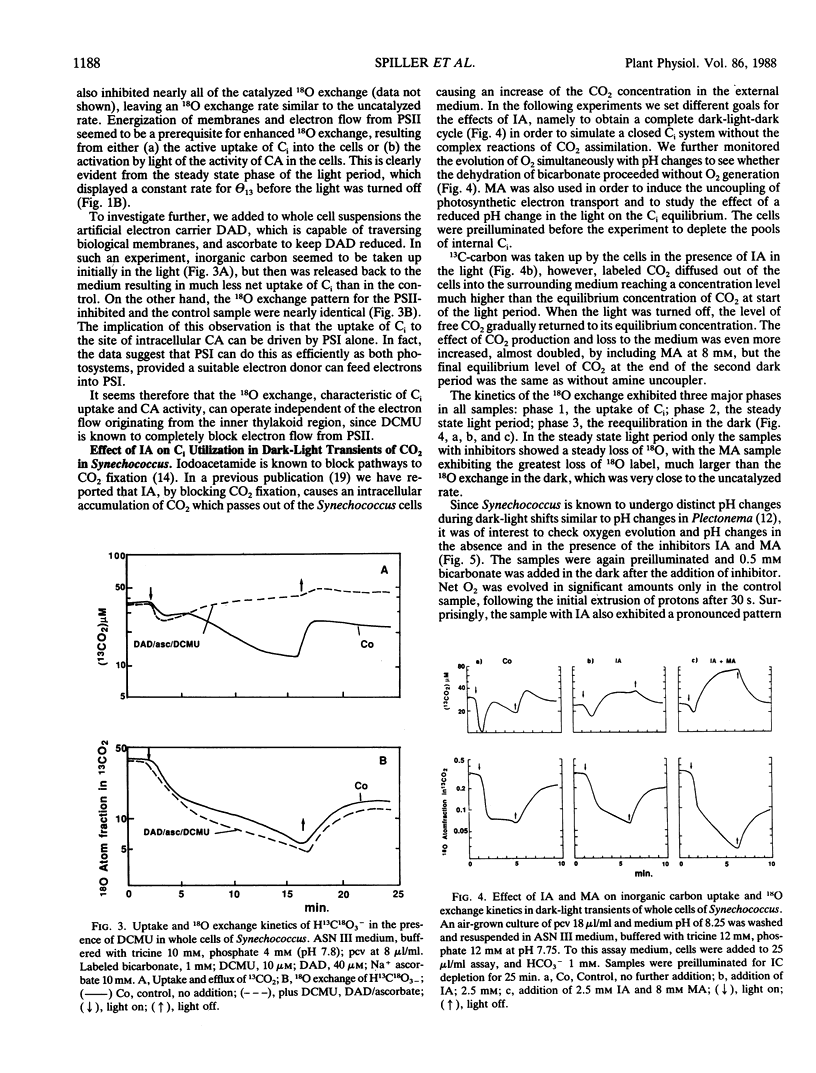
The role of the photosystems in the exchange of 18O between species of inorganic carbon and water was studied in suspensions of the cyanobacterium Synechococcus sp. (UTEX 2380) using membrane-inlet mass spectrometry. This 18O exchange is caused by the hydration-dehydration cycle of CO2 and is catalyzed by carbonic anhydrase. We observed the complex 18O exchange kinetics including dark-light-dark transients in suspensions of whole cells and found these to be identical to the 18O exchange kinetics of physiologically fully active spheroplast preparations. There was no enhancement effect of inorganic nitrogen on inorganic carbon accumulation. Membrane preparations exhibited no uptake of inorganic carbon and very little carbonic anhydrase activity, although these membranes were photosynthetically fully competent. DCMU, the inhibitor of photosystem II, eliminated almost entirely the 18O exchange activity of whole cells in the light. But this effect of DCMU could be reversed by addition of the electron donor couple 3,6-diaminodurene/ascorbate, suggesting the involvement of photosystem I in the events leading to 18O exchange. Iodoacetamide, an inhibitor of CO2 fixation, enhanced the 18O exchange in whole cell suspensions and inhibited neither the uptake of inorganic carbon nor the dehydration of bicarbonate in the light. The proton carrier carbonylcyanide m-chlorophenylhydrazone and the inhibitors diethylstilbestrol and N,N′ -dicyclohexyl carbodiimide affecting the membrane potential, totally abolished 18O exchange in the light. From 18 O-labeled inorganic carbon experiments we conclude that one of the roles of photosystem I is to provide the active uptake of inorganic carbon into the cells, where carbonic anhydrase catalyzes the interconversion between CO2 and HCO3− resulting in the 18O exchange from inorganic carbon to water.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson M. A. Inhibitors of proton pumping: effect on passive proton transport. Plant Physiol. 1986 May;81(1):55–59. doi: 10.1104/pp.81.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner G., Horner F. pH Changes in the Cytoplasm of the Blue-Green Alga Anacystis nidulans Caused by Light-dependent Proton Flux into the Thylakoid Space. Plant Physiol. 1976 Dec;58(6):717–718. doi: 10.1104/pp.58.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Fong F., Heath R. L. Carbonic anhydrase of spinach: studies on its location, inhibition, and physiological function. Plant Physiol. 1975 Mar;55(3):468–474. doi: 10.1104/pp.55.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H., Wiley C. W. Kinetics of carbonic anhydrase in whole red cells as measured by transfer of carbon dioxide and ammonia. Mol Pharmacol. 1970 Jul;6(4):430–440. [PubMed] [Google Scholar]
- Masamoto K., Nishimura M. Estimation of internal pH in cells of blue-green algae in the dark and under illumination. J Biochem. 1977 Aug;82(2):483–487. [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Energy transduction in the photosynthetic membranes of the cyanobacterium (blue-green alga) P-lectonema boryanum. J Biol Chem. 1978 May 10;253(9):3281–3286. [PubMed] [Google Scholar]
- Silverman D. N. Carbonic anhydrase: oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- Silverman D. N., Tu C. K., Roessler N. Diffusion-limited exchange of 18O between CO2 and water in red cell suspensions. Respir Physiol. 1981 Jun;44(3):285–298. doi: 10.1016/0034-5687(81)90024-4. [DOI] [PubMed] [Google Scholar]
- Spiller H. Photophosphorylation capacity of stable spheroplast preparations of anabaena. Plant Physiol. 1980 Sep;66(3):446–450. doi: 10.1104/pp.66.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]


