Abstract
A randomized, prospective, double-blind, double-dummy, multicenter study investigated the efficacy and safety of 10 days of oral therapy with grepafloxacin at 400 mg once daily, grepafloxacin at 600 mg once daily, or ciprofloxacin at 500 mg twice daily in 624 patients with acute bacterial exacerbations of chronic bronchitis. At the end of treatment, clinical success (cure or improvement) was achieved for 93% (140 of 151), 88% (137 of 156), and 91% (145 of 160) of patients in the groups receiving grepafloxacin at 400 mg, grepafloxacin at 600 mg, and ciprofloxacin, respectively (clinically evaluable population). At follow-up (14 to 28 days posttreatment), the clinical success rates were 87% (124 of 143), 81% (122 of 151), and 80% (123 of 154) in the groups receiving grepafloxacin at 400 mg and 600 mg and ciprofloxacin, respectively. A total of 379 pathogens were isolated from 290 patients, with the most common isolates being Moraxella catarrhalis (21%), Staphylococcus aureus (20%), Haemophilus influenzae (18%), and Streptococcus pneumoniae (7%). For the evaluable population, successful bacteriologic response was obtained at the end of treatment for 96% (92 of 96), 98% (87 of 89), and 92% (82 of 90) of patients receiving grepafloxacin at 400 mg, grepafloxacin at 600 mg, and ciprofloxacin, respectively, and was maintained in 86% (82 of 95), 88% (78 of 89), and 82% (69 of 84) of patients, respectively, at follow-up. All pretreatment S. pneumoniae isolates were susceptible to grepafloxacin, but two strains were resistant to ciprofloxacin. All treatments were well tolerated, with the most frequently reported drug-related adverse events being nausea, taste perversion, and headache. All drug-related adverse events in the grepafloxacin groups were mild or moderate in severity. This study demonstrates that 10-day courses of grepafloxacin given at 400 or 600 mg once daily were as effective, clinically and bacteriologically, as ciprofloxacin given at 500 mg twice daily for the treatment of acute bacterial exacerbations of chronic bronchitis.
Chronic bronchitis is a common inflammatory condition of the lower respiratory tract which afflicts more than 7 million Americans (30). Patients may experience frequent and severe acute bacterial exacerbations which are generally characterized by increased cough, dyspnea, and sputum production. The pathogens most commonly associated with acute bacterial exacerbations of chronic bronchitis (ABECB) include Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, and Haemophilus parainfluenzae (2, 19). Empiric therapy with oral antibiotics is normal practice in the treatment of this condition, but there is growing concern regarding the efficacies of currently available antibiotic therapies. In particular, there is an increasing incidence worldwide of β-lactamase-producing H. influenzae and M. catarrhalis, making β-lactam antimicrobial agents less effective, as well as an increasing incidence of S. pneumoniae strains resistant to penicillin and other antibiotics (1, 3).
Grepafloxacin is a new oral fluoroquinolone currently under investigation for the treatment of community-acquired respiratory tract infections such as ABECB. It has potent, broad-spectrum in vitro activity against organisms associated with lower respiratory tract infections, including gram-positive and gram-negative bacteria as well as intracellular organisms, such as Mycoplasma, Chlamydia, and Legionella species (19a, 23, 31). In particular, grepafloxacin has increased activity against S. pneumoniae and methicillin-susceptible Staphylococcus aureus compared with those of ciprofloxacin and other fluoroquinolones (20, 23, 27). Grepafloxacin has an extended elimination half-life of 12 h in humans (14), allowing for once-daily dosing, and is extensively distributed into respiratory tissues (11).
The aims of the present study were to compare the efficacies and safety of 10-day regimens of grepafloxacin at 400 or 600 mg once daily with ciprofloxacin at 500 mg twice daily for the treatment of patients with ABECB.
MATERIALS AND METHODS
This was a randomized, double-blind, double-dummy, comparative study performed at 33 centers throughout the United States. The study was approved by institutional review boards, and patients gave their written informed consent before participating in the study.
Patient selection.
Patients were screened for eligibility to enter the study at an initial pretreatment visit within 48 h of the start of treatment. In order to be eligible for enrollment in the study, patients had to be 18 to 80 years of age and have an established diagnosis of chronic bronchitis, defined as chronic or recurrent productive cough present on most days for a minimum of 3 months in a year and for not less than 2 successive years. Patients must have had an acute bacterial exacerbation of their chronic bronchitis, characterized by the presence of at least two of the following symptoms: an increase in the frequency and/or an increase in the severity of cough; an increase in sputum production; changes in the purulence of the sputum; an increase in chest congestion, as indicated by the presence of adventitious sounds (rales, rhonchi, and wheezes); chills and/or fever; and onset of or increase in dyspnea.
Patients were excluded from the study if they had a history of allergy to quinolone derivatives; were pregnant or breast-feeding; had respiratory tract disease or infection that required parenteral antimicrobial therapy or systemic high-dose steroids; had active bronchiectasis, pneumonia, or cystic fibrosis; had chest X-ray signs of pneumonia, abscesses, tumor, or active tuberculosis; had hepatic disease or severe renal impairment; had a history of seizures; had gastrointestinal disease that could affect drug absorption; had treatment with a quinolone or an oral nonquinolone antibiotic within the preceding 3 days or a long-acting parenteral antibiotic within the previous 4 weeks unless it was administered for less than 24 h or the patient received no more than one dose or unless the infecting organism was demonstrated to be resistant to the prior antibiotic and the patient was considered to be a treatment failure; had received treatment with another investigational drug within the preceding 4 weeks; had a pretreatment plasma theophylline level of >20 μg/ml; or were terminally ill or immunocompromised. Concomitant treatment with antimicrobial therapy other than topical or antifungal agents was prohibited. In addition, patients taking probenecid and warfarin were excluded, because it had not yet been studied whether there is an interaction between grepafloxacin and these drugs. Patients taking fluconazole and diflunisal were also excluded due to the possible interaction between quinolones and compounds with the difluorophenyl structure.
Antimicrobial therapy.
Patients were randomized to receive one of three treatments for 10 days: grepafloxacin at 400 mg once daily, grepafloxacin at 600 mg once daily, or ciprofloxacin at 500 mg twice daily. Since this study had a double-blind and double-dummy design, patients received placebo tablets and placebo capsules to maintain blinding of the medications of different dosage forms. A study medication was taken twice daily for 10 days: the morning dose comprised three tablets plus two capsules, while the evening dose was two capsules. The morning dose of study drug was taken before or with breakfast and at least 2 h before any dose of antacids or sucralfate.
Clinical and bacteriologic evaluations.
Patients were assessed on day 5 during treatment, 3 to 5 days after treatment ended (end-of-treatment visit), and 14 to 28 days after treatment ended (follow-up visit). Patients were withdrawn from the study, placed on alternative therapy, and rated as clinical failures if their clinical condition had worsened by day 3 or was unchanged by day 5.
Clinical assessments were made on the basis of changes in the frequency and/or the severity of cough; changes in sputum quality; changes in dyspnea and wheezing; and adventitious sounds, breath sound intensity, and prolongation of the expiratory phase, all of which were graded according to predefined scales. The presence or absence of chest pain and discomfort, fever, chills, and/or friction rub was also recorded.
The clinical response at the end of treatment was assessed as (i) cure (disappearance of the signs and symptoms of acute infection), (ii) improvement (a reduction in the severity and/or number of signs and symptoms of the acute infection), and (iii) failure (insufficient lessening of the signs and symptoms of acute infection). Patients whose clinical response was assessed as failure were withdrawn and started on alternative therapy. Clinical response at follow-up (14 to 28 days posttreatment) was assessed as (i) persistent resolution (condition as good as or better than that at the end of treatment), (ii) mild relapse (not quite as good as that at the end of treatment), (iii) relapse (recurrence of an acute bacterial exacerbation), and (iv) indeterminate (evaluation not possible). Clinical success was defined as cure or improvement at the end-of-treatment analysis and persistent resolution or mild relapse at follow-up.
A sputum specimen for culture was obtained before starting treatment. A Gram stain of the sputum sample (containing ≤10 epithelial cells and >25 leukocytes per low-power field) was used to determine that the specimen was adequate for microbiologic evaluation. Acceptable sputum samples were sent to a central laboratory for bacterial culture and susceptibility testing. Respiratory pathogens were identified according to the methods currently accepted by the American Society for Microbiology (5). Each species of Haemophilus, Moraxella, and Staphylococcus that was isolated and identified was tested for β-lactamase production by the cefinase disk method (Becton-Dickinson Microbiology Systems, Cockeysville, Md.). In addition, all staphylococcal isolates were tested for methicillin resistance by using 1-μg oxacillin disks according to the current standards of the National Committee for Clinical Laboratory Standards (NCCLS). MICs were determined by the broth microdilution method by procedures recommended by NCCLS (21). The susceptibilities of the organisms to grepafloxacin were determined by using the following tentative breakpoint concentrations: Haemophilus spp., ≤0.06 μg/ml (28); S. pneumoniae, ≤1.0 μg/ml (16); other organisms, ≤2.0 μg/ml (breakpoint for intermediate susceptibility, 4.0 μg/ml; breakpoint for resistance, ≥8.0 μg/ml) (4). The susceptibilities of the organisms (except S. pneumoniae) to ciprofloxacin were determined by using NCCLS-approved breakpoints (22). For Haemophilus spp. and other organisms, the approved ciprofloxacin breakpoint for susceptibility is ≤1.0 μg/ml (breakpoint for intermediate susceptibility, 2.0 μg/ml; breakpoint for resistance, ≥4.0 μg/ml). In the absence of NCCLS-approved ciprofloxacin breakpoints for S. pneumoniae, the susceptibilities of isolates of this organism were evaluated by using the approved breakpoints for other organisms.
The bacteriologic response at the end of treatment and at follow-up was assessed as follows: (i) eradication (eradication of causative organism from the sputum after therapy was stopped or within 3 to 5 days of completing treatment); (ii) presumed eradication (absence of culture material for evaluation because patient was clinically improved and no adequate sputum was produced); (iii) persistence (presence of causative organism at the end of therapy or within 5 days posttreatment); (iv) presumed persistence (the need for additional antimicrobial therapy due to continued infection in the absence of microbiological data); (v) superinfection (presence of a new organism during or at the end of therapy or within 5 days posttreatment with the new organism judged to be causing a new pulmonary infection documented by clinical findings); (vi) relapse (presence of the causative organism in the sputum and clinical symptoms after one negative culture); (vii) eradication with reinfection (elimination of the initial infecting pathogen followed by replacement with a new species or a new serotype or biotype of the same organism in a culture of a specimen after the completion of therapy; reinfection was documented by clinical findings); (viii) colonization (a positive sputum specimen culture not associated with clinical signs and symptoms of an ABECB, an increase in bacterial numbers in sputum, and the absence of increased numbers of leukocytes in a Gram stain of the sputum were considered to be colonization and not representative of actual infection); or (ix) indeterminate (not evaluable). If more than one pretreatment pathogen was identified, the bacteriologic response was assessed separately for each pathogen.
Safety evaluations.
At each visit, clinical laboratory tests (chemistry, hematology, and urinalysis) were performed on blood and urine samples, and adverse events were assessed for severity, frequency, outcome, and relationship to a study drug.
For patients concomitantly receiving theophylline, plasma theophylline levels were monitored by the Acculevel Theophylline Test (Syntex Medical Diagnostics, Palo Alto, Calif.). In patients in whom theophylline levels were 10 to 20 μg/ml at enrollment, the theophylline dose was reduced by 50%. If theophylline levels were above 20 μg/ml, the patient was excluded from enrollment in the study. Once the patient was in the study, if the theophylline level rose above 20 μg/ml, the dose was adjusted as clinically indicated.
Statistical analysis.
The Cochran-Mantel-Haenszel test was used to compare the differences between each grepafloxacin group and the ciprofloxacin group for patient demographic and pretreatment baseline characteristics (intent-to-treat population) and individual signs and symptoms of infection in patients with a documented bacterial infection. The bacteriologic success rate, based on the number of pathogens, was calculated at the end of treatment and at follow-up.
The equivalence of each grepafloxacin group in comparison with the ciprofloxacin group was assessed by calculating the two-tailed 95% confidence intervals (CIs) for the difference in outcomes by the Mantel-Haenszel method. Equivalence was established if the 95% CI crossed zero and the lower bound of the CI was within −10% for response rates of >90%, within −15% for response rates of 80 to 89%, and within −20% for response rates of <80%. The treatment-by-center interaction was assessed by the Breslow-Day test.
RESULTS
A total of 624 patients entered the study; 207 of these patients were randomized to receive 10 days of treatment with grepafloxacin at 400 mg once daily, 204 patients were randomized to receive grepafloxacin at 600 mg once daily, and 213 patients were randomized to receive ciprofloxacin at 500 mg twice daily. Five hundred fifteen patients completed the study, and 109 patients were withdrawn prematurely (Table 1). Fewer patients in the group receiving grepafloxacin at 400 mg (4%) than in the group receiving grepafloxacin at 600 mg (6%) or the group receiving ciprofloxacin (9%) were withdrawn due to a lack of response. Significantly fewer patients in the group receiving grepafloxacin at 400 mg than in the group receiving ciprofloxacin were withdrawn for this reason (P = 0.037). Twenty patients were withdrawn due to adverse events, and 19 of these patients discontinued the study medication. A total of 24 patients were withdrawn for other reasons, with the most common being the use of other antibiotics or other protocol-prohibited medications. No patient was withdrawn from the study as a consequence of treatment-emergent laboratory abnormalities. During the posttreatment period, three patients in each of the grepafloxacin groups and eight patients in the ciprofloxacin group were withdrawn due to relapses.
TABLE 1.
Reasons for withdrawal
| Reason for withdrawal | No. (%) of patients
|
|||
|---|---|---|---|---|
| Grepafloxacin
|
Ciprofloxacin at 500 mg twice daily (n = 213) | Overall (n = 624) | ||
| 400 mg once daily (n = 207) | 600 mg once daily (n = 204) | |||
| Lack of responsea | 8 (4) | 13 (6) | 20 (9) | 41 (7) |
| Adverse event | 6 (3) | 8 (4) | 6 (3) | 20 (3) |
| General unwillingness to participate | 2 (1) | 4 (2) | 2 (1) | 8 (1) |
| Lost to follow-up | 4 (2) | 4 (2) | 1 (0.5) | 9 (1) |
| Noncomplianceb | 2 (1) | 1 (0.5) | 4 (2) | 7 (1) |
| Other | 10 (5) | 7 (3) | 7 (3) | 24 (4) |
| Total | 32 (16) | 37 (18) | 40 (19) | 109 (18) |
Assessed as treatment failures.
Defined as failure to take at least 80% of the first 7 days of medication or failure to report for at least three of the four required during-treatment and posttreatment clinic visits.
All 624 patients who entered the study were included in the intent-to-treat population for safety analysis. The demographic and baseline characteristics of the patients in the three treatment groups in the intent-to-treat population were comparable (Table 2). The overall mean age of the patients was 47.6 years (range, 18 to 82 years), and the majority of patients (88%) were Caucasian. The number of nonsmokers was unusually high compared with the number usually seen among patients with chronic bronchitis. Many of the patients were receiving concomitant medication, including central nervous system agents (58%), autonomic drugs (46%), hormones and synthetic substitutes (31%), and cardiovascular drugs (26%).
TABLE 2.
Baseline and demographic characteristics of patients (intent-to-treat population)
| Characteristic | Grepafloxacin at 400 mg once daily | Grepafloxacin at 600 mg once daily | Ciprofloxacin at 500 mg twice daily |
|---|---|---|---|
| No. of patients | 207 | 204 | 213 |
| Race (no. [%] of patients) | |||
| Caucasian | 179 (86) | 180 (88) | 189 (89) |
| Black | 25 (12) | 21 (10) | 21 (10) |
| Other | 2 (1) | 3 (2) | 3 (1) |
| Sex (no. [%] of patient) | |||
| Male | 93 (45) | 100 (49) | 96 (45) |
| Female | 113 (55) | 104 (51) | 117 (55) |
| Mean ± SD age (yr [range]) | 47.7 ± 15.3 (18–82) | 48.4 ± 18.0 (18–77) | 46.8 ± 14.7 (18–80) |
| Mean ± SD wt (kg) | 80.5 ± 20.8 | 79.6 ± 18.7 | 80.2 ± 21.5 |
| Smoking habits (no. [%] of patients) | |||
| Nonsmoker | 48 (23) | 40 (20) | 39 (18) |
| Ex-smoker | 61 (30) | 55 (27) | 52 (24) |
| Current smoker | 97 (47) | 109 (53) | 122 (57) |
A total of 379 pretreatment pathogens were isolated from the 290 patients in the modified intent-to-treat population. The most common isolates were M. catarrhalis (21%), S. aureus (20%), H. influenzae (18%), S. pneumoniae (7%), and H. parainfluenzae (6%). These commonly isolated pathogens appeared to be similarly distributed among the three treatment groups. Eighty-five percent of M. catarrhalis isolates and 30% of H. influenzae isolates produced β-lactamase. S. aureus was the second most common pretreatment pathogen, and all S. aureus isolates were susceptible to methicillin. The frequency of occurrence of S. aureus appeared to be particularly high in this study, but this may have been related to the criteria used to determine the acceptability of sputum samples. Although the proportion of infections caused by S. pneumoniae was relatively low, the incidence of recovery of this organism varies from year to year, and rates of pneumococcal infection similar to those found in the present study have been observed in other studies (17, 24). In addition, recovery of S. pneumoniae from transported clinical samples is relatively difficult. Bacteria other than H. influenzae, S. pneumoniae, and M. catarrhalis were considered pathogens if they were present on Gram staining and/or were the predominant organism cultured. Since in many cases S. aureus is associated with the presence of another organism, its true pathogenesis was difficult to ascertain for many of the patients. All pretreatment isolates with the exception of 20 organisms from 18 patients were susceptible to both grepafloxacin and ciprofloxacin; the exceptions included strains of Pseudomonas aeruginosa, H. parainfluenzae, and Stenotrophomonas maltophilia, which demonstrated resistance or intermediate susceptibility to grepafloxacin and/or ciprofloxacin, and two strains of S. pneumoniae, which were resistant to ciprofloxacin but susceptible to grepafloxacin.
Clinical response.
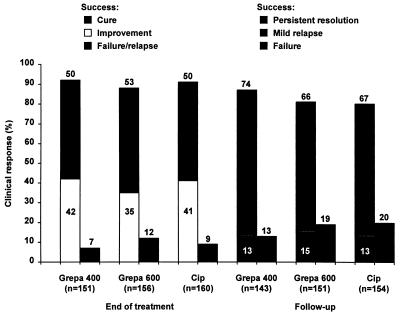
The clinical responses of the patients in the three treatment groups at the end of treatment and at follow-up are summarized in Fig. 1. Of the 467 clinically evaluable patients for whom efficacy data were available at the end of treatment, 93% of patients in the group receiving grepafloxacin at 400 mg, 88% of patients in the group receiving grepafloxacin at 600 mg, and 91% of patients in the group receiving ciprofloxacin were considered clinical successes (cure or improvement). The 95% CI confirmed the equivalence of the groups receiving grepafloxacin at 400 mg and ciprofloxacin (95% CI = −4.5 and 8.9%), the groups receiving grepafloxacin at 600 mg and ciprofloxacin (95% CI = −10.0 and 4.4%), and the groups receiving grepafloxacin at 400 and 600 mg (95% CI = −1.9 and 12.3%). None of the comparisons of cured or improved subcategories of response demonstrated significant differences, and the confidence intervals demonstrated equivalence. At the follow-up visit (14 to 28 days posttreatment), clinical success (persistent resolution or mild relapse) was demonstrated for 87% of patients in the group receiving grepafloxacin at 400 mg, 81% of patients in the group receiving grepafloxacin at 600 mg, and 80% of patients in the group receiving ciprofloxacin. Once again, the 95% CI confirmed the equivalence of the groups receiving grepafloxacin at 400 mg and ciprofloxacin (95% CI = −1.7 and 15.4%), the groups receiving grepafloxacin at 600 mg and ciprofloxacin (95% CI = −7.5 and 10.3%), and the groups receiving grepafloxacin at 400 and 600 mg (95% CI = −2.7 and 14.3%).
FIG. 1.
Clinical response at the end of treatment and at follow-up (14 to 28 days posttreatment) for patients treated with grepafloxacin (Grepa) at 400 mg once daily (n = 151 and 143, respectively), grepafloxacin at 600 mg once daily (n = 156 and 151, respectively), or ciprofloxacin (Cip) at 500 mg twice daily (n = 160 and 154, respectively) (clinical efficacy evaluable population).
(i) Clinical responses of patients with pretreatment pathogens exhibiting possible resistance to either study drug.
Six pathogens with resistance or intermediate susceptibility to grepafloxacin were isolated from four patients subsequently randomized to grepafloxacin treatment. P. aeruginosa alone was isolated from two of these patients, P. aeruginosa plus S. maltophilia was isolated from one patient, and P. aeruginosa plus H. parainfluenzae was isolated from one patient. All four of these patients had successful clinical outcomes at the end of treatment, although one patient infected only with P. aeruginosa had a relapse by the follow-up visit, at which time P. aeruginosa was again isolated. The bacteriologic responses of the other three patients were as follows: for the patient infected with P. aeruginosa only, success; for the patient infected with P. aeruginosa and S. maltophilia, failure and success, respectively; and for the patient infected with P. aeruginosa and H. parainfluenzae, failure and success, respectively. However, it should be noted that the roles of both P. aeruginosa and S. maltophilia in ABECB are unclear. Ciprofloxacin-resistant S. pneumoniae strains were isolated from two patients, one of whom was subsequently randomized to treatment with grepafloxacin at 600 mg and one of whom was randomized to treatment with ciprofloxacin. Both of these patients had successful clinical outcomes at the end of treatment and at follow-up, although the patient treated with ciprofloxacin was classified as a bacteriologic failure at follow-up.
(ii) Clinical signs and symptoms of acute infection.
The individual clinical signs and symptoms of acute infection (cough severity; breath sound intensity; prolongation of expiratory phase; sputum color, viscosity, and thickness; wheezing severity; chest pain; and chills) were all improved at the end of treatment and at follow-up, and these improvements were comparable for the patients in the three treatment groups (data not shown). Patients in all three treatment groups exhibited a reduction in the mean sputum purulence by the end of treatment, and this continued through the follow-up period.
Bacteriologic response.
At the end of treatment, bacteriologic success rate for the microbiologically evaluable patients (eradication, presumed eradication, and eradication plus contamination or colonization) was 96% for the group receiving grepafloxacin at 400 mg, 98% for the group receiving grepafloxacin at 600 mg, and 92% for the group receiving ciprofloxacin (Table 3). The 95% CI confirmed the equivalence of the groups receiving grepafloxacin at 400 mg and ciprofloxacin (95% CI = −3.7 and 13.0%), the groups receiving grepafloxacin at 600 mg and ciprofloxacin (95% CI = −2.1 and 15.0%), and the groups receiving grepafloxacin at 400 and 600 mg (95% CI = −9.2 and 5.6%). The bacteriologic success rates at follow-up remained high for all three treatment groups (86, 88, and 82%, respectively; Table 3). Equivalence was seen between the groups receiving grepafloxacin at 400 mg and ciprofloxacin (95% CI = −5.9 and 15.3%) and between the groups receiving grepafloxacin at 600 mg and ciprofloxacin (95% CI = −4.8 and 17.4%).
TABLE 3.
Bacteriologic response at the end of treatment and at follow-up in microbiologically evaluable patients treated with grepafloxacin (400 or 600 mg once daily) or ciprofloxacin (500 mg twice daily)
| Time and response | Grepafloxacin at 400 mg once daily | Grepafloxacin at 600 mg once daily | Ciprofloxacin at 500 mg twice daily |
|---|---|---|---|
| End-of-treatment | |||
| Total no. of evaluable pathogens | 96 | 89 | 90 |
| Success (no. [%] of patients) | 92 (96) | 87 (98) | 82 (92) |
| Eradication | 11 (12) | 17 (19) | 9 (10) |
| Presumed eradication | 77 (80) | 68 (76) | 73 (81) |
| Eradication + contamination or colonization | 4 (4) | 2 (2) | 0 |
| Reinfection | 0 | 0 | 0 |
| Failure (no. [%] of patients) | 4 (4) | 2 (2) | 8 (9) |
| Presumed persistence | 3 (3) | 2 (2) | 3 (3) |
| Persistence | 0 | 0 | 1 (1) |
| Relapse | 1 (1) | 0 | 4 (4) |
| Follow-up | |||
| Total no. of evaluable pathogens | 95 | 89 | 84 |
| Success (no. [%] of patients) | 82 (86) | 78 (88) | 69 (82) |
| Eradication | 4 (4) | 12 (14) | 2 (2) |
| Presumed eradication | 74 (78) | 62 (70) | 63 (75) |
| Eradication + contamination or colonization | 4 (4) | 4 (5) | 1 (1) |
| Reinfection | 0 | 0 | 3 (4) |
| Failure (no. [%] of patients) | 13 (14) | 11 (12) | 15 (18) |
| Presumed persistence | 10 (11) | 6 (7) | 11 (13) |
| Persistence | 1 (1) | 0 | 0 |
| Relapse | 2 (2) | 5 (6) | 4 (5) |
Bacteriologic response for individual pathogens.
The bacteriologic success rates at the end of treatment and at follow-up (Table 4) for H. influenzae, H. parainfluenzae, M. catarrhalis, and S. aureus isolates were similar among the three treatment groups. At 3 to 5 days posttreatment, the success rate for S. pneumoniae was 94% for patients treated with grepafloxacin (for both groups combined) and 60% for those receiving ciprofloxacin. At 2 to 4 weeks posttreatment these figures were reduced to 81 and 50%, respectively. Thus, the bacteriologic success rate for S. pneumoniae appeared to be lower for the ciprofloxacin group than for either grepafloxacin group at both time points, although the number of pneumococcal isolates detected in this study was too small for this to be clinically meaningful. Two of the three pneumococcal strains isolated from ciprofloxacin-treated patients who failed bacteriologically at follow-up were susceptible to the drug (MICs, 1 μg/ml), and one was classified as resistant (MIC, 4 μg/ml). All of the pathogens found to be resistant to grepafloxacin pretreatment, with the exception of the three strains of P. aeruginosa described above, were successfully eradicated. Clinical efficacy did not always correlate with bacteriologic outcome or in vitro susceptibility. This is not unusual and may be related to other factors such as host response and possibly patient-to-patient variation in the absorption and distribution of drug.
TABLE 4.
Bacteriologic success at follow-up for individual pathogens in microbiologically evaluable patients treated with grepafloxacin (400 or 600 mg once daily) or ciprofloxacin (500 mg twice daily)
| Pretreatment pathogen | No. of pathogens successfully treated/total no. of pathogens (%)
|
||
|---|---|---|---|
| Grepafloxacin at 400 mg once daily | Grepafloxacin at 600 mg once daily | Ciprofloxacin at 500 mg twice daily | |
| H. influenzae | 17/19a (90) | 15/16 (94) | 15/18 (83) |
| β-Lactamase negative | 10/11 (91) | 11/11 (100) | 12/12 (100) |
| β-Lactamase positive | 7/7 (100) | 4/5 (80) | 3/6 (50) |
| H. parainfluenzae | 4/4 (100) | 4/4 (100) | 5/5 (100) |
| M. catarrhalis | 18/20a (90) | 18/21 (86) | 15/16 (94) |
| β-Lactamase negative | 4/4 (100) | 1/1 (100) | 4/4 (100) |
| β-Lactamase positive | 13/15 (87) | 17/20 (85) | 11/12 (92) |
| S. aureus | 11/15 (73) | 14/17 (82) | 14/17 (82) |
| S. pneumoniae | 6/8 (75) | 7/8 (88) | 2/5 (40) |
One H. influenzae isolate and one M. catarrhalis isolate were not tested for β-lactamase production.
Adverse events.
All patients who took study drugs were included in safety analyses. All three treatment regimens were well tolerated. The most frequently reported drug-related treatment-emergent adverse events were nausea, taste perversion, headache, dizziness, diarrhea, and vomiting (Table 5). Nausea was considered drug related for 102 of 114 (89%) patients who reported it, although it was mild to moderate for 88% of patients. The incidence was significantly greater for the group receiving grepafloxacin at 600 mg than for the group receiving ciprofloxacin (P = 0.014). Taste perversion (usually described by patients as a metallic or bitter taste) was considered drug related for all but two patients; a significantly greater incidence was observed among patients in both grepafloxacin groups than among patients in the ciprofloxacin group and in the group receiving grepafloxacin at 600 mg than in the group receiving grepafloxacin at 400 mg. Headache was considered to be drug related for approximately half of the patients receiving grepafloxacin who reported it and for 80% of the patients receiving ciprofloxacin, but its incidence did not differ significantly between the treatment groups. Photosensitivity reactions were seen in only one patient in the group receiving grepafloxacin at 400 mg (1 of 207; <1%) and in 6 of 204 (3%) patients in the group receiving grepafloxacin at 600 mg (data not shown); most of these reactions were described as sun sensitivity or sunburn, and only one patient required the application of topical cream. No photosensitivity reactions were reported among the patients in the ciprofloxacin group. The incidence of photosensitivity reactions was significantly greater in the group receiving grepafloxacin at 600 mg than in the group receiving ciprofloxacin (P = 0.033).
TABLE 5.
Most frequently reported drug-related treatment-emergent adverse eventsa
| Adverse event | No. (%) of patients
|
||
|---|---|---|---|
| Grepafloxacin at 400 mg once daily (n = 207) | Grepafloxacin at 600 mg once daily (n = 204) | Ciprofloxacin at 500 mg twice daily (n = 213) | |
| Nausea | 30 (15) | 45 (22%)b | 27 (13%)b |
| Taste perversion | 26 (13)c,d | 54 (27%)d,e | 8 (4%)c,e |
| Headache | 16 (8) | 16 (8) | 23 (11) |
| Dizziness | 17 (8) | 19 (9) | 12 (6) |
| Diarrhea | 12 (6) | 14 (7) | 14 (7) |
| Vomiting | 4 (2)f | 18 (9)f | 9 (4) |
P values are provided only where significant differences were observed.
P = 0.014.
P = 0.001.
P < 0.001.
P < 0.001.
P = 0.002.
Eight serious adverse events were reported by six patients. One patient in the group receiving grepafloxacin at 400 mg reported cholecystitis and two patients in the group receiving grepafloxacin at 600 mg reported depression, but none of these adverse events was considered to be drug related. Of the three patients in the ciprofloxacin group with serious adverse events, one each reported dyspnea (of unknown relationship to the study drug), asthma (not drug related), and dyspnea with rhinitis and pain (not drug related). No deaths were reported in this study.
A total of 26 patients discontinued the study medication due to drug-related adverse events, but not all patients were excluded from further analyses: 7 of 207 (3.4%) patients in the group receiving grepafloxacin at 400 mg, 15 of 204 (7.4%) patients in the group receiving grepafloxacin at 600 mg and 4 of 213 (1.9%) patients in the group receiving ciprofloxacin. This difference was statistically significant when the groups receiving grepafloxacin at 600 mg, and ciprofloxacin are compared (P = 0.015). The most frequent causes of discontinuation were nausea in 14 of 26 patients (3 in the group receiving grepafloxacin at 400 mg, 8 in the group receiving grepafloxacin at 600 mg, and 3 in the group receiving ciprofloxacin), vomiting in 8 of 26 patients (1 in the group receiving grepafloxacin at 400 mg, 4 in the group receiving grepafloxacin at 600 mg, and 3 in the group receiving ciprofloxacin), taste perversion in 7 of 26 patients (2 in the group receiving grepafloxacin at 400 mg, and 5 in the group receiving grepafloxacin at 600 mg), dizziness in 6 of 26 patients (3 in each of the grepafloxacin groups), and diarrhea in 4 of 26 patients (2 in the group receiving grepafloxacin at 400 mg group and 1 each in the groups receiving grepafloxacin at 600 mg and ciprofloxacin).
(i) Clinical laboratory tests and vital signs.
No unique or unexpected laboratory findings were seen. Treatment with grepafloxacin or ciprofloxacin resulted in slight decreases in neutrophil and leukocyte counts (consistent with improvement of infection) and increases in platelet counts and creatinine and uric acid concentrations. The observed changes were consistent with expected laboratory results for quinolones. No clinically meaningful differences were seen between treatment groups in the mean change from pretreatment values for temperature, systolic or diastolic blood pressure, pulse rate, or respiratory rate.
(ii) Plasma theophylline concentration.
The concomitant administration of grepafloxacin and theophylline appeared to be well tolerated in the 71 patients who received both drugs. Six patients exhibited a plasma theophylline concentration of ≥20 μg/ml in the first 15 days of the study: two patients in the group receiving grepafloxacin at 400 mg and four patients in the group receiving grepafloxacin at 600 mg. Adverse experiences were reported by three patients: one patient in the group receiving grepafloxacin at 600 mg (the patient had a theophylline concentration of 24.9 μg/ml and reported nausea) and two patients in the group receiving grepafloxacin at 400 mg (one patient had a theophylline concentration of 23.0 μg/ml and reported anorexia, insomnia, somnolence, and nausea, and the other patient had a theophylline concentration of 20.3 μg/ml and reported back pain and taste perversion). None of the adverse events was considered serious, and none of the serious side effects associated with theophylline concentrations of ≥20.0 μg/ml were observed. None of the 23 patients receiving ciprofloxacin and theophylline exhibited a plasma theophylline concentration of ≥20.0 μg/ml.
DISCUSSION
The primary goals of antimicrobial therapy in patients with ABECB are to resolve the infection as quickly as possible and to keep the patient free of any further exacerbation for as long as possible. Ineffective therapy not only leads to high failure rates but also may contribute to shorter infection-free periods, longer periods of morbidity, and a progressive decline in lung function (7). This damage is mediated by toxins produced by the infecting organism and by the host response to infection in patients who have impaired mucociliary clearance (10).
The results of this study indicate that 10-day courses of grepafloxacin at 400 or 600 mg once daily are as effective as ciprofloxacin at 500 mg twice daily in the treatment of ABECB. Both grepafloxacin and ciprofloxacin treatments gave high clinical success rates at the end of treatment (88 to 93 and 91%, respectively). More importantly, clinical success was maintained for up to 4 weeks after the end of treatment for 81 to 87 and 80% of patients receiving grepafloxacin and ciprofloxacin, respectively. The 600-mg dose of grepafloxacin was associated with a higher incidence of adverse events than either the 400-mg dose of grepafloxacin or ciprofloxacin. This may have contributed to the slightly lower clinical response rate observed in the group receiving grepafloxacin at 600 mg because, as adverse events increase, patients are less likely to report “improvement.” It was not reflected in the bacteriological response rate, which was similar for patients receiving both grepafloxacin doses.
The most common pathogens isolated from patients enrolled in the present study were M. catarrhalis, S. aureus, H. influenzae, S. pneumoniae, and H. parainfluenzae, which is generally consistent with previous reports describing the most common organisms associated with ABECB (2, 19), the exception being the higher incidence of S. aureus seen in the present study. Only 6 of 379 pretreatment pathogens were resistant to ciprofloxacin or grepafloxacin. Two strains of S. pneumoniae were resistant to ciprofloxacin, but all isolates were susceptible to grepafloxacin.
Ciprofloxacin is one of the most extensively studied quinolones for the treatment of respiratory tract infections. In a previous study with patients with ABECB, ciprofloxacin given orally at 750 mg twice daily for 14 days resulted in clinical cure for all patients evaluated. Relapses occurred for 15% of the patients and were due to H. influenzae and S. pneumoniae, although ciprofloxacin exhibited good antimicrobial activity against the major respiratory pathogens both at the end of therapy and posttherapy (9). A lower dose of ciprofloxacin (500 mg twice daily) also resulted in clinical cure, although the relapse rate, again due to S. pneumoniae and H. influenzae, was higher (21%) (8). In a more seriously ill group of patients, ciprofloxacin given orally at 500 mg twice daily for 10 days resulted in a clinical cure rate of 70%, although 1 week after follow-up this had decreased to 50% (12). The results reported for ciprofloxacin in the present study are consistent with those reported in similar previous studies.
In the present study the overall bacteriologic success rates for grepafloxacin and ciprofloxacin were equivalent at the end of treatment and at 14 to 28 days posttreatment. The differences in bacteriologic failure rates between the groups receiving grepafloxacin at 400 and 600 mg at the end of treatment (4 versus 2%) and at follow-up (14 versus 12%) were not significant. S. pneumoniae eradication rates appeared to be better for grepafloxacin than ciprofloxacin both at the end of treatment and at follow-up, although the number of pneumococcal isolates detected in this study was very small. This trend may be a reflection of the difference in in vitro activity against S. pneumoniae between ciprofloxacin and grepafloxacin (25, 30). The in vitro spectrum of grepafloxacin also includes penicillin-resistant isolates, which are increasing in prevalence worldwide (1). Recent data indicate that between 9 and 10% of isolates in the United States are resistant to penicillin (13), and in some states, the incidence of resistant pneumococci is nearly 30% (6).
In recent years, extensive efforts have been made to develop new fluoroquinolones with improved activities against gram-positive organisms, such as S. pneumoniae and S. aureus, and with better pharmacokinetic profiles. Grepafloxacin appears to meet both of these objectives, having superior activity compared to that of ciprofloxacin against gram-positive organisms (18, 20, 23), extensive penetration into respiratory tissues (11), and a longer elimination half-life (12 h), allowing for once-daily administration (14).
Since good patient compliance with therapy is a key factor in the successful treatment of any infection, once-daily treatment with antibiotics such as grepafloxacin may have compliance advantages over twice-daily treatment with agents such as ciprofloxacin (15).
The ability to tolerate both study drugs was comparable among the treatment groups. The most common adverse events associated with the use of fluoroquinolones are gastrointestinal disturbances such as nausea, diarrhea, dyspepsia, and abdominal pain; skin reactions such as rash; and blood effects such as decreased leukocyte or neutrophil counts (26). In the present study, grepafloxacin demonstrated a safety profile similar to that reported for other fluoroquinolones and appeared to be well tolerated. The most common drug-related adverse events in patients treated with grepafloxacin were nausea, taste perversion, and headache. The majority of events were mild to moderate in severity and required no treatment. There were few serious adverse events in the present study, and none was considered to be related to the study medication.
In conclusion, this study has shown that grepafloxacin given orally at 400 or 600 mg once daily is as effective, both clinically and bacteriologically, as ciprofloxacin given orally at 500 mg twice daily for the treatment of patients with ABECB. The once-daily dosing schedule required for grepafloxacin may have advantages in terms of compliance. The potential advantages of grepafloxacin over ciprofloxacin against S. pneumoniae remain to be confirmed in studies with larger numbers of patients in whom this is the pathogen causing infection.
ACKNOWLEDGMENTS
The participation of the following investigators in this study is gratefully acknowledged: V. Acampora, Cherry Hill, N.J.; J. M. Adelgrass, Carrollton, Tex.; J. Anders, Champaign, Ill.; S. Bowman, Clearwater, Fla.; S. Bradshaw, Daly City, Calif.; F. Branditz, Zanesville, Ohio; R. M. Champion, Birmingham, Ala.; D. Cheung, Indianapolis, Ind.; G. Collins, Charlotte, N.C.; C. A. DeAbate, Metairie, La.; J. E. Gaidry, San Diego, Calif.; J. Grady, Boulder, Colo.; B. Harwood, Vancouver, Wash.; S. Heatley, Redwood City, Calif.; P. d’Hemecourt, Bethesda, Md.; K. Humphries, Dallas, Tex., and Fort Worth, Tex.; R. P. Miller, Roseville, Calif.; M. Noonan, Portland, Oreg.; P. Poirier, Phoenix, Ariz.; J. Ravi, Orlando, Fla.; W. Reeves, Atlanta, Ga.; J. Salisbury, N.J.; R. Santiago, San Antonio, Tex.; C. Scott, South Euclid, Ohio; W. M. Shapiro, San Diego, Calif.; W. Sloan, Jr., Atlanta, Ga.; B. Sklar, Alameda, Calif.; R. Tanaka, Modesto, Calif.; D. J. Thompson, Wauwatosa, Wis.; and Y. Zakkay, Tampa, Fla.
This study was supported by the Otsuka Pharmaceutical Company.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Ball, P. 1995. Epidemiology and treatment of chronic bronchitis and its exacerbations. Chest 108(Suppl.):43–52. [DOI] [PMC free article] [PubMed]
- 3.Baquero, F., and E. Loza. 1994. Antibiotic resistance of microorganisms involved in ear, nose, and throat infections. Pediatr. Infect. Dis. J. 13(Suppl. 1):9–14. [DOI] [PubMed]
- 4.Barry A L. Program and abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. In vitro activities of OPC-17116 and five other fluoroquinolones, abstr. 779; p. 240. [Google Scholar]
- 5.Bartlett J G, Ryan K J, Smith T F, Wilson W R. Cumitech 7A, Laboratory diagnosis of lower respiratory tract infections. Coordinating ed., J. A. Washington II. Washington, D.C: American Society for Microbiology; 1987. [Google Scholar]
- 6.Block S, Hendrick J, Wright P, Finger R, Leggiadro R, Appleton M, et al. Drug-resistant Streptococcus pneumoniae—Kentucky and Tennessee. Morbid Mortal Weekly Rep. 1993;43:23–25. [PubMed] [Google Scholar]
- 7.Chodosh, S. 1987. Acute bacterial exacerbations in bronchitis and asthma. Am. J. Med. 82(Suppl. 4A):154–163. [PubMed]
- 8.Chodosh, S. 1991. Use of quinolones for the treatment of acute exacerbations of chronic bronchitis. Am. J. Med. 91(Suppl. 6A):93S–100S. [DOI] [PubMed]
- 9.Chodosh, S., J. Tuck, K. D. Stottmeier, and D. Pizzuto. 1989. Comparison of ciprofloxacin with ampicillin in acute infectious exacerbations of chronic bronchitis. Am. J. Med. 87(Suppl. 5A):107S–112S. [DOI] [PubMed]
- 10.Cole P. Evaluating the clinical outcomes of respiratory infection. Int J Antimicrob Agents. 1993;3:S15–S19. doi: 10.1016/0924-8579(93)90031-y. [DOI] [PubMed] [Google Scholar]
- 11.Cook P J, Andrews J M, Wise R, Honeybourne D, Moudgil H. Concentrations of OPC-17116, a new fluoroquinolone antibacterial, in serum and lung compartments. J Antimicrob Chemother. 1995;35:317–326. doi: 10.1093/jac/35.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Davies B I, Maesen F P V, Baur C. Ciprofloxacin in the treatment of acute exacerbations of chronic bronchitis. Eur J Clin Microbiol. 1986;5:226–231. doi: 10.1007/BF02013995. [DOI] [PubMed] [Google Scholar]
- 13.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30 center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efthymiopoulos, C., S. L. Bramer, and A. Maroli. Pharmacokinetics of grepafloxacin after oral administration of single and repeat doses in healthy young males. Clin. Pharm. 33(Suppl. 1):1–8. [DOI] [PubMed]
- 15.Eisen S A, Miller D K, Woodward R S, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881–1884. [PubMed] [Google Scholar]
- 16.Fuchs P C, Barry A L, Brown A D. Tentative interpretive criteria for testing the susceptibility of Streptococcus pneumoniae to eight fluoroquinolones. Diagn Microbiol Infect Dis. 1996;26:23–27. doi: 10.1016/s0732-8893(96)00161-7. [DOI] [PubMed] [Google Scholar]
- 17.Henry D, Ruoff G E, Rhudy J, Puopolo A, Drehobl M, Schoenberger J, Giguere G, Collins J J. Effectiveness of short-course therapy (5 days) with cefuroxime axetil in treatment of secondary bacterial infections of acute bronchitis. Antimicrob Agents Chemother. 1995;39:2528–2534. doi: 10.1128/aac.39.11.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imada T, Miyazaki S, Nishida M, Yamaguchi K, Goto S. In vitro and in vivo antibacterial activities of a new quinolone, OPC-17116. Antimicrob Agents Chemother. 1992;36:573–579. doi: 10.1128/aac.36.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson J A. Pathogenesis of bacterial infections of the respiratory tract. Br J Biomed Sci. 1995;52:157–161. [PubMed] [Google Scholar]
- 19a.Kenny G E, Cartwright F D. Susceptibilities of Mycoplasma hominis, Mycoplasma pneumoniae, and Ureaplasma urealyticum to a new quinolone, OPC17116. Antimicrob Agents Chemother. 1993;37:1726–1727. doi: 10.1128/aac.37.8.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marco F, Jones R N, Hoban D J, Pignatari A C, Yamane N, Frei R. In-vitro activity of OPC-17116 against more than 6000 consecutive clinical isolates: a multicentre international study. J Antimicrob Chemother. 1994;33:647–654. doi: 10.1093/jac/33.3.647. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, 5th informational supplement, document M100-S5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, 6th informational supplement, document M100-S6. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 23.Neu H C, Fang W, Gu J-W, Chin N-X. In vitro activity of OP-17116. Antimicrob Agents Chemother. 1992;36:1310–1315. doi: 10.1128/aac.36.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notario G, Siepman N, Devcich K, Guay D R P. Comparative safety and efficacy of clarithromycin and three oral cephalosporins in the treatment of outpatients with bacterial bronchitis. Curr Ther Res. 1993;54:628–640. [Google Scholar]
- 25.Pankuch G A, Jacobs M R, Appelbaum P C. Activity of CP 99,219 compared with DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J Antimicrob Chemother. 1995;35:230–232. doi: 10.1093/jac/35.1.230. [DOI] [PubMed] [Google Scholar]
- 26.Paton J H, Reeves D S. Adverse reactions to the fluoroquinolones. Adv Drug React Bull. 1992;153:575–578. [Google Scholar]
- 27.Sader H S, Erwin M E, Jones R N. In vitro activity of OPC-17116 compared to other broad-spectrum fluoroquinolones. Eur J Clin Microbiol Infect Dis. 1992;11:372–381. doi: 10.1007/BF01962081. [DOI] [PubMed] [Google Scholar]
- 28.Sewell D L, Barry A L. Tentative criteria for determining the in vitro susceptibilities of Haemophilus influenzae, including quality control parameters to two fluoroquinolones (grepafloxacin and PD 131628) Diagn Microbiol Infect Dis. 1995;21:175–179. doi: 10.1016/0732-8893(95)00021-2. [DOI] [PubMed] [Google Scholar]
- 29.Skudlarska B A, Scott G C. The role of antibiotics in the management of acute exacerbations of chronic bronchitis (answers to some commonly asked questions) Mol Med. 1992;89:289–293. [PubMed] [Google Scholar]
- 30.Spangler S K, Jacobs M R, Pankuch G A, Appelbaum P C. Susceptibility of 170 penicillin-susceptible and penicillin-resistant pneumococci to six oral cephalosporins, four quinolones, desacetylcefotaxime, Ro 23-9429 and RP 67829. J Antimicrob Chemother. 1993;31:273–280. doi: 10.1093/jac/31.2.273. [DOI] [PubMed] [Google Scholar]
- 31.Wise R, Andrews J M, Brenwald N. The in vitro activities of OPC-17116, a new 5-methyl substituted quinolone. J Antimicrob Chemother. 1993;31:487–504. doi: 10.1093/jac/31.4.497. [DOI] [PubMed] [Google Scholar]