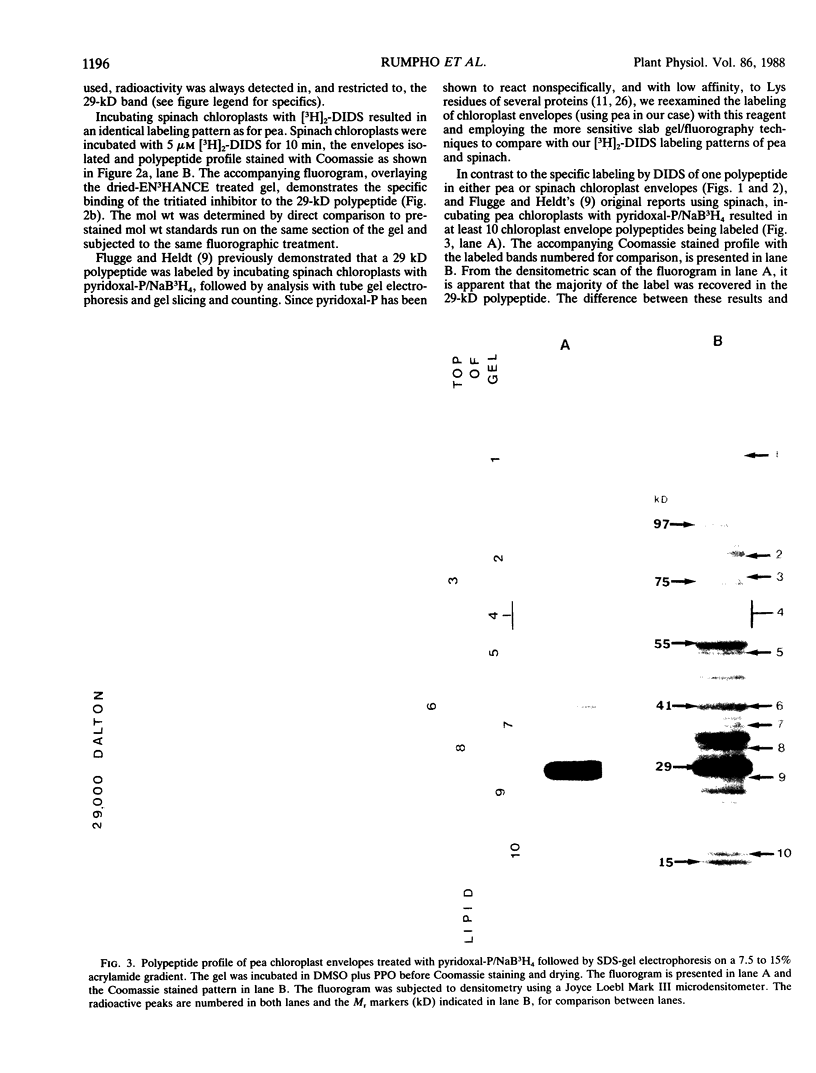
Abstract
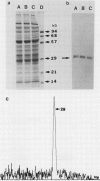
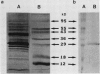
The phosphate translocator protein of C3 and C4 mesophyll chloroplast envelopes was specifically labeled using the anion exchange inhibitor, 1,2-ditritio-1,2-(2,2′ -disulfo-4,4′ -diisothiocyano) diphenylethane ([3H]2-DIDS). Intact mesophyll chloroplasts were isolated from the C3 plants, Spinacia oleracea L. (spinach) and Pisum sativum L. (pea), and the C4 plant, Zea mays L. (corn). Chloroplasts were incubated with 5 to 50 μm [3H]2-DIDS and, in addition, pea chloroplasts were also incubated with pyridoxal phosphate/tritiated sodium borohydride. The chloroplasts were washed, the envelopes isolated and solubilized. Following sodium dodecyl sulfate polyacrylamide gel electrophoresis, label from bound [3H]2-DIDS was detected only in the 28- to 30-kilodalton protein (proposed C3 phosphate translocator) for both C3 and C4 chloroplasts, as demonstrated by fluorography. In contrast, when pyridoxal phosphate/tritiated sodium borohydride was used to label pea chloroplasts, radioactivity was detected in several other bands in addition to the 29-kilodalton polypeptide. These findings suggest that DIDS is a much more specific inhibitor than reagents previously employed to study the phosphate translocator and could be used to isolate and characterize the differences in the C3 and C4 phosphate translocator protein(s).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. I. Electrophoretic and immunochemical analyses. J Biol Chem. 1983 Nov 10;258(21):13273–13280. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Hatch M. D. Transport of 3-phosphoglyceric acid, phosphoenolpyruvate, and inorganic phosphate in maize mesophyll chloroplasts,, and the effect of 3-phosphoglyceric acid on malate and phosphoenolpyruvate production. Arch Biochem Biophys. 1981 Oct 15;211(2):743–749. doi: 10.1016/0003-9861(81)90511-7. [DOI] [PubMed] [Google Scholar]
- Falke J. J., Chan S. I. Molecular mechanisms of band 3 inhibitors. 1. Transport site inhibitors. Biochemistry. 1986 Dec 2;25(24):7888–7894. doi: 10.1021/bi00372a015. [DOI] [PubMed] [Google Scholar]
- Flügge U. I., Heldt H. W. Specific labelling of a protein involved in phosphate transport of chloroplasts by pyridoxal-5'-phosphate. FEBS Lett. 1977 Oct 1;82(1):29–33. doi: 10.1016/0014-5793(77)80878-8. [DOI] [PubMed] [Google Scholar]
- Hamasaki N., Matsuyama H., Hirota-Chigita C. Characterization of phosphoenolpyruvate transport across the erythrocyte membrane. Evidence for involvement of band 3 in the transport system. Eur J Biochem. 1983 May 16;132(3):531–536. doi: 10.1111/j.1432-1033.1983.tb07394.x. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Transport in C4 mesophyll chloroplasts: evidence for an exchange of inorganic phosphate and phosphoenolpyruvate. Biochim Biophys Acta. 1977 Dec 23;462(3):603–612. doi: 10.1016/0005-2728(77)90104-9. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Anderson M. P., Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein. Localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem. 1986 Jul 5;261(19):9002–9010. [PubMed] [Google Scholar]
- Keifer D. W., Franceschi V. R., Lucas W. J. Plasmalemma Chloride Transport in Chara corallina: Inhibition by 4,4'-Diisothiocyano-2,2'-Disulfonic Acid Stilbene. Plant Physiol. 1982 Nov;70(5):1327–1334. doi: 10.1104/pp.70.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Compans R. W., Reich E. A specific labeling procedure for proteins on the outer surface of membranes. J Biol Chem. 1972 Oct 25;247(20):6432–6437. [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E. Characterization of 4,4'-Diisothiocyano-2,2'-disulfonic Acid Stilbene Inhibition of 3-Phosphoglycerate-Dependent O(2) Evolution in Isolated Chloroplasts : Evidence for a Common Binding Site on the C(4) Phosphate Translocator for 3-Phosphoglycerate, Phosphoenolpyruvate, and Inorganic Phosphate. Plant Physiol. 1985 Jul;78(3):537–544. doi: 10.1104/pp.78.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E. Inhibition of 3-Phosphoglycerate-Dependent O(2) Evolution by Phosphoenolpyruvate in C(4) Mesophyll Chloroplasts of Digitaria sanguinalis (L.) Scop. Plant Physiol. 1984 Nov;76(3):711–718. doi: 10.1104/pp.76.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. G., Brailsford M. A., Beechey R. B. Identification of the phosphate-translocator from maize mesophyll chloroplasts. Biochem Biophys Res Commun. 1987 Feb 27;143(1):164–169. doi: 10.1016/0006-291x(87)90645-0. [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M., Cline K., Keegstra K. Analysis of pea chloroplast inner and outer envelope membrane proteins by two-dimensional gel electrophoresis and their comparison with stromal proteins. Plant Physiol. 1983 Nov;73(3):569–575. doi: 10.1104/pp.73.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Woldegiorgis G., Voss S., Shrago E., Werner-Washburne M., Keegstra K. An adenine nucleotide-phosphoenolpyruvate counter-transport system in C3 and C4 plant chloroplasts. Biochem Biophys Res Commun. 1983 Nov 15;116(3):945–951. doi: 10.1016/s0006-291x(83)80233-2. [DOI] [PubMed] [Google Scholar]