HIGHLIGHTS
-
•
We conducted a systematic review of reviews and assessment of causality.
-
•
Most evidence was from observational studies.
-
•
Physical activity is inversely related to incident depression and anxiety.
-
•
Depression and anxiety are probably causally related to physical inactivity.
Keywords: Physical activity, anxiety, depression, mental health, risk factor
Abstract
Introduction
Globally, depressive and anxiety disorders are the leading contributors to mental ill health. Physical activity reduces symptoms of depression and anxiety and has been proposed as an adjunct treatment therapy for depression and anxiety. Prospective studies suggest that physical activity may reduce the incidence of depression and anxiety. We conducted a systematic review of reviews with the aim to provide a comprehensive overview of available epidemiologic evidence on the strength of the association between physical activity and incident cases of depression and anxiety and to assess the likelihood of these associations being causal.
Methods
We searched Embase and PubMed databases for systematic reviews published between January 1, 2000 and March 19, 2020 that reported findings on the strength of association between physical activity and incidence of depression and anxiety. We updated this search to October 15, 2022. Two reviewers independently assessed the methodologic quality of the included reviews using the Assessment of Multiple Systematic Reviews rating scale. We carried out a narrative synthesis of the evidence. We used the Bradford Hill criteria to assess the likelihood of associations being causal.
Results
The initial search yielded 770 articles, of which 4 remained for data extraction. Two of the included reviews were scored as high quality, and 2 were scored as low quality. From the 2 included reviews that reported pooled estimates, people with high physical activity levels were found to have a decreased risk of incident depression (adjusted RR=0.83, 95% CI=0.76, 0.90) and reduced odds of developing anxiety (adjusted OR=0.74,95% CI=0.62, 0.88) when compared with those with low physical activity levels. We assessed physical activity to be probably causally related to both depression and anxiety.
Discussion
Our evidence is drawn from systematic reviews of observational data. Further high-quality studies, such as randomized control trials, would help to strengthen the evidence base of the associations between physical activity and depression and anxiety. Nonetheless, our findings provide empirical support for the consideration of physical activity in strategies for the prevention of mental ill health.
Graphical Abstract
INTRODUCTION
Globally, in 2019, mental disorders were the seventh leading cause of disability attributing to 970 million prevalent cases, depressive and anxiety disorders being the leading contributors to this burden.1 It is estimated that lost productivity because of anxiety and depression costs the global economy $1 trillion each year.2 People experiencing severe mental health conditions also die up to 2 decades prematurely owing to preventable physical conditions.3,4 The coronavirus disease 2019 (COVID-19) pandemic has probably made matters worse.5,6
Staying active regularly has been identified as essential for good mental health.7,8 Specifically, physical activity (PA) reduces the symptoms of depression and anxiety9,10 and has been proposed as an adjunct treatment therapy for depression and anxiety.11, 12, 13, 14 From a preventive approach, prospective studies suggest that PA may reduce the incidence of depression and anxiety.15,16 A comprehensive review of this body of literature could help determine the best estimates of the measure of strength of the associations between PA and incidence of depression and anxiety. Previous systematic reviews of reviews and meta-analyses have focused on the topic of PA and mental health.9,17,18 One review investigated the relationship between PA and multiple physical or mental health outcome in adults aged ≥60 years,17 but the authors only included 1 study that focused on our outcomes (incident depression only).19 A second study identified and summarized findings from systematic reviews examining PA and depression, anxiety, and self-esteem outcomes in children and youth.9 However, they did not present measures of the strength of the associations. Moreover, all primary studies that they included examined PA and reduction of symptoms of existing depression and anxiety. The third is an update of a previous review in which Biddle and colleagues18 present evidence on PA and mental health and assess the extent to which associations can be considered causal. Their review is restricted to children and adolescents, and the investigation is not specific to incident cases of depression and anxiety because it includes reduction of symptoms of existing symptoms. Our study focuses on a comprehensive overview of the measures of strength of the associations between PA and incident cases of depression and anxiety across all ages and assesses the likelihood of these associations being causal. If the association between PA and depression and anxiety is confirmed and judged to be causal, this would support the adoption of PA in strategies for the prevention of mental ill health globally. In addition, estimates of the strength of these associations can be used in cost-effectiveness studies that model the impact of measures that increase PA on mental health outcomes.
Globally, it is estimated that 27.5% of adults do not meet the WHO PA guidelines,20,21 and people living in socioeconomically disadvantaged communities tend to be less active.22,23 Combined with the large burden of mental ill health, this implies that even a modest effect of PA on depression and anxiety could result in a significant benefit to health and health equity from interventions that improve the levels of PA.
This study was part of a broader project commissioned by the New South Wales Ministry of Health, Australia, to value the health benefits of active transport. We developed the New South Wales Active Transport Health model and sought to include all relevant health outcomes that have sufficiently strong epidemiologic evidence of an association with active transport. Against this background, we conducted this systematic review of reviews with the aim to provide a comprehensive overview of the available epidemiologic evidence on the strength of the association between incident cases of depression, anxiety, and PA; to assess the likelihood of these associations being causal; and if justified, to derive estimates that can be used in studies estimating the health benefits of PA and active transport.
METHODS
Our study protocol took a 2-step approach: first, a systematic review of reviews was conducted to establish the available epidemiologic evidence on the strength of the association between PA and incident cases of depression and anxiety, and second, if an association was found, an assessment of the likelihood of these associations being causal was performed (Appendix Figure 1, available online). The protocol was not submitted for peer reviewed publication or registration. However, it was prepared before carrying out the systematic review of reviews.
Search Strategy and Inclusion Criteria
We carried out our search in Embase and PubMed, 2 leading health and social science databases. The search terms covered the 2 concept areas: PA/active transport and mental health problems (Table 1). The search strategy was informed by guidelines for systematic reviews.24 We prepared the review protocol according to the PRISMA-P 2015 statement.25 The search strategy followed an iterative process. It was developed in consultation with a research librarian and tested and modified in PubMed and Embase. The medical subject heading search terminology in PubMed was used to inform the selection of search terms. We also reviewed the reference lists from included reviews for suitable reviews that met the inclusion criteria. We selected the reviews on the basis of predefined inclusion and exclusion criteria agreed upon by 4 reviewers (MNW, HM, FH, and JLV) (Table 2).
Table 1.
Search Terms Used in the Systematic Review of Reviews
| Exposure and health outcome search terms |
|---|
| (“physical activity” OR “physical exercise” OR walking OR bicycling OR “active transport”) AND (“mental health” OR “mental disorders” OR anxiety OR “anxiety disorder*” OR depression OR “depressive disorder*”) |
Table 2.
Inclusion and Exclusion Criteria
| Criteria |
|---|
| Participants/population: the review included studies reporting results representative of whole populations who did not have any of the study outcomes (depression and anxiety) at baseline and covering either all ages, multiple age categories across all ages (children, youth, as well as adults), or specific age groups so long as they are representative of the whole population at those ages. Study designs: systematic reviews with or without meta-analyses Exposure: reviews reporting findings on physical activity (physical activity) as exposure Outcomes: reviews reporting findings on incident cases of depression and anxiety as outcomes. Publication status: peer reviewed studies published or in print with full text publicly available. Timeline: reviews published in the year 2000 to March 19, 2020. We later updated our search to October 15, 2022. Language: English Reviews that provided risk estimates (RR, hazard ratio, or OR) with CIs or SEs or the data to calculate these. |
Study Selection
The identified studies were imported to EndNote x9 software. We removed the duplicate records. After the study selection process, the full-text documents of reviews meeting the inclusion criteria were retrieved and saved into EndNote.
MNW ran the search and identified records from PubMed and Embase databases. HM ran the same search for confirmation. MNW screened the titles and abstracts of identified reviews on the basis of the inclusion and exclusion criteria (Table 1). This screening was checked by 2 reviewers (HM and JLV). Two authors (MNW and HM) then screened the full texts of reviews that met the inclusion criteria after screening of title and abstract. The final list of reviews was discussed and agreed upon by all authors. The reasons for exclusion of reviews after full-text review were documented.
Data Extraction
Four reviewers (MNW, HM, FH, and JLV) achieved consensus on which data to extract from the included reviews. One reviewer (MNW) extracted data from the full texts. Two other reviewers (HM and JLV) cross-checked the data extraction variables. The main areas of data extraction from each included review are shown in Appendix Table 1 (available online).
Data Synthesis
We carried out a narrative synthesis of the evidence and report our systematic review of reviews in adherence with the Preferred Reporting Items for Overviews of Reviews guideline.26 In our initial protocol, a modified version of GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) guidelines27 was proposed for reporting, but this was updated to Preferred Reporting Items for Overviews of Reviews. We graded the evidence to support a judgment of a causal relationship using the World Cancer Research Fund grading system28 that is informed by the Bradford Hill criteria.27,29 This criteria has been used elsewhere to assess for causal associations between PA and mental health in children and adults18 and in the Global Burden of Disease studies.30 We adopted grades of convincing (strong), probable, possible (suggestive), and insufficient evidence (Appendix Table 2, available online). Two reviewers (MNW and FH) independently assessed the methodologic quality of the included systematic reviews using the Assessment of Multiple Systematic Reviews rating scale.31 Differences were resolved through discussion. Ethics approval was not required for the systematic review of reviews.
Updated Search and Study Selection
The updated search was run on October 15, 2022, and the identified records were saved onto EndNote x9 software. These records were then imported to Covidence software32 where duplicates were removed, and the screening process was carried out by MNW. This was checked and discussed with JLV.
RESULTS
Study Selection
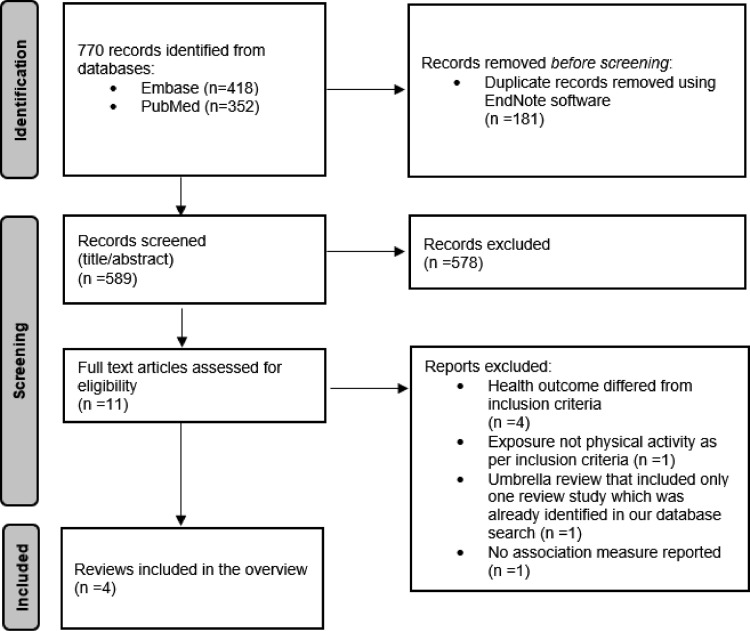
We identified 770 articles in the initial database search (418 records from Embase and 352 records from PubMed) (see Figure 1 for the PRISMA33 flow diagram and Appendix Table 3, available online, for the search algorithm). A total of 589 records remained after removing duplicates. After screening of title and abstract, 578 records were excluded, and 11 remained for full-text analysis. Seven studies were further excluded with reason after full-text screening (Appendix Table 4, available online), leaving a total of 4 studies for data extraction.19,34, 35, 36
Figure 1.
PRISMA flow diagram.
The results from the updated October 15, 2022 search are not included in our initial overview. We report the results separately and explain this in the results and discussion sections.
Study Characteristics
We provide details of the characteristics of included studies in Table 3.19,34, 35, 36 In summary, 3 included studies conducted systematic reviews with meta-analyses,19,35,36 and 1 conducted a systematic review.34 All included studies reported both male and female participants. All the 4 included evidence from prospective cohort studies only, and almost all the primary cohort studies were from high income countries. All included reviews reported primary evidence for adults and children, but 2 studies did not provide specific details on age.19,36 Apart from 1 review that did not report participant numbers,34 the included reviews covered large samples (ranging from 80,000 to 266,939).19,35,36 Follow-up time for primary studies in 1 review ranged from 1 to 27 years,34 and the other 3 reported average follow-up time ranging from 3.5 to 7.4 years.19,35,36 Nearly all primary studies included in the 4 reviews used self-reported PA measures. As indicated in Table 3, the included reviews reported a variety of measures used to assess the outcomes: depression and anxiety. We assessed the included primary studies in each of the 4 reviews for the degree of overlap and report our findings in Table 4.19,34, 35, 36
Table 3.
A Summary of the Characteristics of Included Reviews
| Review | Search dates | Study design of primary studies | Total person years of follow-up/participants | Follow-up time | Location of primary cohort studies as reported | Average age of participants (years) | Sex | Physical activity measurement | Measure of health outcome investigated | Covariates included in multivariable adjusted estimates | Risk of bias tool used in each review | Assessment of publication bias in each review | Statistical heterogeneity | AMSTAR assessment score for each review |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mammen and Faulkner34 | January 1976 to December 2012 | Prospective cohort studies | Not stated. Harvest plot presents lowest category of study sample size as <1,000 subjects and highest as >10,000 subjects. | Reported: ranged from 1 to 27 years | Majority of studies in North America (n=14) and Europe (n=13). The study SF tables included the following countries: Sweden, Norway, Finland, Australia, Netherlands, U.S. | No age restriction reported. The study SF tables indicated population ages from 11 years to 100 years | Mixed (20 studies), female only (5 studies), male only (4 studies) | Subjective physical activity measures of aerobic activity. Only one study objectively measured physical activity via ergometer cycling | Majority of studies: validated measures such as Center for Epidemiologic Studies Depression Scale (CESD); other studies DSM-IV. More direct measures: via physician diagnosis (n=3); hospital discharge register (n=2); or use of antidepressants (n=1) | Under the study quality assessment, authors indicate the studies that assessed for confounding variables (n=11). In discussion, 3 low quality primary studies are reported as not accounting for BMI and social economic status | Critical Appraisal Skills Programme | Narrative | A harvest plot | Low |
| Schuch et al.19 | Database inception to October 18, 2017 | Prospective cohort studies | n=266,939 | person-years 1,837,794 | Average of 7.4 years | Australia, Norway, Germany, U.S., Iceland, Canada, Ghana, India, Mexico, Russia, South Korea, England, Italy, UK, Netherlands, Sweden, Taiwan, Japan, Denmark, Korea, Spain, | Average age not stated. No age restriction reported. Results table shows age group at baseline as "adults, children, adolescents, older adults." | Mixed: median proportion of males across studies, 47% | Self-report questionnaire, such as the International Physical Activity Questionnaire, single or multiple questions on participation in exercise, sports, or physical activity. Only one study objectively measured physical activity via pedometers | Semi-structured diagnostic instruments or self-reported physician diagnosis of depression | Analysis for adjusted and unadjusted measures conducted separately. In subgroup analyses, adjusted for age and sex, BMI, Smoking, and baseline depressive symptoms | Newcastle-Ottawa scale. | Begg and Mazumdar and Egger tests and corrected for this using the Duval and Tweedie trim and fill method | Q and I2 Scores of 25% (low heterogeneity), 25%–50% (moderate), and >50% (high) |
High |
| McDowell et al.35 | Database inception to June 2018 | Prospective cohort studies | >80,000 unique individuals | Median follow-up, 4.75 years From their results table, least follow-up time= 1 year, longest follow-up time, 16 years |
Europe (n=18), Australia (n=2), North America (n=2), Asia (n=2), | A total of 43.7 (25.6–48.4) years. Two studies covered only children or adolescents | Mixed: median proportion of female participants =54.5% (49%–61%) | Self-report measure assessed at baseline alone (n=18) or as change over time (n=6) | Anxiety symptoms (n=8), a screening level for anxiety symptoms indicative of a disorder (n=10), self-reported diagnosis of anxiety disorder (n=1), and diagnosis of an anxiety disorder (n=5) | Analyses of AORs and unadjusted ORs from cohort studies were run separately. Covariates included in multivariable adjusted estimated not stated by the review authors | Q-Coh | Not reported | Q statistic and I2 | Low |
| Schuch et al.36 | Database inception to October 10, 2018 | Prospective cohort studies | n=75,831 | 357,424 person–years | Median of 3.5 years (IQR=2.0–6.5) | Germany, Australia, UK, Sweden, Korea, U.S., Ireland, Spain, The Netherlands, | Average age not stated. No age restriction reported. Results table shows age group at baseline as "adults, children, adolescents, older adults." | Mixed: median males=50.1% | No study used an objective measure to evaluate physical activity | Structured or semistructured diagnostic instruments or self‐reported physician diagnosis of anxiety disorders (n=10 studies), and cut‐offs of anxiety screening instruments (n=4) | Analysis for adjusted OR and OR were conducted separately. In subgroup analyses, adjusted for age and sex, BMI and smoking, | Newcastle‐Ottawa scale | Begg and Mazumdar and Egger tests and corrected through the Duval and Tweedie trim and fill method | Q and I2 statistic Scores of 25% (low heterogeneity), 25%–50% (moderate), and >50% (high) |
High |
Note: The search was conducted on March 19, 2020. The search terms, algorithm, and identified records are reported in Appendix Table 3 (available online).
AMSTAR, Assessment of Multiple Systematic Reviews; UK, United Kingdom.
Table 4.
Overlap of Primary Studies Across Reviews
| Outcome | Review | Search dates | Number of included primary studies | Number of overlapping primary studies between reviews |
|---|---|---|---|---|
| Depression | Mammen and Faulkner34 | January 1976–December 2012 | 30 | 14 |
| Schuch et al.19 | Database inception to October 18, 2017 | 49 | ||
| Anxiety | McDowell et al.35 | Database inception to June 2018 | 24 | 10 |
| Schuch et al.36 | Database inception to October 10, 2018 | 14 |
The search was conducted on March 19, 2020. The search terms, algorithm and identified records are reported in Appendix Table 3 (available online)
Methodologic Quality of the Included Systematic Reviews
Using Assessment of Multiple Systematic Reviews criteria,31 we scored the methodologic quality for 2 of the included reviews as high19,36 and 2 as low34,35 (Table 3). The independent assessment results from the 2 reviewers are presented in Appendix Table 5 (available online).
Review Findings
The Association Between Physical Activity and Depression
Mammen and Faulkner34 included prospective, longitudinal design articles examining the relationships between PA and depression with a minimum of 1 year follow-up. They included only primary studies that excluded individuals with depression at baseline.
They found a significant inverse relationship between baseline PA and depression at follow-up in 25 of the 30 primary studies included in their review. For instance, compared with not engaging in PA, engaging in PA for up to 150 minutes/week was associated with 8%–63% decreased risk of future depression (n=3 studies), whereas engaging in >150 minutes/week was associated with 19%–27% decreased risk of future depression (n=3 studies). Most primary studies that found a protective role were rated of high or modest methodologic quality. The use of inconsistent self-reported measures of PA between primary studies prevented Mammen and Faulkner34 from examining the dose–response relationships between PA and depression. However, they presented a narrative overview of findings from 7 primary studies that reported dose–response relationships, of which 5 were of high quality. For instance, their findings show that 1 high-quality primary study found that as little as 10–29 minutes of daily PA was preventive in the onset of depression (RR=0.90). Furthermore, higher levels of daily PA were associated with further decreases in the risk of developing depression (60–90 minutes/day, RR=0.84; >90 minutes/day, RR=0.80). The review authors considered this as promising evidence that shows that any level of PA, including low levels, can prevent future depression. In addition, the authors report that 4 primary studies concluded that the protective effect of PA on depression is specific to women and girls. Of their 30 included primary studies, 5 revealed null findings for the association between PA and depression. Of these 5, only 1 was considered high quality. They attributed the low and modest scores to the failure to control for significant covariates that have been linked with depression, such as body mass and SES. They discussed that in 4 of these studies, the lack of precision of estimating PA dosage might have explained the null findings. The authors discuss that gray research literature and studies not in English language were excluded, thus findings may be due to publication bias.34
Our second included review was a meta-analysis of prospective cohort studies.19 Authors found a significant inverse relationship between baseline PA and depression at follow-up. They included a total of 49 primary studies with an average follow-up time of 7.4 years. Only primary studies that had at least 1 year of follow-up were included. Their pooled estimates showed that people with high levels of PA had decreased risks of developing depression compared with people with low levels of PA (adjusted RR=0.83, 95% CI=0.76, 0.90, I2=0.00 and adjusted OR=0.83, 95% CI=0.79, 0.88, I2=0.00). The protective PA effect was seen for people of all ages (youths, working-age adults, elderly persons). Their findings suggested that the potential protective association of PA for men is similar to that for for women.19 In their subgroup analyses, they showed that the protective effects of PA were found in primary studies in which the different aspects of PA (intensity, frequency, volume) were measured individually or when 2 or more aspects (metabolic equivalents/composite) were considered. Correction for publication bias slightly reduced the associations they reported, but the association remained significant (adjusted OR=0.85; 95% CI=0.81, 0.89; adjusted RR=0.86; 95% CI=0.78, 0.96).
In all subgroup analyses, Schuch and Vancampfort19 found protective effects for adults and older persons, but they reported mixed findings in their subgroup analyses of primary studies specific for children/adolescents (adjusted OR=0.91, 95% CI=0.84, 0.99, I2=0.00 [3 cohorts], crude OR=0.50, 95% CI=0.21, 1.20, I2=0.00 [2 cohorts] and crude RR/hazard ratio=0.54, 95% CI=0.25, 1.15, I2=0.00 [2 cohorts]). The authors explored heterogeneity using subgroup and meta-regression analysis. They found that none of the investigated moderators significantly explained the variance of the effects of PA on depression onset in any of their analyses.
In their review, the definitions of low or high PA as well as the aspects of PA that were captured by each instrument (intensity, frequency, volume, or ≥2) varied widely between the included primary studies. These limitations prevented the review from establishing a minimum or an optimal dosage of PA necessary to decrease the odds of incident depression. Nonetheless, the authors considered the available evidence to support the conclusion that PA can offer protection against the emergence of depression. Although significant publication bias was found in their analyses, the authors indicate that adjusting for this did not change the magnitude of the associations that they found.19
Our assessment of the evidence from the 2 included reviews against Bradford Hill's criteria for causality27,29 suggested that depression is probably causally related to physical inactivity (Table 5).
Table 5.
Assessing the Evidence Against Causal Criteria
| Criterion | Findings for physical activity and depression | Findings for physical activity and anxiety |
|---|---|---|
| (1) Temporality criterion: exposure preceded outcome in the included cohort studies. Inclusion of prospective based studies allowed for the examination of the temporal sequence between baseline levels of physical activity and primary prevention of depression, anxiety at follow-up. |
Mammen and Faulkner34 and Schuch et al.19 included primary results from prospective cohort studies. We considered that the evidence from these 2 reviews meets the temporality criteria where the exposure (physical activity) is seen to precede the effect (depression). | Both reviews by Schuch, Stubbs36 and McDowell and Dishman35 included only prospective cohort studies with a follow-up period of >1 year. These 2 reviews assess prospective evidence where exposure (physical activity) preceded the effect (anxiety) in time. This evidence meets the temporality criterion. |
| (2) Strength of association We used an adaptation of measures by Webb, Bain27 to guide our classification where relative risk (RR) >3.0 (<0.33) are classified as moderately strong, >5.0 (<0.2), strong. Additional measures: RR=1.5–2.9 (0.34–0.67) classified as modest and RR<1.5 (>0.67), weak association (Appendix 1, available online, and Appendix Table 2, available online). Though the strength of association facilitates assessment for possible causal relationship, a strong association is neither necessary nor sufficient for causality, and weakness is neither necessary nor sufficient for concluding absence of causality. |
The relative effects reported in the included reviews were considered weak. Weakness of an association makes the risk of alternative explanations greater but does not preclude causality. Measures of association: Mammen and Faulkner34 Out of 30 included primary studies, 25 reported a significant inverse relationship between PA and incident depression while 5 found no relationship. The point estimates for all studies are not reported. Below is a summary of estimates reported. engaging in <150 minutes/week and >150 minutes/week were associated with an 8%–63% (n=3) and 19%–27% (n=3) decreased risk of future depression, respectively. Being physically active for >240 minutes/week (n=2) and 420 minutes/week (n=1) were protective against subsequent depression. As little as 10–29 minutes (RR=0.90) of daily PA was preventive in the onset of depression and, higher levels of daily PA (60–90 minutes/day, RR=0.84; >90 minutes/day, RR=0.80) were significantly associated with decreased risk of developing depression (n=1). Engaging in PA for >30 minutes/day reduced the odds of subsequent depression by 48% (n=1). Even “low” walking levels were associated with a decreased risk of depression of up to nearly 60% (n=2). Even walking at an average pace of <20 minutes/day and >40 minutes/day was protective against depression of up to 6% and 17%, respectively (n=1). Being physically active less than twice per week was associated with an increased risk (OR=1.34) of developing depression (n=1). Being active one to 2 times/week or more than once per week was associated with a decreased risk of depression of up to 40% (n=2). Schuch et al.19 Authors reported adjusted odds ratios for 36 cohorts from the 34 unique primary studies that provided this data. Of these, a total of 32 cohorts reported point estimates <1 and 4 reported point estimates >1. Below are the estimates from the meta-analysis: Compared with people with low levels of physical activity, those with high levels had lower odds of developing depression (AOR=0.83,95% CI=0.79,0.88, I2=0.00, (n=36); OR=0.59, 95% CI=0.51, 0.68, I2=52.38, (n=19) and had decreased risks on adjusted and crude relative risk analyses (adjusted RR=0.83, 95% CI=0.76, 0.90, I2=0.00, [n=18]; RR=0.68, 95% CI=0.60, 0.78, I2=33.40 [n=17]). Physical activity had a protective effect against the emergence of depression in youths (AOR=0.90, 95% CI=0.83, 0.98, I2=0.00), in adults (AOR=0.78, 95% CI=0.70, 0.87, I2=0.00), and in elderly persons (AOR=0.79, 95% CI=0.72, 0.86, I2=0.00). Protective effects against depression were found across geographic regions, with adjusted odds ratios ranging from 0.65 to 0.84 in Asia, Europe, North America, and Oceania, and against increased incidence of positive screen for depressive symptoms (AOR=0.84, 95% CI=0.79, 0.89, I2=0.00) or major depression diagnosis (AOR=0.86, 95% CI=0.75, 0.98, I2=0.00). |
The strength of association between physical activity and anxiety was considered weak. The mean odds ratio was considered modest in strength though with a very wide CI. OR values >0.67 were classified as weak associations. Measures of association: McDowell, Dishman35 Self-reported anxiety symptoms: mean crude OR= 0.84 (95% CI=0.76, 0.93, I2=47.31%), mean AOR= 0.87 (95% CI=0.77, 0.99, I2=48.67%). All AORs (n=9) were <1.00. Diagnosis of Any Anxiety Disorder: mean OR was 0.66 (95% CI=0.53, 0.82, I2=62.26%). All AORs (n=3) were <1.00. Diagnosis of Generalized Anxiety Disorder: mean OR was 0.54 (95% CI=0.32, 0.92, I2=0.00%). All AORs (n=3) were <1.00. Schuch and Stubbs36 Authors reported AORs from 11 primary stuies that provided this data. All 11 reported point estimates < 1. Below are the estimates from the meta-analysis across 14 cohorts of 13 unique prospective studies People with high self- reported physical activity (versus low physical activity) were at reduced odds of developing anxiety (AOR=0.74, 95% CI=0.62, 0.88, I2=23.96; crude OR=0.80, 95% CI=0.69, 0.92, I2=0.00). Protective effects for anxiety were evident in Asia (AOR =0.31, 95% CI= 0.10, 0.96, I2=0.00) and Europe (AOR=0.82, 95% CI=0.69, 0.97, I2=11.36); for children/adolescents (AOR=0.52, 95% CI=0.29, 0.90, I2=0.00) and adults (AOR=0.81, 95% C =0.69, 0.95, I2=12.18). |
| (3) Consistency | Consistent findings were observed across the 2 included reviews. In their discussions, the authors of the 2 reviews indicated that consistency had been witnessed in the various primary studies that had been carried out in different populations. | In the review by McDowell and Dishman35 all crude and adjusted associations included in their meta-analyses indicated inverse associations between physical activity and subsequent anxiety. Similarly, Schuch and Stubbs36 provide evidence of the protective effects of self‐reported physical activity on anxiety development referencing previous cross‐sectional studies. We found that there is evidence for repeated observation of an association between physical activity and anxiety from other studies in different populations under different circumstances. These findings support the criterion of consistency. |
| (4) Dose–response relationship A dose–response relationship supports a causal interpretation.27,29 |
A dose–response relationship was reported in several primary studies included in the review by Mammen and Faulkner.34 Schuch and Vancampfort19 did not investigate a dose–response relationship owing to the varied definitions of low or high physical activity in their included primary studies. | Schuch and Stubbs36 did not investigate a dose response relationship between physical activity exposure and anxiety. In the review by McDowell and Dishman35 a total of 11 primary studies assessed for a dose response relationship between physical activity and various anxiety outcomes. All of which reported lower odds of anxiety outcomes for increased amounts of physical activity. In all, there is modest evidence of a dose–response relationship. |
| (5) Biological plausibility | Mammen and Faulkner34 and Schuch, Vancampfort19 highlight that it is likely that no single mechanism can explain the protective effect of physical activity in the development of depression. They discuss that a range of biochemical and psychosocial factors are likely responsible, including biological mechanisms through, which exercise increases neurogenesis and reduces inflammatory and oxidant markers and activates the endocannabinoid system; a neuromodulatory system involved in several mental disorders. Moreover, they report that people with depression have decreased hippocampal volumes and levels of markers of neurogenesis, and increased levels of inflammatory and oxidant markers. There is evidence that physical activity may regulate these abnormalities, increasing hippocampal volume and neurogenesis levels, as well as adjusting the imbalance between antiinflammatory and proinflammatory and oxidant markers. Also, physical activity may directly increase psychological factors such as self-esteem or perceptions of physical competence. Improved levels of fitness lead to both subjective and objective improvements in physical health status. In both reviews, the authors recommend that future research should investigate these underlying biological and psychological mechanisms.19,34 We concluded that the available evidence meets the biological plausibility criterion. |
Though the mechanisms are largely unclear, Schuch and Stubbs36 and McDowell and Dishman35 presented evidence of potential biological processes that may underlie the protective effect of physical activity on incident anxiety. Physical activity is known to influence similar pathways as those seen to play a role in the pathogenesis of anxiety disorders. Some of these biological processes include inflammation, oxidative and nitrogen stress, and subsequent alteration of neurotrophins, neurogenesis, and neuroplasticity. For instance, physical activity may promote neuroregeneration, or the balance between inflammatory/anti‐inflammatory and oxidative/antioxidative markers. This may protect against anxiety. From a psychological perspective, physical activity may reduce the risk of developing anxiety through reduced anxiety sensitivity or improved psychological factors such as increased self-efficacy regarding the ability to exert control over potential threats. |
| (6) Specificity | This criterion is not met—physical inactivity does not invariably lead to depression, and depression is not the only health condition associated with inactivity. However, this criterion was thought of in relationship to infectious agents, and seldom applies. | As with depression, this criterion is not met but of questionable relevance. |
| (7) Coherence | We considered that the interpretation of the association between physical activity and depression does not conflict with what is known of the natural history and biology of depression. | The interpretation for the association of physical activity and anxiety does not conflict with what is known of the natural history and biology of anxiety. |
| Assessed against Bradford Hill's criteria for causality.27,29 Assessment of grade of evidence: Convincing (strong)/probable/possible (suggestive)/insufficient | We graded the evidence as strong enough to support a judgment of a probable causal relationship, with higher levels of physical activity probably leading to a lower risk of depression. | We considered that the evidence supports a judgment of a probable causal relationship. |
I2, I-squared statistic; PA, physical activity.
The Association Between Physical Activity and Anxiety
A systematic review and meta-analysis of prospective cohort studies found that PA was associated with lower odds of self-reported anxiety symptoms (AORs=0.87; 95% CI=0.77, 0.99, n=9, I2=48.67%), diagnosis of any anxiety disorder (mean OR=0.66; 95% CI=0.53, 0.82, n=3, I2=62.26%), and diagnosis of generalized anxiety disorder (mean OR=0.54; 95% CI=0.32, 0.92, n=3, I2=0.00%).35 The authors included a total of 24 prospective cohort studies, of which 13 were included in the meta-analyses. McDowell et al.35 found a moderate degree of heterogeneity for outcomes of self-reported anxiety symptoms and a diagnosis of any anxiety disorder. The small number of primary studies included in their analyses did not allow a quantitative examination (i.e., meta-regression analysis) of potential sources of variability. They did not assess publication bias.35
A similar finding was seen in the meta-analysis by Schuch and colleagues.36 They included a total of 14 cohorts of 13 unique prospective studies in their review. High self‐reported PA levels were associated with lower rates of incident anxiety than low PA levels (AOR=0.74, 95% CI=0.62, 0.88, n=11, I2=23.96). After adjusting for publication bias, this association decreased but remained significant (AOR=0.86, 95% CI=0.69, 0.99).
The authors explored heterogeneity using subgroup and meta-regression analysis. They identified no significant moderators through meta‐regression. In subgroup analysis, the authors presented findings from 1 cohort specific for children/adolescents (AOR=0.52, 95% CI=0.29, 0.90, I2=0.00). They found evidence of publication bias both for adjusted and crude analysis, but the results remained significant after adjusting for publication bias, and the overall effect size did not change significantly.36
Table 5 presents a summary of the assessment against Bradford Hill's criteria for causality.27,29 We graded the evidence from the 2 included reviews as probable evidence to support that anxiety is causally related to physical inactivity.
Updated Search
We ran an updated search from the year 2020 (database settings did not allow us to start from 19 March, the day the previous search was run) to October 15, 2022. We report the search algorithm in Appendix Table 6 (available online). We identified 669 articles (371 records from Embase and 298 records from PubMed). A total of 479 records remained after removing duplicates. We excluded 454 records at the title and abstract screening stage. We screened the full text of the remaining 25 records. In total, 20 records were excluded with reason, leaving a total of 5 reviews for data extraction37, 38, 39, 40, 41 (see Appendix Figure 2, available online, for the PRISMA33 flow diagram and Appendix Table 7, available online, for a list of excluded records and reasons for exclusion). Of the 5 included studies, 4 investigated the outcome incident depression,38, 39, 40, 41 and one investigated incident anxiety.37 We document the findings from the updated search in Table 6.
Table 6.
Findings From the Updated Search
| Review | Study aim | Included primary studies and study overlap | Study findings | Data extraction and AMSTAR scores |
|---|---|---|---|---|
| Zimmermann et al.37 | To identify studies that examined the modifiable risk and protective factors for anxiety disorders among adults in the general population. | A total of 19 primary studies included in their review. Of these, 2 prospective studies were for the outcome: incident anxiety. These 2 primary studies were included in previous reviews from our initial search. | Authors conducted a qualitative synthesis. One primary study found that when compared with no exercise, regular exercise was found to be a protective factor for agoraphobia and specific phobia. Nonregular physical activity was a protective factor only for agoraphobia. The second primary study found that general physical activity did not predict the onset of any anxiety disorder, but sports participation appeared to be a protective factor in the onset of any anxiety disorder. |
We extracted data for the 2 primary studies as reported in the Zimmermann et. al. review. This is reported in Appendix Table 8 (available online) where we give a summary of the characteristics of included reviews from the updated search. Low-quality review. |
| Brady et al.38 | To systematically review the literature on accelerometer-measured physical activity and health outcomes in adults. | Of 52 primary studies included in their review, only 1 was related to our review outcome (incident depression), a new primary cohort study published in 2020. | From the one primary study included for the outcome, depression, women in the lowest tertile of light physical activity (68.1 min/day) had a statistically significant increased risk of developing depressive symptoms, compared with those in the highest tertile (130.0 min/day) (HR=1.98, 95% CI=1.19, 3.29) but not in men (p-interaction<0.01). (light physical activity tertiles, mean [SD]: lowest: 68.1 [11.3] min/day, middle 95.3 [6.5] min/day, highest 130.0 [20.9] min/day) |
Because only 1 primary study in their review reported on our study outcome, we did not extract data for this review or assess for review quality. |
| Dishman et al.39 | They quantified the cumulative association of customary physical activity with incident depression and with an increase in subclinical depressive symptoms over time as reported from prospective observational studies They define incident depression by clinical diagnosis or cut points on depression screening tests. |
A total of 111 reports included in their review. From their report, we could not distinguish between primary studies reporting incident depression and those reporting subclinical depressive symptoms over time. We did not carry out an analysis of the included primary studies. |
Authors defined incident depression by clinical diagnosis or cut points on depression screening tests. The authors found that both odds of incident cases of depression or an increase in subclinical depressive symptoms were reduced after exposure to physical activity. They report that results were materially the same for incident depression and subclinical symptoms (OR=0.69, 95% CI=0.63, 0.75, I2=93.7, and adjusted OR=0.79, 95% CI=0.75, 0.82, I2=87.6). |
We did not carry out data extraction for our selected data items or assess review for quality. This was because we could not distinguish between primary studies reporting incident depression and those reporting subclinical depressive symptoms over time. From additional information in their supplementary file, our inclusion criterion for population was not fully met for some of the primary studies. |
| Guo et al.40 | To quantify the relationship between different physical exercise doses and the risk of depression | A total of 12 primary studies all reporting on incident depression. Of these, 8 prospective studies are included in previous reviews from our initial search. |
Authors found a nonlinear relationship between leisure time physical activity and the risk of incident depression. The categorical dose–response association: Compared with the lowest LTPA category, the risk of incident depression was 23% lower for all LTPA categories (RR=0.77, 95% CI=0.68, 0.86, I2=69%) and 27%, 17%, and 8% lower for light, moderate, and highest-dose LTPA participants (RR=0.73, 95% CI=0.64, 0.82, I2=43%; RR=0.83, 95% CI=0.78, 0.87, I2=46%; RR=0.93, 95% CI=0.86, 0.99, I2=79%, respectively). Continuous dose–response association: When physical activity was <25 MET-h/week, they found that the RR of depression risk was reduced by 3% for every 5 MET-h/week increase (RR=0.97, 95% CI=0.95, 0.98). When physical activity was >25 MET-h/week, the increased physical activity did not further reduce the risk of depression, that is, the risk of depression increased by 4% for every 5 MET-h/week increase (RR=1.04, 95% CI=1.02, 1.05). |
We extracted data and give a summary of the characteristics of study in Appendix Table 8 (available online) Low-quality review |
| Pearce et al.41 | To systematically review and meta-analyze the dose–response association between physical activity and incident depression from published prospective studies of adults. | A total of 15 primary studies all reporting on incident depression. Ten of these primary studies are included in previous reviews from our initial search. |
The authors found an inverse curvilinear dose–response association between physical activity and incident depression, with steeper association gradients at lower activity volumes. (those accumulating half the recommended volume of physical activity by 18% [95% CI=13%, 23%] lower risk of depression. Those accumulating the recommended volume had 25% [95% CI=18%, 32%] lower risk, diminishing additional potential benefits and greater uncertainty at higher volumes of physical activity.) |
We extracted data and give a summary of the characteristics of study in Appendix Table 8 (available online) Low-quality review. |
For the updated search conducted on October 15, 2022, data extraction and assessment of the methodologic quality of the included systematic reviews were done by MNW. This was checked and discussed with JLV.
AMSTAR, Assessment of Multiple Systematic Reviews; HR, hazard ratio; I2, I-squared statistic; LTPA, leisure-time physical activity; MET-h/week, MET hours per week, min, minute.
DISCUSSION
This umbrella review provides a comprehensive and systematic overview of epidemiologic evidence, up to March 2020, on the strength of the association between PA and incident cases of depression and anxiety and assesses the likelihood of these associations being causal. Four reviews were included: 2 for the outcome depression (which included 79 primary prospective cohort studies in total) and 2 for anxiety (which included 37 primary prospective cohort studies in total).
The consistent findings from the 4 reviews provide sufficient evidence to support an association between PA and incident cases of depression and anxiety. When assessed against Bradford Hill's criteria for causality,27,29 we found a probable causal relationship, with higher levels of PA leading to a lower risk of depression and anxiety. Our umbrella review expands the evidence in the area of PA and mental health. Previous systematic review of reviews and meta-analyze have reported associations between PA and depression with various estimates of the strength of these associations.9,17,18 Regarding causality, Biddle et al.18 found partial support for a causal association between depression and PA. For the association between PA and anxiety, Biddle et al.18 found that there was an association but cautioned that only a small number of primary studies had been included in their review. They concluded that a full analysis of causality was not possible owing to the small number of primary studies found. Dale et al.9 classified the associations between PA and anxiety as unclear owing to a small number of primary studies included in their review. The measures of strength of the associations reported in these previous systematic reviews of reviews are not comparable with the measures that we have reported in our study because of the difference in definition of outcomes investigated. Whereas we restricted our outcomes to incident cases of depression and anxiety, previous systematic reviews of reviews included existing diagnosis or symptoms of depression and anxiety.9,18 There have been 2 recent published overviews that are similar to our review of reviews. One is a systematic meta-review of top-tier evidence examining how PA, sleep, dietary patterns, and tobacco smoking impact on the risk and treatment outcomes across a range of mental disorders.42 They found that the evidence supports the use of PA in primary prevention and clinical treatment of mental disorders. For the outcomes incident depression and anxiety, they included 3 reviews,19,35,36 which were included in this umbrella review.
The second one is an umbrella review of universally delivered preventive interventions for depression with an aim to provide an overview of the meta-analytic literature. Several preventive interventions were included in the study: psychological interventions, school-based interventions, electronic health interventions, and PA interventions. The authors give a detailed report of findings on PA interventions and conclude that there is meta-analytic evidence that PA can prevent depression.43
Limitations
Our review protocol was not submitted for peer-reviewed publication or for registration; however, the protocol was prepared according to set guidelines and protocol for systematic reviews, including the selection of studies on the basis of a predefined set of inclusion and exclusion criteria.24,25 We restricted inclusion to peer reviewed studies. Although this enhances the rigor of evidence, findings may be susceptible to publication bias. Furthermore, we only included reviews published in the English language. Three included reviews also reported evidence of publication bias in their primary studies.19,34,36 In 2 reviews that adjusted for publication bias, authors found that it did not change the magnitude of the associations.19,36
A few other limitations of our study and the underlying evidence are worth discussing. For the strength of the associations between PA and depression and anxiety, we rely on the evidence from previous studies—primary cohort studies summarized in systematic reviews with pooled estimates. To reduce the possibility of reverse causality (undetected illness at baseline causing lower PA), each of the 4 reviews included in our study only covered primary studies with a follow-up period of >1 year. For depression, the primary studies excluded participants with depression at baseline. This reduced the risk of selection bias. However, the review authors noted that depression is a recurrent disorder, and previous depressive episodes were not well documented in the primary studies. In 1 review,19 a subgroup analysis of primary studies that controlled for baseline depressive symptom severity also showed significant associations between high PA levels and lower incidence of depression. This makes it less likely that reverse causation explains the association found.
Being an umbrella review, a potential limitation of our study is the overlap in the primary research studies across the included reviews (Table 3). In addition, most of the primary studies reported in the 4 included reviews were from high-income countries and regions. Studies in low- and middle-income countries are warranted to establish any variations in the context-specific measures of association between PA and depression and anxiety.
Another limitation is that imprecise measurement of PA might have weakened the associations with health outcomes. The authors of the 4 reviews report that most of the primary studies used self-reported PA, which is imprecise. Imprecise measurement of exposure leads to regression dilution bias.44 The use of self-report questionnaires may also have introduced recall bias; PA tends to be overestimated.45,46 Furthermore, the review authors reported that most of the primary studies assessed PA at baseline only and that a few assessed PA levels at several points in time. Tracking PA levels in a cohort across time permits estimation of change in exposure across follow-up, reducing the risk of misclassification bias (but increasing the risk of reverse causation). PA classification criteria differed across primary studies, and aspects of PA captured by each instrument varied widely between primary studies (intensity, frequency, volume). This prevented the authors of the 4 included reviews from assessing dose–response relationships.
Although different measures for depression and anxiety were used in the included primary studies, the review authors report that most of the primary studies assessed the health outcomes through well-validated high-quality measures. In the review by Schuch et al.,19 subgroup analyses showed that PA decreased the risk of developing depression, regardless of whether this was based on self-report measures or major depression diagnosis from structured clinical diagnostic interviews.
Some caution is required because some covariates may not have been assessed in the 4 reviews. Examples of such covariates are genetic factors; familial history of depression and anxiety; other risk factors for depression and anxiety, such as obesity, poor diet, and use of tobacco; and other clinical comorbidities. However, despite the limitations encountered in synthesis of primary studies, authors of the 4 reviews consistently provided evidence to support that engaging in PA protects against incident cases of depression and anxiety. They also presented respective measures of the strength of these associations.
Our initial search was on March 19, 2020, and it should be noted that our study includes a limited number of reviews published up to March 2020, which may have resulted in an increased risk of publication bias. However, we have systematically updated our search to October 15, 2022 and identified a few new reviews as reported in our results section. The measures of the strength of associations reported in these additional reviews are in line with our findings and strengthen our conclusion on the association between PA and depression. Similar to our initial search, there were few primary studies and reviews found on the outcome anxiety. This has also been the case in previous studies.9,18 Our study is unique in the explicit consideration of causality and the link with our companion paper that quantifies the impact of changes in the Australian population's PA levels on the burden of anxiety and depression and healthcare costs in Australia.47
Implications of the Results for Practice and Policy
Our findings provide qualified support for the inclusion of PA in potential strategies for the prevention of mental ill health. Given the large burden of mental health problems, even small improvements can have a significant effect at population level. True benefits of measures that increase PA levels are likely to be even greater because the mental health benefits would be additional to those accrued from reduction of other diseases associated with PA such as cardiovascular disease, diabetes, several types of cancer, and other chronic health conditions.48,49
Strenuous PA may lead to repeated intense stress on the joints or acute high stress. This could damage cartilage, ligaments, and other joint structures. Such strenuous PA can be seen in extreme sports but may be unlikely in the kind of PA levels recommended in various guidelines for health. In the long term, recommended levels of PA may prevent injury through increased muscle strength and flexibility, hence protecting the spine and joints from injuries. Increased PA through active transport such as walking or bicycling on or along busy roads could increase injury risk and may result in harm through exposure to air pollution. Physical inactivity could be a factor in the causal path to anxiety and depression. For instance, people experiencing incapacitation may develop anxiety and depression owing to the inability to participate in PA. Tailored PA interventions may be needed for various groups with specific needs. The measures of association reported in most studies are drawn from samples representative of the general population controlled for various variables. Hence, the effect from a PA intervention may differ between smaller proportion of populations experiencing incapacitation and larger proportions not experiencing that.
Unanswered Questions and Future Research
Further research could strengthen the evidence base for sex- and age-specific measures of association between PA and depression and anxiety. Future research is needed to improve the estimates of the dose–response relationship between PA and depression and anxiety. Preferably, exposure should be objectively measured and should capture the frequency, intensity, type (e.g., aerobic, strength), and duration of PA. As outcomes, continuous measures of depressive mood and anxiety are preferable to dichotomous measures.34,35 Where possible, PA should be measured several times across the study—not only at baseline—to capture impact of habitual or sustained PA.50 Such studies should aim for representative samples and should fully account for participants lost to follow-up.35 Future studies should also assess the size and distribution of the benefits for different socioeconomic and ethnic groups.
CONCLUSIONS
Our evidence is drawn from systematic reviews of observational data. Further high-quality studies, such as randomized control trials, would help to strengthen the evidence base of the associations between PA and depression and anxiety. Nonetheless, our findings provide empirical support for the consideration of PA in strategies for the prevention of mental ill health.
CRediT authorship contribution statement
Mary Njeri Wanjau: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Holger Möller: Conceptualization, Methodology, Investigation, Formal analysis, Writing – review & editing. Fiona Haigh: Conceptualization, Methodology, Investigation, Formal analysis, Writing – review & editing. Andrew Milat: Conceptualization, Writing – review & editing. Rema Hayek: Conceptualization, Writing – review & editing, Project administration. Peta Lucas: Conceptualization, Writing – review & editing. J. Lennert Veerman: Conceptualization, Methodology, Investigation, Formal analysis, Resources, Writing – review & editing, Supervision, Funding acquisition.
Acknowledgments
ACKNOWLEDGMENTS
The authors acknowledge the NSW (New South Wales) Ministry of Health for funding our study. We would like to thank the NSW Ministry of Health and the cross-agency advisory group for their review and input into the findings of our systematic review of reviews. The NSW Ministry of Health initiated the larger project to identify a best practice method to cost the health benefits of active transport, which this study was part of. The authors acknowledge Jasmin Spinoso, Research Support Officer in the School of Medicine and Dentistry, Griffith University for her support in developing the graphical abstract. The authors acknowledge and thank the reviewers and journal editors for their review and helpful comments.
The funder had no say in design/interpretation of the study.
This work was funded by the New South Wales Ministry of Health.
Declaration of interest: none.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.focus.2023.100074.
Appendix. Supplementary materials
REFERENCES
- 1.Network. Global Burden of Disease Collaborative. Global Burden of Disease Study 2019 (GBD 2019) Results, 2020, Institute for Health Metrics and Evaluation (IHME). Seattle, WA. https://vizhub.healthdata.org/gbd-results/
- 2.The Lancet Global Health Mental health matters. Lancet Glob Health. 2020;8(11):e1352. doi: 10.1016/S2214-109X(20)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oude Voshaar RC, Aprahamian I, Borges MK, et al. Excess mortality in depressive and anxiety disorders: the Lifelines Cohort Study. Eur Psychiatry. 2021;64(1):e54. doi: 10.1192/j.eurpsy.2021.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM. Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. Br J Psychiatry. 2011;199(6):453–458. doi: 10.1192/bjp.bp.110.085100. [DOI] [PubMed] [Google Scholar]
- 5.COVID-19 Mental Disorders Collaborators Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawel A, Shou Y, Smithson M, et al. The effect of COVID-19 on mental health and wellbeing in a representative sample of Australian adults [published correction appears in Front Psychiatry. 2021;11:619331] Front Psychiatry. 2020;11(1026) doi: 10.3389/fpsyt.2020.579985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . WHO; Geneva, Switzerland: Published November 14, 2013. Global action plan for the prevention and control of NCDs 2013–2020.https://www.who.int/nmh/events/ncd_action_plan/en/ [Google Scholar]
- 8.ISPAH International Society for Physical Activity and Health The Bangkok Declaration on physical activity for global health and sustainable development. Br J Sports Med. 2017;51(19):1389–1391. doi: 10.1136/bjsports-2017-098063. [DOI] [PubMed] [Google Scholar]
- 9.Dale LP, Vanderloo L, Moore S, Faulkner G. Physical activity and depression, anxiety, and self-esteem in children and youth: an umbrella systematic review. Ment Health Phys Act. 2019;16:66–79. doi: 10.1016/j.mhpa.2018.12.001. [DOI] [Google Scholar]
- 10.Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9(3):366–378. doi: 10.1080/17437199.2015.1022901. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(9):964–974. doi: 10.4088/JCP.13r08765. [DOI] [PubMed] [Google Scholar]
- 12.Anderson E, Shivakumar G. Effects of exercise and physical activity on anxiety. Front Psychiatry. 2013;4:27. doi: 10.3389/fpsyt.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104–111. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belvederi Murri M, Ekkekakis P, Magagnoli M, et al. Physical exercise in major depression: reducing the mortality gap while improving clinical outcomes. Front Psychiatry. 2018;9:762. https://doi.org/10.3389/fpsyt.2018.00762. [DOI] [PMC free article] [PubMed]
- 15.McDowell CP, Dishman RK, Vancampfort D, et al. Physical activity and generalized anxiety disorder: results from the Irish Longitudinal Study on Ageing (TILDA) Int J Epidemiol. 2018;47(5):1443–1453. doi: 10.1093/ije/dyy141. [DOI] [PubMed] [Google Scholar]
- 16.Ku PW, Fox KR, Chen LJ. Physical activity and depressive symptoms in Taiwanese older adults: a seven-year follow-up study. Prev Med. 2009;48(3):250–255. doi: 10.1016/j.ypmed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham C, O’ Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30(5):816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 18.Biddle SJH, Ciaccioni S, Thomas G, Vergeer I. Physical activity and mental health in children and adolescents: an updated review of reviews and an analysis of causality. Psychol Sport Exer. 2019;42:146–155. doi: 10.1016/j.psychsport.2018.08.011. [DOI] [Google Scholar]
- 19.Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175(7):631–648. doi: 10.1176/appi.ajp.2018.17111194. [DOI] [PubMed] [Google Scholar]
- 20.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants [published correction appears in Lancet Glob Health. 2019;7(1):e36] Lancet Glob Health. 2018;6(10):e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 22.Bauman AE, Reis RS, Sallis JF, et al. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 23.WHO . WHO; Geneva, Switzerland: Published 2018. Global action plan on physical activity 2018–2030: more active people for a healthier world.https://apps.who.int/iris/bitstream/handle/10665/272722/9789241514187-eng.pdf [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Chichester, United Kingdom: 2019. https://training.cochrane.org/handbook [Google Scholar]
- 25.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;378 doi: 10.1136/bmj-2022-070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb P, Bain C, Page A. 4th ed. Cambridge University Press; Cambridge, United Kingdom: 2019. Essential Epidemiology: an Introduction for Students and Health Professionals. [Google Scholar]
- 28.World Cancer Research Fund International; London, United Kingdom: Published 2018. Judging the evidence.https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/judging-the-evidence/ [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3/e (paperback) Lippincott; Philadelphia, PA: 2008. https://books.google.com.au/books?id=Bt7puQEACAAJ [Google Scholar]
- 30.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019 [published correction appears in Lancet. 2020;396(10262):1562] Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Covidence Systematic Review Software. Veritas Health Innovation. www.covidence.org.
- 33.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45(5):649–657. doi: 10.1016/j.amepre.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 35.McDowell CP, Dishman RK, Gordon BR, Herring MP. Physical activity and anxiety: a systematic review and meta-analysis of prospective cohort studies. Am J Prev Med. 2019;57(4):545–556. doi: 10.1016/j.amepre.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Schuch FB, Stubbs B, Meyer J, et al. Physical activity protects from incident anxiety: a meta-analysis of prospective cohort studies. Depress Anxiety. 2019;36(9):846–858. doi: 10.1002/da.22915. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann M, Chong AK, Vechiu C, Papa A. Modifiable risk and protective factors for anxiety disorders among adults: a systematic review. Psychiatry Res. 2020;285 doi: 10.1016/j.psychres.2019.112705. [DOI] [PubMed] [Google Scholar]
- 38.Brady R, Brown WJ, Hillsdon M, Mielke GI. Patterns of accelerometer-measured physical activity and health outcomes in adults: a systematic review. Med Sci Sports Exerc. 2022;54(7):1155–1166. doi: 10.1249/MSS.0000000000002900. [DOI] [PubMed] [Google Scholar]
- 39.Dishman RK, McDowell CP, Herring MP. Customary physical activity and odds of depression: a systematic review and meta-analysis of 111 prospective cohort studies. Br J Sports Med. 2021;55(16):926–934. doi: 10.1136/bjsports-2020-103140. [DOI] [PubMed] [Google Scholar]
- 40.Guo Z, Li R, Lu S. Leisure-time physical activity and risk of depression: a dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore) 2022;101(30):e29917. doi: 10.1097/MD.0000000000029917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce M, Garcia L, Abbas A, et al. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(6):550–559. doi: 10.1001/jamapsychiatry.2022.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–380. doi: 10.1002/wps.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoare E, Collins S, Marx W, et al. Universal depression prevention: an umbrella review of meta-analyses. J Psychiatr Res. 2021;144:483–493. doi: 10.1016/j.jpsychires.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]
- 45.Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46(1):99–106. doi: 10.1249/MSS.0b013e3182a0595f. [DOI] [PubMed] [Google Scholar]
- 46.Shook RP, Gribben NC, Hand GA, et al. Subjective estimation of physical activity using the international physical activity questionnaire varies by fitness level. J Phys Act Health. 2016;13(1):79–86. doi: 10.1123/jpah.2014-0543. [DOI] [PubMed] [Google Scholar]
- 47.Wanjau MN, Möller DH, Haigh F, et al. Physical activity and depression and anxiety disorders in Australia: a lifetable analysis. AJPM Focus. 2022;2(2):100030. doi: 10.1016/j.focus.2022.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dishman RK, Vandenberg RJ, Motl RW, Nigg CR. Using constructs of the transtheoretical model to predict classes of change in regular physical activity: a multi-ethnic longitudinal cohort study. Ann Behav Med. 2010;40(2):150–163. doi: 10.1007/s12160-010-9196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.