HIGHLIGHT
-
•
Growing evidence supports the health benefits of more physical activity (PA) and step counts.
-
•
This systematic review included large cohort studies that used different activity monitors.
-
•
Moderate-to-vigorous PA reduced all-cause and cardiovascular mortality in adults.
-
•
Higher steps per day lowered the risk of all-cause mortality among older persons.
-
•
Initial health benefits were observed for light-intensity PA.
Keywords: Physical activity, sedentary behavior, step count, review, meta-analysis, cohort study
Abstract
Introduction
This review synthesized evidence from prospective cohort studies on the association of device-measured physical activity and sedentary behavior with cardiovascular disease and all-cause mortality among adults.
Methods
Five databases were searched from 2000 through April 29, 2020. Study quality was appraised using the NIH Quality Assessment Tool. Pooled hazard ratio and 95% CI were obtained from random-effects meta-analyses. Subgroup analyses by age and sex were conducted for studies on all-cause mortality.
Results
Of 29 articles included in the systematic review, 5 studies on cardiovascular disease mortality and 15 studies on all-cause mortality were included in meta-analyses. Comparing the highest with the lowest exposure categories, the pooled hazard ratios (95% CIs) for cardiovascular disease mortality were 0.29 (CI=0.18, 0.47) for total physical activity, 0.37 (CI=0.25, 0.55) for moderate-to-vigorous physical activity, 0.62 (0.41–0.93) for light physical activity, and 1.89 (CI=1.09, 3.29) for sedentary behavior. The pooled hazard ratios (95% CIs) for all-cause mortality were 0.42 (CI=0.34, 0.53) for total physical activity, 0.43 (CI=0.35, 0.53) for moderate-to-vigorous physical activity, 0.58 (CI=0.43, 0.80) for light physical activity, and 1.58 (CI=1.19, 2.09) for sedentary behavior. The pooled hazard ratio (95% CI) for all-cause mortality was 0.35 (CI=0.29, 0.42) for steps per day, but the studies available for analysis were conducted in older adults. The results of subgroup analyses were consistent with the main results.
Discussion
Rapidly accumulating evidence suggests that more physical activity and less sedentary behavior are associated with a lower risk of cardiovascular disease and all-cause mortality. Similar beneficial relationships were found for step counts and all-cause mortality among older adults. Future studies employing standardized research methodologies and up-to-date data processing approaches are warranted to recommend specific amounts of physical activity and limits to sedentary behavior.
INTRODUCTION
Lack of physical activity (PA) and prolonged sedentary behavior (SB) have been associated with higher hazards of all-cause mortality1, 2, 3 and cardiovascular diseases (CVDs).2,4, 5, 6, 7, 8 However, past reviews were largely developed on the basis of studies of self-reported PA and SB where measurement errors because of inaccurate recall and social desirability bias were inevitable.9 Moreover, most questionnaires only capture PA of moderate-to-vigorous intensity for at least 10 minutes continuously, and few assess PA of lighter intensity or shorter duration.10,11
To overcome the limitations of self-report using questionnaires, objective approaches to estimate PA and SB using devices such as pedometers and accelerometers worn on various body parts have become increasingly popular in epidemiologic studies since the early 21st century.12,13 Leveraging such devices, researchers are able to continuously capture movement behaviors of large populations with higher temporal resolution over multiple days under free-living conditions. The movement behaviors can subsequently be represented as total PA (TPA) or further classified into distinct categories of intensity-based PA, that is, vigorous-intensity PA (VPA), moderate-to-vigorous-intensity PA (MVPA), light-intensity PA (LPA), daily step counts, and SB. It has also been shown that device-measured data offers higher reliability and smaller measurement errors and provides in-depth information and a long-term understanding of PA or SB patterns.14,15
Several published reviews have added new insights to the current body of knowledge, which is in line with the growing interest in using devices for estimating PA and SB. A harmonized meta-analysis of 8 studies using Actigraph or Actical accelerometers revealed that lower PA and higher SB were associated with a higher hazard of all-cause mortality.16 Besides PA of higher intensity levels, the literature also suggests that having more time spent on lighter forms of PA may confer health benefits. A meta-analysis reported that engaging in at least 3 hours of LPA per day was associated with a lower hazard of all-cause mortality.17 Furthermore, LPA was also associated with better cardiometabolic health.18 In addition to the commonly known PA and SB measures that are classified on the basis of intensity levels, device-enabled counting of steps represents another approach to quantifying PA or SB. Evidence on positive health implications of accumulating daily step counts is emerging and has been reported in recent systematic reviews and meta-analyses.19,20
Although these reviews have advanced our understanding of the long-term health implications of movement behaviors, they also present opportunities for further research. First, the harmonized analysis16 focused on only 2 types of devices; therefore, it does not capture prospective cohort studies conducted worldwide that are using various types and models of activity-tracking devices. Second, the relationship between LPA and long-term health outcomes, especially CVD outcomes, has not received much attention. Third, new findings from recent large cohort studies have not been considered in previous reviews.21, 22, 23, 24, 25 To address these knowledge gaps, we conducted a systematic review of prospective cohort studies to provide a comprehensive overview of the relationship between device-measured exposures (i.e., PA and SB) and the associated long-term health outcomes (i.e., CVD outcomes and all-cause mortality) among adults. In this review, we covered the entire spectrum of PA measures to include LPA and step counts in addition to TPA and MVPA. We further conducted a meta-analysis to compare the highest with the lowest categories of PA or SB in relation to CVD mortality and all-cause mortality and express these associations in hazard ratios (HRs) and 95% CIs.
METHODS
Following the PRISMA guidelines,26 we conducted a literature search of 5 databases (Embase, MEDLINE, Web of Science Core Collection, SportDiscus, and Google Scholar) from the year 2000 through April 29, 2020. The search strategy includes a combination of Medical Subject Headings and keyword terms: (physical activity OR steps OR step count OR stepping OR MVPA OR moderate-to-vigorous OR inactive OR inactivity OR inactivities OR exercise OR sedentar*) AND (objectively OR acceleromet* OR pedomet* OR wearable* OR tracker* OR actigraph OR activpal OR actical OR axivity OR inclinomet* OR heart rate monitor* OR fitness trackers). An age filter (i.e., adult) was applied. The detailed search strategy is provided in Appendix Figure 1 (available online). Manual searches of bibliographies of review articles were conducted to identify additional studies. Two reviewers (SJL, NAP) screened all eligible titles and abstracts, independently reviewed and identified relevant full-text articles for inclusion, and assessed the quality of the included articles. All discrepancies were discussed with a third reviewer (FMR) to reach a consensus. We updated the search until September 2, 2022 in MEDLINE and discussed the additional findings in the context of our main results.
Population- or community-based prospective observational studies of adults were included. Studies were excluded if they purposively selected participants with specific diseases (e.g., cancer, mental illnesses, disabilities), occupations (e.g., workforce at specific workplace settings, members of the military, athletes), or institutionalized individuals. Studies that were conducted in controlled environments such as exercise laboratories, editorial articles, commentaries, or review articles were also excluded.
Device-measured exposures included in this review were (1) PA (e.g., duration or volume of TPA, VPA, MVPA, moderate-intensity PA [MPA], LPA, and MET-based measurements such as MET-hour or MET-minute), (2) SB (e.g., duration of SB), and (3) step counts. Considered outcomes were (1) CVD outcomes (e.g., cardiovascular-specific mortality, myocardial infarction, coronary heart disease, ischemic heart disease, acute coronary syndrome, coronary heart diseases, stroke, or other CVDs) and (2) all-cause mortality. Studies that reported associations between any or all the exposure measures mentioned earlier with the outcomes of interest were included.
SJL and FMR created the data extraction form, which contained study characteristics (first author, site of study, publication year, follow-up duration), population characteristics (analysis sample size, proportion of male versus female, and age of study population), device characteristics (brand name and model, wear location and duration, definition of wear time), definitions of exposure and outcome, methods of outcome ascertainment, analysis methods, adjusted variables, main results, and quality ratings of included studies. Definitions and measurements of the outcomes were HR, risk ratio or related statistics, and 95% CIs. The most completely adjusted HR or risk ratio was extracted. SJL extracted information from the included full-text papers, and NAP performed a detailed check of the extracted data.
Two authors (SJL and NAP) independently appraised the methodologic quality of all included studies using a quality rating list on the basis of the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies27 that aims to assess selection bias, information bias, measurement bias, and confounding in cohort studies. First, each question in the 14-item questionnaire was given a Yes; No; or Other: cannot determine, not applicable, or not reported. As recommended by the guidelines, the overall quality of each study was categorized as good, fair, or poor instead of rated using the sum of scores. In this review, a study was rated as good when there was an overall low risk of biases and confounding, as fair when there was a moderate risk of biases (e.g., moderate rate of dropout, selection of participants was based on completeness of data, among others), and as poor when there was a high risk of biases (e.g., high rate of dropout, key confounders were not accounted for in analysis). Any disagreements in quality ratings between the 2 reviewers were resolved in a consensus meeting with a third reviewer (FMR).
When studies reported estimates for both MPA and MVPA, we extracted effect sizes on the basis of MVPA only. Studies reporting risk estimates relative to the highest category of PA were recalculated to set the lowest PA category as the reference group.28
For studies that reported exposures in categories, the effects between the highest and the lowest categories were included in the meta-analysis and compared by exposure subtypes (i.e., TPA, MVPA, LPA, SB, and step counts). When multiple publications from the same cohort were found, the most appropriate study was selected on the basis of the relevance of exposure and outcome measures, study methodology, or sample size. Studies reporting risk estimates independently on the basis of participants’ characteristics (e.g., men and women) were treated as separate observations. Pooled estimates were calculated for an exposure subtype if there were at least 2 data points.
Meta-analyses were performed by NN, and random-effects models were used to calculate summary HRs and 95% CIs. The risk ratio reported in 1 original study was treated as HR in the meta-analysis. The average of the natural logarithm of the HRs was estimated, and the HR from each study was included using random effects weighting.29 Forest plots were generated and presented in the order of exposure type (i.e., TPA, MVPA, LPA, SB, and step counts) for each type of outcome to facilitate visual inspection. Chi-square tests and I-square statistics were examined to assess the amount of heterogeneity in study results30 where the I-square values of 25%, 50%, and 75% correspond to low, moderate, and high levels of heterogeneity, respectively.31 Individual study-specific HR and 95% CI were indicated by the black dots and the horizontal line, respectively, and the size of the gray squares corresponded to the weight of the study in the meta-analysis. The center of the diamond indicated the pooled HRs, and the width of the diamond indicated the corresponding 95% CI. The certainty of evidence was rated on the basis of the Grading of Recommendations Assessment, Development, and Evaluation approach.32
Additional meta-analyses were performed. First, to improve the comparability of effect estimates across various types of PA measures (i.e., TPA, MVPA, and LPA), we conducted an additional analysis by specifically including studies that reported all the 3 types of PA measures. Second, 2 sensitivity analyses were performed: (1) for CVD mortality by including 1 study that considered both fatal and nonfatal CVD events as an outcome and (2) for all-cause mortality by excluding 1 study that estimated follow-up duration on the basis of the time since baseline assessment at recruitment instead of the actual start time of device-based measurements. Third, subgroup analyses were conducted for all-cause mortality only because there was a lack of studies on CVD outcomes. Two factors were investigated, that is, age and sex. Publication bias by exposure subtype was assessed on the basis of 2 statistical tests (i.e., Egger's two-tailed test for asymmetry33 and Begg's rank correlation test34) and on the basis of the asymmetry of funnel plots (with pseudo 95% confidence limits).35
All analyses were carried out using Stata, Version 12.1 (StataCorp, College Station, TX). Results of statistical analyses with 2-sided p<0.05 were considered statistically significant.
RESULTS
Our search of the literature initially identified 31,055 articles. Of these, 13,606 duplicates were removed, and 17,449 titles and abstracts were screened. Subsequently, 16,364 articles were excluded during the screening of titles and abstracts owing to ineligible exposure, outcome, or study design. Finally, 85 full-text articles were screened, and 29 of these articles were included in this systematic review (Figure 1). Of the 29 included articles, 4 studies examined CVD, 19 studies examined all-cause mortality, and 6 studies examined both CVD and all-cause mortality (Table 1). Among the 10 studies on CVD outcomes, 5 studies focused on CVD-specific mortality, whereas the other 5 studies defined CVD outcomes by including the incidence of fatal and nonfatal CVD events and mortality because of CVD.
Figure 1.
Study selection flow diagram.
CVD, cardiovascular disease; PA, physical activity.
Table 1.
Characteristics of All Included Studies
| Source publication |
Follow-up duration (year) |
Analysis sample size (n) | Age (years) |
Device |
Exposure |
Outcome | Overall quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year, site | Female (%) | TPA | MVPA | LPA | SB | Step count | |||||
| Lee36,a 2018, U.S. |
2.3 (SD: NR) | 16,741 100% |
72.0 (SD: 5.7) | ActiGraph GT3X+ |
√ | √ | √ | √ | ACM | Good | |
| CE Matthews37 2016, U.S. |
6.6 (SD: NR) | 4,840 50.3% |
56.8 (SE: 0.4) | ActiGraph AM-7164 |
√ | √ | √ | ACM | Good | ||
| Dohrn38,a 2018, SW |
14.2 (SD: 1.9) | 828 56.0% |
66.7 (SD: 10.2) | ActiGraph 7,164 |
√ | √ | √ | √ | ACM, CM |
Fair | |
| Evenson39,a 2016, U.S. |
6.8 (IQR: 5.7–7.8) |
3,809 54.6% |
55.3 (SE: 0.4) | Actigraph AM-7164 |
√ | √ | √ | √ | ACM, CM |
Good | |
| Dempsey22 2020, UK |
5.7 (SD: NR) (CVD)b 6.6 (SD: NR) (ACM) |
5,580 (CVD)b 5,249 (ACM) 58.5% |
68.9 (SD: 7.2) | ActiGraph GT1M / GT3X+ |
√ | √ | √ | √ | ACM, CVD Eventc |
Good | |
| J Tarp23 2020, U.S. |
10.8 (IQR: 9.7–11.8) |
3,542 54.0% |
56.1 (SD: 11.4) | ActiGraph 7,164 |
√ | √ | √ | √ | ACM | Good | |
| BJ Jefferis40,a 2018, UK |
5.0 (IQR: 0.2–6.1) |
1,181 0% |
78.4 (SD: 4.6) | Actigraph GT3x |
√ | √ | √ | √ | ACM | Fair | |
| Saint-Maurice 201841 2018, U.S. |
6.6 (SD: NR) | 4,840 50.3% |
≥40.0 | Actigraph AM-7164 |
√ | √ | √ | ACM | Fair | ||
| Bielemann42,a 2019, Br |
2.7 (IQR: 2.5–2.8) |
973 62.2% |
60–69 (50.9%) 70–79 (34.6%) ≥80 (14.2%) |
GENEActiv |
√ | √ | √ | ACM | Fair | ||
| MJ LaMonte43,a 2018, U.S. |
3.1 (SD: 0.7) | 6,382 100% |
78.6 (SD: 6.7) | Actigraph GT3X+ |
√ | √ | √ | ACM, CM |
Good | ||
| KE Ensrud44,a 2014, U.S. |
4.5 (SD: 1.0) | 2,918 0% |
79.0 (SD: 5.2) | SenseWear pro armband | √ | √ | √ | ACM, CM |
Good | ||
| KR Fox45,a 2015, UK |
4.1 (SD: 1.1) | 213 48.8% |
≥70.0 | Actigraph GT1Ms |
√ | √ | √ | ACM | Fair | ||
| BJ Jefferis46,a 2019, UK |
4.9 (range: 0.1–6.1) |
1,181 0% |
78.4 (Range: 71–92) |
Actigraph GT3x |
√ | √ | √ | √ | CVD Eventd |
Good | |
| KM Diaz47,a 2017, U.S. |
4.0 (range: 0.1–6.1) |
7,985 54.1% |
63.5 (SD: 8.5) | Actical |
√ | ACM | Fair | ||||
| Dohrn48 2019, SW |
14.4 (SD: 1.6) | 1,176 55.0% |
45.3 (SD: 14.5) | ActiGraph 7,164 |
√ | √ | √ | √ | CVD Evente |
Good | |
| K Bakrania49 2017, UK |
5.7 (SD: NR) | 683 36.6% |
63.6 (SD: 7.8) | ActiGraph GT3X |
√ | √ | ACM | Fair | |||
| AZ. LaCroix50 2019, U.S. |
3.5 (range: 0.01–4.9) |
5,861 100% |
78.5 (SD: 6.7) | Actigraph GT3X+ |
√ | √ | CVD Eventf |
Good | |||
| PD Loprinzi51 2017, U.S. |
6.6 (SD: NR) | 5,575 51.6% |
46.3 (SE: 0.5) | ActiGraph 7,164 |
√ | ACM | Fair | ||||
| J Klenk59,a 2016, GE |
4.0 (SD: NR) | 1,271 43.6% |
75.6±6.5 | ActivPAL |
√ | √ | ACM | Good | |||
| PD Loprinzi52 2016, U.S. |
7.7 (IQR: 7.3–8.3) |
1,658 52.6% (alive), 46.6% (not alive) |
Range: 40–85 | Actigraph 7,164 |
√ | ACM | Fair | ||||
| YV Chudasama53,a 2019, UK |
6.9 (range: 3.8–9.1) |
95,616 54.9%b, 56.6%h |
65.7g (IQR: 59.5–69.9) 62.3h (IQR: 55.2–67.6) |
Axivity AX3 |
√ | ACM | Good | ||||
| T Dwyer54 2015, AU |
10.0 (SD: NR) | 2,576 52.4% |
58.8 (SD: 13.2) | Omron HJ-003, HJ-102 Yamax SW-200 |
√ | ACM | Good | ||||
| N Yamamoto55,a) 2018, JP |
9.8 (Range: 1–11) |
419 45.6% |
71.0 (SD: 0) | Yamasa EC-100S |
√ | ACM | Fair | ||||
| Lee56,a 2019, U.S. |
4.3 (SD: NR) | 16,741 100% |
72.0 (SD: 5.7) | Actigraph GT3X+ |
√ | ACM | Good | ||||
| AA Wanigatunga57 2019, U.S. |
4.4 (SD: 2.2) | 529 47.8% |
75.8 (SD: 7.2) | Actiheart |
√ | ACM | Fair | ||||
| S Oftedal25 2019, AU |
9.6 (range: 0.2–13.1) |
1,697 49.3% |
65.4 (SD: 7.1) | Yamax SW-200 |
√ | ACM | Fair | ||||
| CM Koolhaas58,a 2017, NE |
7.5 (IQR: 6.6–8.3) |
1,839 54.4% |
T1: 60.1 (SD: 8.0) T2: 63.7 (SD: 9.5) T3: 65.2 (SD: 10.6) |
Actiwatch AW4 |
√ | ACM | Good | ||||
| Bellettiere24 2019, U.S. |
4.9 (SD: NR) | 5,471 100% |
Q1: 76.3 (SD: 6.2) Q2: 78.1 (SD: 6.6) Q3: 78.9 (SD: 6.6) Q4: 80.9 (SD: 6.5) |
ActiGraph GT3x+ |
√ | CVD Eventb |
Fair | ||||
| PF Saint-Maurice21,a 2020, U.S. |
10.1 (SD: NR) | 4,840 53.5% |
56.8 (SE: 0.4) | ActiGraph 7,164 |
√ | ACM, CM | Good | ||||
Follow-up duration and age are reported as mean (SD or SE), median (IQR), or range.
Studies included in the meta-analysis.
CVD events (include the first occurrence of an MI, revascularization, hospitalized angina, heart failure, stroke, or death attributable to any CVD among women without that event) and CHD events (nonfatal MI or coronary death) as a separate endpoint.
Total fatal and nonfatal incidents of CVD.
Fatal and nonfatal CHD, stroke, heart failure.
CVD events and mortality.
Coronary heart disease, revascularization, carotid artery disease, hospitalized angina, congestive heart failure, stroke, or death from other CVDs.
With multimorbidity.
Without multimorbidity.
ACM, all-cause mortality; AU, Australia; BR, Brazil; CHD, coronary heart disease; CM, cardiovascular disease‒specific mortality; CVD, cardiovascular disease; GE, Germany; JP, Japan; LPA, light-intensity physical activity; MI, myocardial infarction; MVPA, moderate-to-vigorous intensity physical activity; NE, Netherlands; NR, not reported; Q, quartile; SB, sedentary behavior; SE, standard error; SW, Sweden; T, Tertile; TPA, total physical activity; UK, United Kingdom.
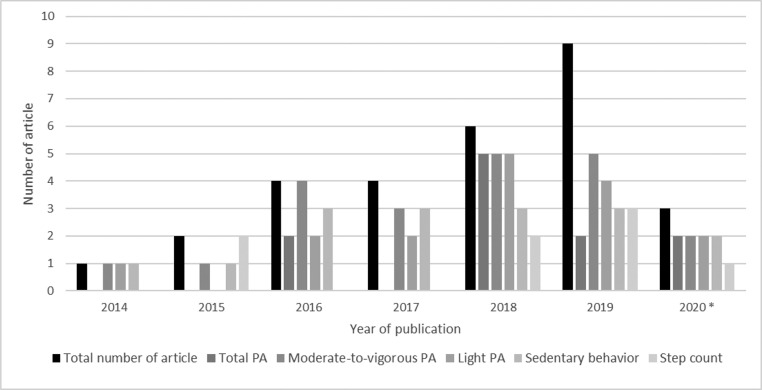
All 29 included studies were published since 2014, with an increasing number of articles published across the years (Figure 2). The studies involved a total of 206,970 adults and were conducted in various parts of the world, that is, the U.S. (n=15),21,23,24,36,37,39,41,43,44,47,50, 51, 52,56,57 the United Kingdom (n=6),22,40,45,46,49,53 Australia (n=2),25,54 Sweden (n=2),38,48 Germany (n=1),59 the Netherlands (n=1),58 Brazil (n=1),42 and Japan (n=1).55 Sample sizes varied across studies: <1,000 participants (n=6),38,42,45,49,55,57 1,000–10,000 participants (n=20),21, 22, 23, 24, 25,37,39, 40, 41,43,44,46, 47, 48,50, 51, 52,54,58,59 and >10,000 participants (n=3).36,53,56 The mean follow-up duration ranged from 2.3 to 14.4 years. Five studies included only females,24,36,43,50,56 3 studies included only males,40,44,46 and 21 studies included both females and males.21, 22, 23,25,37, 38, 39,41,42,45,47, 48, 49,51, 52, 53, 54, 55,57, 58, 59 Thirteen of the 29 studies specifically recruited adults aged ≥60 years,24,36,40,42, 43, 44, 45, 46,50,55, 56, 57,59 whereas the remaining 16 studies recruited participants with a broader age range. Across these studies, different device brands were used to measure the exposures (Table 1). Overall, 16 of the 29 included studies were rated as having good quality, 13 were rated fair, and no study was rated poor. Key findings of the 29 included studies are presented in Tables 2 and 3 for CVD outcomes and all-cause mortality, respectively. Additional information about the study design and definitions of exposure and outcome measures, covariates included in the mostly adjusted multivariable analysis model of each included study, effect estimates, and quality rating of each study are available in Appendix Tables 1–9 (available online).
Figure 2.
Total number of studies and exposures being examined by publication year up to April 29, 2020.
PA, physical activity.
Table 2.
Associations Between Total PA, Moderate-to-Vigorous PA, Light-Intensity PA, SB, and Step Count and CVD Mortality or CVD Event
| First author year | CVD outcome | Total PA |
MVPA | Light-intensity PA |
SB | Step count |
|---|---|---|---|---|---|---|
| Dohrn 201838 |
CVD mortality | AC/day (multiply by 1,000): T1: HR=1.0 (ref) T2: HR=0.27 (0.06, 1.16) T3: HR=0.09 (0.01, 0.93) |
Minute/day T1: HR=1.0 (ref) T2: HR=0.22 (0.06–0.74) T3: HR=0.11 (0.03–0.56) |
Minute/day T1: HR=1.0 (ref) T2: HR=0.48 (0.19, 1.19) T3: no event |
Minute/day T1: HR=1.0 (ref) T2: HR=1.63 (0.36, 7.28) T3: HR=5.51 (1.43, 21.23) |
NA |
| Evenson 201639 |
CVD mortality | AC/minute Q1: HR=1.0 (ref) Q2: HR=0.49 (0.29, 0.83) Q3: HR=0.42 (0.22, 0.81) Q4: HR=0.35 (0.15, 0.84) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.49 (0.28, 0.84) Q3: HR=0.18 (0.07, 0.46) Q4: HR=0.48 (0.23, 1.03) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=1.06 (0.65, 1.72) Q3: HR=0.68 (0.36, 1.26) Q4: HR=0.90 (0.44, 1.84) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=1.12 (0.54, 2.31) Q3: HR=1.03 (0.50, 2.12) Q4: HR=1.44 (0.71, 2.90) |
NA |
| MJ LaMonte 201843 |
CVD mortality | VMC minute/day T1: risk ratio=1.0 (ref) T2: risk ratio=0.64 (0.44, 0.94) T3: risk ratio=0.29 (0.16, 0.53) |
Minute/day T1: risk ratio=1.0 (ref) T2: risk ratio=0.68 (0.45, 0.99) T3: risk ratio=0.42 (0.24, 0.75) |
Minute/day low-intensity: T1: risk ratio=1.0 (ref) T2: risk ratio=0.69 (0.47, 1.02) T3: risk ratio=0.64 (0.41, 0.99) high-intensity: T1: risk ratio=1.0 (ref) T2: risk ratio=0.50 (0.34, 0.74) T3: risk ratio=0.30 (0.17, 0.51) |
NA | NA |
| Dempsey 202022 |
CVD eventa | Higher levels of TPA were associated with lower incident CVD risk in a nonlinear manner. (Ref: 120 cpm/day) |
Higher levels of MVPA were associated with lower incident CVD risk in a nonlinear manner. (Ref: no MVPA) |
Association was attenuated after covariate and MVPA adjustment (Ref: 3 hours/day) |
Positive associations with a steeper relationship beyond 11 hours/day, attenuated after adjusted for covariates and MVPA (ref: 8 hours/day) | NA |
| KE Ensrud 201444 |
CVD mortality | NA | Minute/day Q1 (most active): HR=1.0 (ref) Q2: HR=1.60 (0.82, 3.11) Q3: HR=1.79 (0.94, 3.42) Q4: HR=2.86 (1.50, 5.45) |
Minute/day Q1 (most active): HR=1.0 (ref) Q2: HR=1.29 (0.67, 2.49) Q3: HR=1.92 (1.04, 3.55) Q4: HR=1.73 (0.90, 3.34) |
Minute/day Q1 (least sedentary): HR=1.0 (ref) Q2: HR=1.59 (0.91, 2.75) Q3: HR=1.12 (0.63, 2.00) Q4: HR=1.71 (0.99, 2.97) |
NA |
| Bellettiere 201924 |
CVD eventb | NA | NA | NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=1.40 (1.03, 1.89) Q3: HR=1.40 (1.03, 1.89) Q4: HR=1.53 (1.09, 2.14) |
NA |
| Dohrn 201948 |
CVD eventc (c) | AC/day (multiply by 1000): 1,000): T1: HR=1.0 (ref) T2: HR=0.70 (0.46, 1.05) T3: HR= 0.67 (0.44, 1.04) |
Minute/day T1: HR=1.0 (ref) T2: HR=0.77 (0.52, 1.19) T3: HR=0.52 (0.33, 0.82) |
Minute/day T1: HR=1.0 (ref) T2: HR=1.03 (0.70, 1.53) T3: HR=0.92 (0.60, 1.42) |
Minute/day T1: HR=1.0 (ref) T2: HR=1.05 (0.68, 1.64) T3: HR=1.41 (0.91, 2.20) |
NA |
| Jefferis 201946 |
CVD eventd | NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=0.62 (0.38, 1.00) Q3: HR=0.28 (0.14, 0.55) Q4: HR=0.34 (0.16, 0.74) every 10-minute/day increase: HR=0.89 (0.80, 0.99) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.93 (0.57, 1.50) Q3: HR=0.59 (0.33, 1.06) Q4: HR=0.97 (0.53, 1.80) every 30-minute/day increase: HR=0.99 (0.89, 1.11) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.70 (0.38, 1.28) Q3: HR=0.81 (0.43, 1.52) Q4: HR=0.94 (0.45, 1.94) every 30-minute/day increase: HR=1.01 (0.90, 1.13) |
Steps/day: Q1: HR=1.0 (ref) Q2: HR=0.75 (0.47, 1.20) Q3: HR=0.44 (0.25, 0.77) Q4: HR=0.34 (0.17, 0.67) Every 1,000 step/day increase: HR=0.86 (0.78, 0.95) |
| LaCroix 201950 |
CVD evente | NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=0.75 (0.61, 0.93) Q3: HR=0.66 (0.52, 0.84) Q4: HR=0.71 (0.54, 0.93) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=1.05 (0.84, 1.30) Q3: HR=0.90 (0.71, 1.14) Q4: HR=0.82 (0.63, 1.07) every 1-hour/day increase: HR=0.92 (0.85, 0.99) |
NA | NA |
| Saint-Maurice 202021 |
CVD mortality | NA | NA | NA | NA | Step/day Qn1: HR=1.0 (ref) Qn2: HR=0.68 (0.60, 0.76) Qn3: HR=0.49 (0.40, 0.60) Qn4: HR=0.40 (0.30, 0.52) Qn5: HR=0.35 (0.24, 0.52) |
Note: For mg, 1 mg = 0.00981 ms–2. Associations were presented by exposure category (e.g., T, Q).
Total fatal and nonfatal incidents of CVD.
CVD events (include the first occurrence of an MI, revascularization, hospitalized angina, heart failure, stroke, or death attributable to any CVD among women without that event) and CHD events (nonfatal MI or coronary death) as a separate endpoint.
CVD events and mortality.
Fatal and nonfatal CHD, stroke, heart failure.
CVD events (include CHD, revascularization, carotid artery disease, hospitalized angina, congestive heart failure, stroke, and other CVD deaths).
AC, accelerometer count derived from Actigraph accelerometer; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; mg, milligravity; MI, myocardial infarction; MVPA, moderate-to-vigorous intensity physical activity; NA, not applicable; PA, physical activity; Q, quartile; Qn, quintile; T, tertile; VMC, vector magnitude counts derived from accelerometer.
Table 3.
Associationsa Between Total PA, Moderate-to-Vigorous PA, Light-Intensity PA, SB, and Step Count and All-Cause Mortality
| First author Year |
Total PA |
MVPA | Light-intensity PA |
SB | Step count |
|---|---|---|---|---|---|
| Lee 201836 |
AC/day Q1: HR=1.0 (ref) Q2: HR=0.79 (0.55, 1.12) Q3: HR=0.73 (0.49, 1.09) Q4: HR=0.44 (0.26, 0.74) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.61 (0.42, 0.89) Q3: HR=0.58 (0.38, 0.89) Q4: HR=0.35 (0.20, 0.61) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.97 (0.67, 1.39) Q3: HR=0.79 (0.52, 1.21) Q4: HR=1.06 (0.69, 1.64) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.97 (0.62, 1.50) Q3: HR=1.18 (0.77, 1.82) Q4: HR=0.92 (0.56, 1.50) |
NA |
| Dohrn 201838 |
AC/day (multiply by 1,000): T1: HR=1.0 (ref) T2: HR=0.55 (0.28, 1.07) T3: HR=0.30 (0.13, 0.70) |
Minute/day T1: HR=1.0(ref) T2: HR=0.58 (0.33, 1.00) T3: HR=0.50 (0.28, 0.90) |
Minute/day T1: HR=1.0 (ref) T2: HR=0.46 (0.27, 0.78) T3: HR=0.34 (0.17, 0.67) |
Minute/day T1: HR=1.0 (ref) T2: HR=1.88 (0.99, 3.55) T3: HR=2.72 (1.40, 5.30) |
NA |
| Evenson 201639 |
AC/minute Q1: HR=1.0 (ref) Q2: HR=0.60 (0.45, 0.80) Q3: HR=0.39 (0.27, 0.57) Q4: HR=0.37 (0.23, 0.59) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.56 (0.41, 0.76) Q3: HR=0.41 (0.27, 0.62) Q4: HR=0.44 (0.28, 0.69) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.89 (0.22, 1.05) Q3: HR=0.70 (0.35, 1.47) Q4: HR=0.73 (0.48, 2.08) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=1.05 (0.71, 1.55) Q3: HR=0.86 (0.58, 1.27) Q4: HR=0.97 (0.65, 1.44) |
NA |
| Saint-Maurice 201841 |
AC/day (multiplied by 1,000) Q1: HR=1.0 (ref) Q2: HR=0.48 (0.38, 0.59) Q3: HR=0.30 (0.23, 0.40) Q4: HR=0.26 (0.16, 0.44) |
AC/day (multiplied by 1,000) Q1: HR=1.0 (ref) Q2: HR=0.54 (0.42, 0.70) Q3: HR=0.29 (0.22, 0.39) Q4: HR=0.28 (0.17, 0.46) |
AC/day (multiplied by 1,000) Q1: HR=1.0 (ref) Q2: HR=0.72 (0.56, 0.91) Q3: HR=0.77 (0.59, 1.02) Q4: HR=0.69 (0.47, 1.00) |
NA | NA |
| Bielemann 201942 |
mg/day Men T1: HR=1.0 (ref) T2: HR=0.43 (0.17, 1.08) T3: HR=0.23 (0.06, 0.84) Women T1: HR=1.0 (ref) T2: HR=0.55 (0.22, 1.42) T3: HR=0.08 (0.01, 0.65) |
Minute/day men T1: HR=1.0 (ref) T2: HR=0.98 (0.45, 2.13) T3: HR=0.22 (0.05, 1.05) women T1: HR=1.0 (ref) T2: HR=0.30 (0.11, 0.82) T3: HR=0.07 (0.01, 0.59) |
Minute/day men T1: HR=1.0 (ref) T2: HR=0.73 (0.30, 1.77) T3: HR=0.26 (0.07, 0.95) Women T1: HR=1.0 (ref) T2: HR=0.87 (0.37, 2.05) T3: HR=0.09 (0.01, 0.67) |
NA | NA |
| LaMonte 201743 |
VMC/day T1: risk ratio=1.0 (ref) T2: risk ratio=0.68 (0.54, 0.85) T3: risk ratio=0.49 (0.37, 0.66) Every 30-minute/day increase: risk ratio=0.88 (0.85, 0.92) |
Minute/day T1: risk ratio=1.0 (ref) T2: risk ratio=0.63 (0.50, 0.79) T3: risk ratio=0.42 (0.30, 0.57) Every 30-minute/day increase: risk ratio=0.67 (0.58, 0.78) |
Minute/day low-intensity: T1: risk ratio=1.0 (ref) T2: risk ratio=0.86 (0.69, 1.08) T3: risk ratio=0.80 (0.62, 1.03) High-intensity: T1: risk ratio=1.0 (ref) T2: risk ratio=0.57 (0.45, 0.71) T3: risk ratio=0.47 (0.35, 0.61) every 30-minute/day increase: risk ratio=0.93 (0.89, 0.97) |
NA | NA |
| Dempsey 202022 |
Association was less consistent and tended toward the null after an initial steep decrease in HRs (Ref: 120 cpm/day) |
Association was less consistent and tended toward the null after an initial steep decrease in HRs (Ref: no MVPA) |
Consistently strong and approximately linear inverse associations (Ref: 3 hours/day) |
Consistently strong and approximately linear inverse associations (Ref: 8 hours/day) |
NA |
| Tarp 202023 |
Median AC/day Q1: HR=1.0 (ref) Q2: HR=0.74 (0.53, 1.04) Q3: HR=0.52 (0.37, 0.73) Q4: HR=0.61 (0.37, 1.01) |
Median minute/day Q1: HR=1.0 (ref) Q2: HR=0.67 (0.47, 0.96) Q3: HR=0.67 (0.47, 0.95) Q4: HR=0.68 (0.39, 1.18) |
Median minute/day Q1: HR=1.0 (ref) Q2: HR=0.82 (0.61, 1.08) Q3: HR=0.93 (0.65, 1.34) Q4: HR=0.74 (0.50, 1.09) |
Median hour/day Q1: HR=1.0 (ref) Q2: HR=1.16 (0.63, 2.15) Q3: HR=0.97 (0.58, 1.64) Q4: HR=1.31 (0.80, 2.17) |
NA |
| Ensrud 201444 |
NA | Minute/day Q1 (most active): HR=1.0 (ref) Q2: HR=1.15 (0.81, 1.65) Q3: HR=1.29 (0.92, 1.83) Q4: HR=1.80 (1.27, 2.54) |
Minute/day Q1 (most active): HR=1.0 (ref) Q2: HR=1.06 (0.73, 1.54) Q3: HR=1.42 (1.00, 2.01) Q4: HR=1.70 (1.19, 2.45) |
Minute/day Q1 (least sedentary): HR=1.0 (ref) Q2: HR=1.26 (0.92, 1.75) Q3: HR=1.12 (0.81, 1.56) Q4: HR=1.56 (1.15, 2.14) |
NA |
| Loprinzi 201751 |
NA | NA | Every 60-minute/day increase: HR=0.84 (0.78, 0.91) |
NA | NA |
| Loprinzi 201652 |
NA | Every 1-minute/day increase: HR=0.97 (0.94, 0.99) |
NA | NA | NA |
| Bakrania 201749 |
NA | Every 10% increasea in minute/day: HR=0.95 (0.91, 0.98) |
NA | Every 10-minute/day increase: HR=0.99 (0.95, 1.03) |
NA |
| Wanigatunga 201957 |
Active hour/day HR=0.91 (0.76, 1.09) |
NA | NA | NA | NA |
| Jefferis 201840 |
NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=1.05 (0.71, 1.57) Q3: HR=0.89 (0.53, 1.47) Q4: HR=0.90 (0.48, 1.70) Every 10-minute/day increase: HR=1.00 (0.92, 1.09) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=0.76 (0.53, 1.10) Q3: HR=0.42 (0.27, 0.68) Q4: HR=0.57 (0.34, 0.95) Every 30-minute/day increase: HR=0.87 (0.80, 0.95) |
Minute/day Q1: HR=1.0 (ref) Q2: HR=1.14 (0.69, 1.91) Q3: HR=1.55 (0.91, 2.64) Q4: HR=2.73 (1.50, 4.95) Every 30-minute/day increase: HR= 1.15 (1.06, 1.26) |
Q1: HR=1.0 (ref) Q2: HR=0.63 (0.43, 0.93) Q3: HR=0.59 (0.39, 0.90) Q4: HR=0.31 (0.17, 0.57) Every 1,000 step/day increase: HR=0.86 (0.80, 0.93) |
| Matthews 201637 |
NA | Minute/day HR=0.60 (0.45, 0.81) |
Minute/day HR=0.84 (0.75, 0.95) |
Minute/day HR=1.03 (0.98, 1.08) |
NA |
| Chudasama 201953 |
NA | Minute/day With multimorbidity T1: HR=1.0 (ref) T2: HR=0.49 (0.29, 0.80) T3: HR=0.29 (0.13, 0.61) without multimorbidity T1: HR=1.0 (ref) T2: HR=0.40 (0.29, 0.57) T3: HR=0.29 (0.19, 0.46) |
NA | NA | NA |
| Fox 201545 |
NA | Minute/day T1: HR=1.0 (ref) T2: HR=1.19 (0.23, 6.24) T3: HR=1.84 (0.32, 10.75) |
NA | Minute/day T1: HR=1.0 (ref) T2: HR=0.99 (0.39, 2.58) T3: HR=1.01 (0.35, 2.98) |
T1: HR=1.0 (ref) T2: HR=3.90 (0.77, 19.70) T3: HR=5.46 (0.91, 32.76) Every 1,000 step/day increase: HR=0.64 (0.44, 0.91) |
| Diaz 201747 |
NA | NA | NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=1.22 (0.74, 2.02) Q3: HR=1.61 (0.99, 2.63) Q4: HR=2.63 (1.60, 4.30) |
NA |
| Klenk 201659 |
NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=0.58 (0.33, 1.02) Q3: HR=0.30 (0.14, 0.66) Q4: HR=0.47 (0.23, 0.99) |
NA | Minute/day Q1: HR=1.0 (ref) Q2: HR=0.98 (0.49, 1.98) Q3: HR=0.59 (0.28, 1.22) Q4: HR=1.52 (0.81, 2.83) |
NA |
| Koolhaas 201758 |
NA | NA | NA | Hour/day Q1: HR=1.0 (ref) Q2: HR=1.21 (0.81, 1.81) Q3: HR=1.50 (0.93, 2.41) Every 1-hour increase: HR=1.04 (0.96, 1.13) |
NA |
| Yamamoto 201855 |
NA | NA | NA | NA | Q1: HR=1.0 (ref.) Q2: HR=0.81 (0.43, 1.54) Q3: HR=1.26 (0.70, 2.26) Q4: HR=0.46 (0.22, 0.96) |
| Dwyer 201554 |
NA | NA | NA | NA | Every 1,000 step/day increase: HR=0.94 (0.90, 0.98) |
| Oftedal 201925 |
NA | NA | NA | NA | Every 1,000 step/day increase: HR=0.93 (0.88, 0.98) |
| Saint-Maurice 202021 |
NA | NA | NA | NA | Q1: HR=1.0 (ref) Q2: HR=0.68 (0.64, 0.72) Q3: HR=0.49 (0.44, 0.55) Q4: HR=0.40 (0.34, 0.46) Q5: HR=0.35 (0.28, 0.45) |
| Lee 201956 |
NA | NA | NA | NA | Q1: HR=1.0 (ref) Q2: HR=0.54 (0.43, 0.69) Q3: HR=0.47 (0.35, 0.62) Q4: HR=0.34 (0.24, 0.48) Per1000 step/day increase: HR=0.82 (0.78, 0.87) |
Note: For mg, 1 mg = 0.00981 ms–2. For association, in each category of exposure, findings are presented in this format: for example, classification of exposure presented in mean±SD, median and IQR, or range; effect size presented in HR or risk ratio and corresponding 95% CI.
AC, accelerometer count derived from Actigraph accelerometer; HR, hazard ratio; mg, milligravity; MVPA, moderate-to-vigorous intensity physical activity; NA, not applicable; PA, physical activity; Q, quartile; T, tertile; VMC, vector magnitude counts derived from accelerometer.
Data were log-transformed to reduce the influence of skewed data. To ensure that the HR represented a 10% increase, a log base of 1.1 (i.e., log1.1 [MVPA time]) was used.
We conducted meta-analyses for 2 outcomes, that is, CVD mortality and all-cause mortality, but not for other CVD outcomes because the definitions of CVD events were inconsistent. Finally, the meta-analyses included 5 studies38,39,43,44,46 for CVD mortality and 15 studies21,36,38, 39, 40,42, 43, 44, 45,47,53,55,56,58,59 for all-cause mortality. Not included in the meta-analyses were studies that did not meet the inclusion criteria for analysis, that is, exposure measure was treated as a binary or noncategorical variable, CVD outcome measure combined both fatal or nonfatal CVD incidence and mortality, subanalysis findings of the original study that had already been included in the main meta-analysis, insufficient outcome cases, or lack of data point for meta-analysis. An overview of the meta-analysis results organized by types of outcome and exposure is presented in Table 4.
Table 4.
Summary of Meta-analysis Results by Types of Outcome and Exposure
| Outcome and exposure measures | Pooled results |
||||
|---|---|---|---|---|---|
| n | HR | 95% CI | Heterogeneity |
||
| I2 (%) | p-value | ||||
| Outcome 1: cardiovascular disease mortality | |||||
| Activity intensity | |||||
| 1. Total PA | 3 | 0.29 | 0.18–0.47 | 0.0 | 0.547 |
| 2. Moderate-to-vigorous PA | 4 | 0.37 | 0.25–0.55 | 9.3 | 0.347 |
| 3. Light PA | 3 | 0.62 | 0.41–0.93 | 0.0 | 0.410 |
| 4. Sedentary behavior | 3 | 1.89 | 1.09–3.29 | 34.7 | 0.216 |
| Step count | |||||
| Steps per day | 1 | ―a | ―a | ||
| Outcome 2: all-cause mortality | |||||
| Main analysis | |||||
| Activity intensity | |||||
| 1. Total PA | 6 | 0.42 | 0.34–0.53 | 4.4 | 0.389 |
| 2. Moderate-to-vigorous PA | 11 | 0.43 | 0.35–0.53 | 31.8 | 0.136 |
| 3. Light PA | 8 | 0.58 | 0.43–0.80 | 50.5 | 0.049 |
| 4. Sedentary behavior | 7 | 1.58 | 1.19–2.09 | 61.7 | 0.007 |
| Step count | |||||
| Steps per day | 4 | 0.35 | 0.29–0.42 | 0.0 | 0.874 |
| Additionalb analysis | |||||
| Activity intensity | |||||
| 1. Total PA | 6 | 0.42 | 0.34–0.53 | 4.4 | 0.389 |
| 2. Moderate-to-vigorous PA | 6 | 0.41 | 0.33–0.51 | 0.0 | 0.494 |
| 3. Light PA | 6 | 0.54 | 0.33–0.90 | 63.6 | 0.017 |
Less than 2 observations were available.
Included only studies that reported outcomes for total PA, moderate-to-vigorous intensity PA, and light-intensity PA.
HR, hazard ratio; N, number of observation point; PA, physical activity.
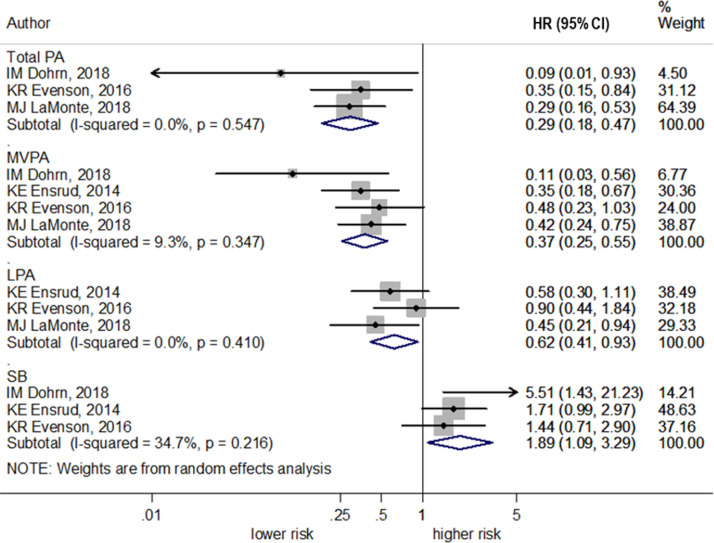
The association between TPA and CVD mortality was investigated in 3 studies.38,39,43 The meta-analysis of these 3 studies38,39,43 revealed a lower hazard of CVD mortality when comparing the highest with the lowest category of TPA (pooled HR=0.29, 95% CI=0.18, 0.47, I2=0.0%, p heterogeneity=0.55) (Figure 3). The meta-analysis included 5 studies36,38,39,42,43 that examined the association of TPA with all-cause mortality. It found a lower hazard of all-cause mortality when comparing the highest category with the lowest category of TPA (pooled HR=0.42, 95% CI=0.34, 0.53, I2=4.4%, p heterogeneity=0.39) (Figure 4).
Figure 3.
Cardiovascular mortality hazards according to PA and SB (highest versus lowest categories). LaMonte et al.43 reported risk estimates in risk ratio.
HR, hazard ratio; LPA, light physical activity; MPVA, moderate-to-vigorous physical activity; PA, physical activity; SB, sedentary behavior.
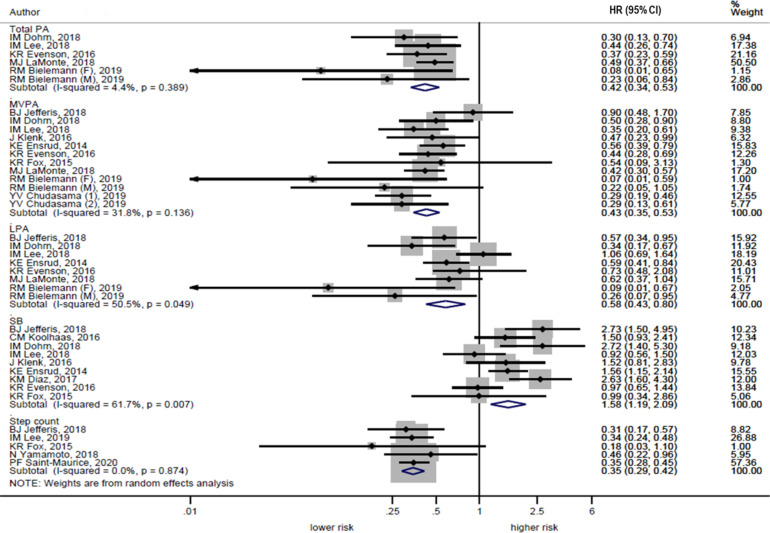
Figure 4.
All-cause mortality hazards according to PA and SB (highest versus lowest categories). LaMonte et al.43 reported risk estimates in risk ratio. Study 1, without multimorbidity; Study 2, with multimorbidity.
F, female; HR, hazard ratio; LPA, light-intensity physical activity; M, male; MPVA, moderate-to-vigorous physical activity; PA, physical activity; SB, sedentary behavior.
The meta-analysis included 4 studies38,39,43,44 that examined the association of MVPA with CVD mortality. It revealed a lower hazard of CVD mortality when comparing the highest with the lowest category of MVPA (pooled HR=0.37, 95% CI=0.25, 0.55, I2=9.3%, p heterogeneity=0.35) (Figure 3). The meta-analysis included 10 studies36,38, 39, 40,42, 43, 44, 45,53,59 that examined the association of MVPA with all-cause mortality. It found a lower hazard of all-cause mortality when comparing the highest category with the lowest category of MVPA (pooled HR=0.43, 95% CI=0.35, 0.53, I2=31.8%, p heterogeneity=0.14) (Figure 4).
The meta-analysis included 3 studies39,43,44 which examined the association of LPA with CVD mortality and found a lower CVD mortality hazard when comparing the highest with the lowest category of LPA (pooled HR=0.62, 95% CI=0.41, 0.93, I2=0.0%, p heterogeneity=0.41) (Figure 3). The meta-analysis included 7 studies36,38, 39, 40,42, 43, 44 that examined the association of LPA with all-cause mortality. It found lower all-cause mortality when comparing the highest category with the lowest category of LPA (pooled HR=0.58, 95% CI=0.43, 0.80, I2=50.5%, p heterogeneity=0.049) (Figure 4).
The meta-analysis included 3 studies38,39,44 that examined the association of SB with CVD mortality. It revealed a higher hazard of CVD mortality when comparing the highest category with the lowest category of SB (pooled HR=1.89, 95% CI=1.09, 3.29, I2=34.7%, p heterogeneity=0.22) (Figure 3). The meta-analysis included 9 studies that examined SB with all-cause mortality. It found a higher hazard of all-cause mortality when comparing the highest category with the lowest category of SB (pooled HR=1.58, 95% CI=1.19, 2.09, I2=61.7%, p heterogeneity=0.01) (Figure 4).
The present review found only 1 study that examined the association of step counts with CVD mortality. Hence, meta-analysis was not conducted for step counts in relation to CVD mortality. The meta-analysis included 5 studies40,45,54, 55, 56 that examined the association of steps per day with all-cause mortality. It found that a lower hazard of all-cause mortality was associated with higher step counts (pooled HR=0.35, 95% CI=0.29, 0.42, I2=0.0%, p heterogeneity=0.87) (Figure 4).
As far as additional analyses, first, we analyzed a subset of 5 studies36,38,39,42,43 that examined the associations of all the 3 PA subtypes (i.e., TPA, MVPA, and LPA) with all-cause mortality (Table 4). Consistent with the results of the main analysis, findings from the subanalysis showed strong beneficial associations for TPA and MVPA but weaker associations for LPA in relation to all-cause mortality. Furthermore, the observed heterogeneity was small for TPA and MVPA but substantial for LPA.
Second, we conducted 2 sensitivity analyses. The first sensitivity analysis was conducted for the associations of MVPA, LPA, and SB with CVD mortality by including 1 study that considered both fatal and nonfatal CVD events as an outcome. Although the association (Appendix Figure 2, available online) remained unchanged for MVPA, the pooled estimates for LPA and SB ceased to be statistically significant. The second sensitivity analysis was conducted for the association of MVPA with all-cause mortality by excluding 1 study that estimated the follow-up duration on the basis of the time since baseline assessment at recruitment instead of the actual start time of device-based measurements. The results of this sensitivity analysis indicated a weaker negative association between MVPA and all-cause mortality, along with a reduction in the degree of heterogeneity (Appendix Figure 3, available online).
Third, we conducted 2 subgroup analyses by (1) age range (broad age range versus older participants defined as the mean age ≥60 years) and (2) sex (female versus male) for all-cause mortality. No substantial differences between these subgroups were found. Overall, the direction of associations in all subgroups (Appendix Table 10, available online) was consistent with the results of the main analysis. However, it was noted that 4 of 5 studies that investigated the association between step counts and all-cause mortality were conducted among older adults, thus limiting the interpretation of our findings of subgroup analysis to the older age group.
Funnel-plot diagrams and statistical tests for publication bias for all-cause mortality were presented in Appendix Figure 4 (available online). There was no indication of publication bias for MVPA, SB, and step counts but a potential bias for TPA (Egger's p=0.003, Begg's p=0.02) and LPA (Egger's p=0.08, Begg's p=0.27).
Regarding certainty of evidence, the evidence profile and summary of findings for 2 outcomes (i.e., CVD mortality and all-cause mortality) were presented in Appendix Table 11 (available online). We did not observe major seriousness in terms of study limitations, indirectness, and imprecision across the studies. The pooled findings largely agreed with the body of knowledge. However, a moderate degree of inconsistency was observed among studies investigating SB and all-cause mortality. This variation in point estimates may be attributable to sex differences in risk reduction and variations associated with the type of SB measures used in the primary studies. Considering the observational study design and the assessments based on Grading of Recommendations Assessment, Development, and Evaluation criteria, the overall certainty of evidence was moderate for evidence that showed a large magnitude of effects with no serious study limitations.
DISCUSSION
We provide a comprehensive overview of prospective cohort studies on the association of device-measured PA and SB with CVD outcomes and all-cause mortality. The outcomes of this systematic review offer holistic insights into long-term health benefits, covering the entire intensity spectrum of PA as well as step counts. The findings suggest that evidence on this topic is emerging rapidly because 18 of the 29 included studies were published since 2018. We found that being more physically active at any intensity, accumulating more daily steps, and spending less time in SB were associated with a substantially lower risk of CVD and all-cause mortality. The meta-analysis of 16 studies suggests that the observed associations with CVD outcomes and all-cause mortality were strong and consistent for TPA, MVPA, and step counts, whereas the associations with LPA and SB were somewhat weaker and less consistent across studies. Some degree of inconsistency was observed in the pooled association of SB with all-cause mortality. This is partly due to sex differences in risk reductions and variations associated with the types of SB measures used in the primary studies. The results of subgroup analysis by age and sex were generally consistent with those of the main results, with the exception of the steps-per-day exposure measure where the available studies were conducted among older adults. Generally, our results are consistent with the findings of earlier systematic reviews and meta-analyses on the basis of self-reported PA or SB.1,2,4, 5, 6, 7 However, heterogeneity across the studies included in our review appears to be lower, possibly attributable to a greater reliability of device-measured exposures and reduced errors during data collection process.
This systematic review has included a large number of prospective cohort studies, covering a variety of devices and data processing protocols used to quantify PA and SB. In terms of intensity-based PA and SB, our findings are largely consistent with the results of the harmonized meta-analysis that included 2 types of devices, that is, actigraph and Actical.16 Although not directly comparable with the review, we observed stronger and more consistent associations for TPA and MVPA. Besides, we also found a beneficial association for LPA, although it was somewhat weaker and less consistent than that of MVPA. These smaller beneficial associations of LPA with cardiometabolic health agree with the findings of an earlier review.18 In line with the current body of evidence, we observed an association between more SB and a higher risk of CVD and all-cause mortality.16 However, the results of this review for SB appeared somewhat less consistent than those for TPA, MVPA, and step counts, possibly owing to the varying definitions of SB among the included studies. In terms of step counts, corroborating the findings of a previous review,19,20 we also observed a substantial inverse association between steps per day and all-cause mortality. However, our findings were mainly based on the available studies conducted among older adults. We also found that very few prospective studies have examined the relationship between step counts and CVD outcomes.
Our systematic review adds important new information. First, this review synthesizes recent evidence in relation to CVD outcomes that have not been investigated in previous reviews and meta-analyses. Second, this review considered a wide range of devices, exposure types, and outcomes, resulting in a more comprehensive overview of the existing evidence. Although the device characteristics and processing methods vary across the primary studies, all of these devices had shown validity in the measurements of exposures.60, 61, 62, 63 Despite these variations, the results of the included studies were largely consistent in terms of the strength and direction of associations. Third, more than half of the included studies were published from 2018 onward, showing rapid growth of evidence,21, 22, 23, 24, 25,42,53,57 which could not have been fully considered by the previous reviews.16,17,64 Fourth, in contrast to MVPA, LPA and accumulating daily step counts through low-intensity walking are examples of PA, which are more achievable by most people. LPA and low-intensity walking both contribute to a large proportion of PA in a typical day of our lives; however, the amount of time spent on LPA cannot be easily recalled, and the logging of daily step counts without the aid of technology is neither easy nor sustainable. Fortunately, research and consumer devices have now made it possible. Such improvements in technology and study methodology have thus enabled this review to investigate potential long-term health implications of LPA and step counts, beyond the focus on MVPA.
Limitations
A few limitations of our systematic review should be acknowledged. First, the findings are influenced by potential biases associated with the sample selection process, the quality of exposure measurements, and loss to follow-up. Unmeasured or residual confounding cannot be eliminated completely, possibly resulting in biases in the estimated associations. Second, the levels of the highest and the lowest categories of PA or SB varied and were not directly comparable across the study population. Such discrepancies can be attributed to individuals’ capacity for performing PA, data processing methods, and selection of cut points. Furthermore, the estimations of SB based on posture may produce results different from that based on activity counts and energy expenditure. However, this meta-analysis was not able to pursue the influence of these factors on the pooled estimates owing to the limited number of studies available for stratified analysis. Although it is easier for users to understand the concept of step counting, caution is advised when interpreting these results because step trackers of different working principles might have introduced variations to the counting of steps.65 Third, the included studies were conducted at different periods, and they spanned across a number of years or a decade. The choice of devices and data processing strategies were therefore influenced by the advancement of science and technology. Hence, the methods adopted in the primary studies conducted in earlier decades might not represent the state of the art. Fourth, although some studies reported that their results did not alter materially after excluding early deaths within 2 years from baseline,36,43,54 reverse causation cannot be eliminated entirely. Fifth, we did not find evidence of publication bias for most of the investigated associations except for the association of TPA and LPA with all-cause mortality. However, given that tests for publication bias were based on a modest number of observations, caution is needed in interpreting these results.
Findings from additional searches
Considering the rapid development and emerging research findings in the field, we updated our original search in September 2022. After screening >2,000 additional references, we identified 7 relevant studies published between April 2020 and September 2, 2022.66, 67, 68, 69, 70, 71 Although none of these studies provide data suitable for our meta-analysis, the additional findings add new knowledge to our synthesized evidence. Building on our main results, for example, a recent finding from the UK biobank6 suggests that the lowest risk for CVD in the cohort was observed at the highest level of TPA, MPA, or VPA. A study conducted among older adults7 further suggests that the risks of CVD and all-cause mortality were lowered for every 30-min/day increment in LPA and MPA but elevated with every 1-hour/day increment in sedentary time.
Similarly, more time spent on SB or prolonged patterns of sedentary accumulation was associated with a higher risk of atrial fibrillation.68 Owing to the complex nature of SB, besides the conventional measures of total time spent on SB, researchers have begun exploring SB from various angles. For instance, a recent publication from the EPIC-Norfolk study suggests that SB bout accumulation instead of SB volume was related to incident CVD.69 Although population characteristics and the measures of SB varied across the studies, similar health benefits were observed for lower sedentary behavior. Findings from a large population-based study with a mean follow-up of 11 years further found that performing more LPA resulted in a significantly greater number of years of life gained.70 This finding holds true for individuals with lower MVPA, suggesting that promoting LPA may be one of the practical recommendations to improve the life expectancy of adults considering that many adults accumulate low levels of MVPA in a typical day.
Our updated review of the literature indicates a growing interest in studying the impact of daily step counts on mortality.71, 72, 73 Findings from additional studies retrieved from the updated search71,72 were consistent with our results, suggesting lower risks of all-cause mortality among participants with higher daily step counts than among those with lower step counts. A study conducted among older persons provides further evidence of lower mortality risks among individuals who increased their daily step counts.73 These research outcomes collectively indicate a considerable growth in the evidence of the health benefits of taking more steps. We also observed the emergence of several distinct ways of analyzing step count data (i.e., step volume, stepping rate, increment of step counts).19,20 However, this meta-analysis specifically included one most relevant primary study from each population cohort that reported steps per day as an exposure measure, a simple measure for potential translation of research into practice, which would also be relevant to individuals who might benefit from an increasingly smaller number of steps each day.
Taken together, these additional findings provide further evidence supporting our main results that more PA, higher step counts, and less sedentary behavior will bring forth positive health outcomes in adults. Compelling new evidence has also emerged to suggest capitalizing on LPA to improve life expectancy among less active adults.
Despite the observational design of included studies, this review shows emerging evidence that is in accord with the body of knowledge that suggest the beneficial effects of being physically active on reducing the risks of chronic diseases and premature deaths. Nonetheless, the potential influence of the genetic composition of adults in the reduction of mortality risks cannot be determined in our review, hence, cannot be dismissed.
Finally, although findings appeared largely consistent across a broad range of study populations, the included primary studies represented a focus on the U.S. and European regions. Furthermore, these study participants were predominantly non-Hispanic Whites, and there was a low representation from Asian countries or ethnicities. Therefore, additional effort is encouraged to investigate the role of ethnicity or race in future population-based studies.
CONCLUSIONS
Evidence on the association of device-measured PA and SB with CVD and all-cause mortality is rapidly accumulating. Our findings suggest that more PA of any intensity and less SB may reduce the risk of CVD and all-cause mortality. In addition to measures of PA that are based on intensity levels, similar beneficial relationships were found for step counts in relation to all-cause mortality, but the studies available for analysis were conducted among older adults. Future efforts employing standardized research methodologies across studies and adopting up-to-date data processing approaches would better define specific amounts of PA and limits to sedentary time to recommend in future guidelines.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to express gratitude to Miss Annelissa Chin from the National University of Singapore Library for her guidance in performing systematic searches through the databases during our initial scoping review and to the Singapore National Library for the access to the SportDiscus database and prompt support from the librarians.
Declarations of interest: none.
CREDIT AUTHOR STATEMENT
Seaw Jia Liew: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. Nicholas A. Petrunoff: Writing – review & editing. Nithya Neelakantan: Meta Analysis, Writing – review & editing. Rob M. van Dam: Supervision, Writing – review & editing. Falk M-uller-Riemenschneider: Conceptualization, Methodology, Supervision, Writing – review & editing.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.focus.2022.100054.
Appendix. Supplementary materials
REFERENCES
- 1.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40(5):1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 2.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–2905. doi: 10.1007/s00125-012-2677-z. [published correction appears in Diabetologia. 2013;56(4):942–943] [DOI] [PubMed] [Google Scholar]
- 3.Hupin D, Roche F, Gremeaux V, et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49(19):1262–1267. doi: 10.1136/bjsports-2014-094306. [DOI] [PubMed] [Google Scholar]
- 4.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahid A, Manek N, Nichols M, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(9) doi: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol. 2012;41(5):1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A, Salahuddin U, Garg S, et al. Continuous dose-response association between sedentary time and risk for cardiovascular disease: a meta-analysis. JAMA Cardiol. 2016;1(5):575–583. doi: 10.1001/jamacardio.2016.1567. [DOI] [PubMed] [Google Scholar]
- 9.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exer Sport. 2000;71(suppl 2):1–14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 10.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ) J Public Health. 2006;14(2):66–70. doi: 10.1007/s10389-006-0024-x. [DOI] [Google Scholar]
- 12.Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamatakis E, Koster A, Hamer M, et al. Emerging collaborative research platforms for the next generation of physical activity, sleep and exercise medicine guidelines: the Prospective Physical Activity, Sitting, and Sleep consortium (ProPASS) Br J Sports Med. 2020;54(8):435–437. doi: 10.1136/bjsports-2019-100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowd KP, Szeklicki R, Minetto MA, et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. Int J Behav Nutr Phys Act. 2018;15(1):15. doi: 10.1186/s12966-017-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey AM, Freedson PS. Utility of consumer physical activity trackers as an intervention tool in cardiovascular disease prevention and treatment. Prog Cardiovasc Dis. 2016;58(6):613–619. doi: 10.1016/j.pcad.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570. doi: 10.1136/bmj.l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku PW, Hamer M, Liao Y, Hsueh MC, Chen LJ. Device-measured light-intensity physical activity and mortality: a meta-analysis. Scand J Med Sci Sports. 2020;30(1):13–24. doi: 10.1111/sms.13557. [DOI] [PubMed] [Google Scholar]
- 18.Chastin SFM, De Craemer M, De Cocker K, et al. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370–376. doi: 10.1136/bjsports-2017-097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7(3):e219–e228. doi: 10.1016/S2468-2667(21)00302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng M, Yang J, Bao M, et al. The relationships between step count and all-cause mortality and cardiovascular events: a dose-response meta-analysis. J Sport Health Sci. 2021;10(6):620–628. doi: 10.1016/j.jshs.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saint-Maurice PF, Troiano RP, Bassett DR, Jr, et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA. 2020;323(12):1151–1160. doi: 10.1001/jama.2020.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dempsey PC, Strain T, Khaw KT, Wareham NJ, Brage S, Wijndaele K. Prospective associations of accelerometer-measured physical activity and sedentary time with incident cardiovascular disease, cancer, and all-cause mortality. Circulation. 2020;141(13):1113–1115. doi: 10.1161/CIRCULATIONAHA.119.043030. [DOI] [PubMed] [Google Scholar]
- 23.Tarp J, Hansen BH, Fagerland MW, Steene-Johannessen J, Anderssen SA, Ekelund U. Accelerometer-measured physical activity and sedentary time in a cohort of U.S. adults followed for up to 13 years: the influence of removing early follow-up on associations with mortality. Int J Behav Nutr Phys Act. 2020;17(1):39. doi: 10.1186/s12966-020-00945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellettiere J, LaMonte MJ, Evenson KR, et al. Sedentary behavior and cardiovascular disease in older women: The Objective Physical Activity and Cardiovascular Health (OPACH) Study. Circulation. 2019;139(8):1036–1046. doi: 10.1161/CIRCULATIONAHA.118.035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oftedal S, Holliday EG, Attia J, et al. Daily steps and diet, but not sleep, are related to mortality in older Australians. J Sci Med Sport. 2020;23(3):276–282. doi: 10.1016/j.jsams.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 27.Quality assessment tool for observational cohort and cross-sectional studies. NIH National Heart Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Updated July 2021. Accessed April 2020.
- 28.Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. John Wiley & Sons; New York, NY: 2011. Introduction to Meta-Analysis. [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines:1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 35.Sedgwick P, Marston L. How to read a funnel plot in a meta-analysis. BMJ. 2015;351:h4718. doi: 10.1136/bmj.h4718. [DOI] [PubMed] [Google Scholar]
- 36.Lee IM, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE. Accelerometer-measured physical activity and sedentary behavior in relation to all-cause mortality: The Women's Health Study. Circulation. 2018;137(2):203–205. doi: 10.1161/CIRCULATIONAHA.117.031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews CE, Keadle SK, Troiano RP, et al. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in U.S. adults. Am J Clin Nutr. 2016;104(5):1424–1432. doi: 10.3945/ajcn.116.135129. [published correction appears in Am J Clin Nutr. 2018;107(3):483] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dohrn IM, Sjöström M, Kwak L, Oja P, Hagströmer M. Accelerometer-measured sedentary time and physical activity-a 15 year follow-up of mortality in a Swedish population-based cohort. J Sci Med Sport. 2018;21(7):702–707. doi: 10.1016/j.jsams.2017.10.035. [published correction appears in J Sci Med Sport. 2019;22(3):246] [DOI] [PubMed] [Google Scholar]
- 39.Evenson KR, Wen F, Herring AH. Associations of accelerometry-assessed and self-reported physical activity and sedentary behavior with all-cause and cardiovascular mortality among U.S. adults. Am J Epidemiol. 2016;184(9):621–632. doi: 10.1093/aje/kww070. [published correction appears in Am J Epidemiol. 2017;186(1):129] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53(16):1013–1020. doi: 10.1136/bjsports-2017-098733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saint-Maurice PF, Troiano RP, Berrigan D, Kraus WE, Matthews CE. Volume of light versus moderate-to-vigorous physical activity: similar benefits for all-cause mortality? J Am Heart Assoc. 2018;7(7) doi: 10.1161/JAHA.118.008815. [published correction appears in J Am Heart Assoc. 2018;7(23):e03714] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bielemann RM, LaCroix AZ, Bertoldi AD, et al. Objectively measured physical activity reduces the risk of mortality among Brazilian older adults. J Am Geriatr Soc. 2020;68(1):137–146. doi: 10.1111/jgs.16180. [DOI] [PubMed] [Google Scholar]
- 43.LaMonte MJ, Buchner DM, Rillamas-Sun E, et al. Accelerometer-measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc. 2018;66(5):886–894. doi: 10.1111/jgs.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ensrud KE, Blackwell TL, Cauley JA, et al. Objective measures of activity level and mortality in older men. J Am Geriatr Soc. 2014;62(11):2079–2087. doi: 10.1111/jgs.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox KR, Ku PW, Hillsdon M, et al. Objectively assessed physical activity and lower limb function and prospective associations with mortality and newly diagnosed disease in UK older adults: an OPAL four-year follow-up study. Age Ageing. 2015;44(2):261–268. doi: 10.1093/ageing/afu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jefferis BJ, Parsons TJ, Sartini C, et al. Does total volume of physical activity matter more than pattern for onset of CVD? A prospective cohort study of older British men. Int J Cardiol. 2019;278:267–272. doi: 10.1016/j.ijcard.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults: a national cohort study. Ann Intern Med. 2017;167(7):465–475. doi: 10.7326/M17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dohrn IM, Welmer AK, Hagströmer M. Accelerometry-assessed physical activity and sedentary time and associations with chronic disease and hospital visits - a prospective cohort study with 15 years follow-up. Int J Behav Nutr Phys Act. 2019;16(1):125. doi: 10.1186/s12966-019-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakrania K, Edwardson CL, Khunti K, et al. Associations of objectively measured moderate-to-vigorous-intensity physical activity and sedentary time with all-cause mortality in a population of adults at high risk of type 2 diabetes mellitus. Prev Med Rep. 2017;5:285–288. doi: 10.1016/j.pmedr.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaCroix AZ, Bellettiere J, Rillamas-Sun E, et al. Association of light physical activity measured by accelerometry and incidence of coronary heart disease and cardiovascular disease in older women. JAMA Netw Open. 2019;2(3) doi: 10.1001/jamanetworkopen.2019.0419. [published correction appears in JAMA Netw Open. 2019;2(5):e194476] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loprinzi PD. Light-intensity physical activity and all-cause mortality. Am J Health Promot. 2017;31(4):340–342. doi: 10.4278/ajhp.150515-ARB-882. [DOI] [PubMed] [Google Scholar]
- 52.Loprinzi PD, Crush E. Sensory impairment, functional balance and physical activity with all-cause mortality. J Phys Act Health. 2016;13(9):980–987. doi: 10.1123/jpah.2015-0692. [DOI] [PubMed] [Google Scholar]
- 53.Chudasama YV, Khunti KK, Zaccardi F, et al. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med. 2019;17(1):108. doi: 10.1186/s12916-019-1339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0141274. [published correction appears in PLoS One. 2015;10(12):e0146202] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18(1):540. doi: 10.1186/s12889-018-5434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med. 2019;179(8):1105–1112. doi: 10.1001/jamainternmed.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanigatunga AA, Di J, Zipunnikov V, et al. Association of total daily physical activity and fragmented physical activity with mortality in older adults. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koolhaas CM, Dhana K, van Rooij FJA, et al. Sedentary time assessed by actigraphy and mortality: the Rotterdam Study. Prev Med. 2017;95:59–65. doi: 10.1016/j.ypmed.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Klenk J, Dallmeier D, Denkinger MD, et al. Objectively measured walking duration and sedentary behaviour and four-year mortality in older people. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duncan S, Stewart T, Bo Schneller M, Godbole S, Cain K, Kerr J. Convergent validity of ActiGraph and Actical accelerometers for estimating physical activity in adults. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kooiman TJM, Dontje ML, Sprenger SR, Krijnen WP, van der Schans CP, de Groot M. Reliability and validity of ten consumer activity trackers. BMC Sports Sci Med Rehabil. 2015;7(1):24. doi: 10.1186/s13102-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberger ME, Buman MP, Haskell WL, McConnell MV, Carstensen LL. Twenty-four hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sports Exerc. 2016;48(3):457–465. doi: 10.1249/MSS.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart T, Narayanan A, Hedayatrad L, Neville J, Mackay L, Duncan S. A dual-accelerometer system for classifying physical activity in children and adults. Med Sci Sports Exerc. 2018;50(12):2595–2602. doi: 10.1249/MSS.0000000000001717. [DOI] [PubMed] [Google Scholar]
- 64.Ku PW, Steptoe A, Liao Y, Hsueh MC, Chen LJ. A threshold of objectively-assessed daily sedentary time for all-cause mortality in older adults: a meta-regression of prospective cohort studies. J Clin Med. 2019;8(4):564. doi: 10.3390/jcm8040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bassett DR, Jr, Toth LP, LaMunion SR, Crouter SE. Step counting: a review of measurement considerations and health-related applications. Sports Med. 2017;47(7):1303–1315. doi: 10.1007/s40279-016-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramakrishnan R, Doherty A, Smith-Byrne K, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK Biobank cohort study. PLoS Med. 2021;18(1) doi: 10.1371/journal.pmed.1003487. [published correction appears in PLoS Med. 2021;18(9):e1003809] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ballin M, Nordström P, Niklasson J, Nordström A. Associations of objectively measured physical activity and sedentary time with the risk of stroke, myocardial infarction or all-cause mortality in 70-year-old men and women: a prospective cohort study. Sports Med. 2021;51(2):339–349. doi: 10.1007/s40279-020-01356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boursiquot BC, Bellettiere J, LaMonte MJ, LaCroix AZ, Perez MV. Sedentary behavior and atrial fibrillation in older women: the OPACH study. J Am Heart Assoc. 2022;11(6) doi: 10.1161/JAHA.121.023833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dempsey PC, Strain T, Winkler EAH, et al. Association of accelerometer-measured sedentary accumulation patterns with incident cardiovascular disease, cancer, and all-cause mortality. J Am Heart Assoc. 2022;11(9) doi: 10.1161/JAHA.121.023845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Del Pozo Cruz B, Biddle SJH, Gardiner PA, Ding D. Light-intensity physical activity and life expectancy: national health and nutrition survey. Am J Prev Med. 2021;61(3):428–433. doi: 10.1016/j.amepre.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Hansen BH, Dalene KE, Ekelund U, et al. Step by step: association of device-measured daily steps with all-cause mortality-a prospective cohort Study. Scand J Med Sci Sports. 2020;30(9):1705–1711. doi: 10.1111/sms.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paluch AE, Gabriel KP, Fulton JE, et al. Steps per day and all-cause mortality in middle-aged adults in the coronary artery risk development in young adults study. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mañas A, Del Pozo Cruz B, Ekelund U, et al. Association of accelerometer-derived step volume and intensity with hospitalizations and mortality in older adults: a prospective cohort study. J Sport Health Sci. 2022;11(5):578–585. doi: 10.1016/j.jshs.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.