Highlights
-
•
Neighborhood socioeconomic disadvantage predicted higher household disadvantage.
-
•
Neighborhood and household-level resources were constrained among Black women.
-
•
Neighborhood through household disadvantage was associated with higher postpartum weight retention.
-
•
Black women had higher postpartum weight retention relative to all other women via disadvantage.
Keywords: Postpartum weight retention, Black women, pregnancy, neighborhood socioeconomic disadvantage, household socioeconomic disadvantage, pregnancy health risk
Abstract
Introduction
Structural racism leads to neighborhood-level socioeconomic disadvantage, which determines adverse birth outcomes. Individual socioeconomic disadvantage is associated with compromised healthy pregnancy outcomes. This study aimed to investigate the pathways by which race, neighborhood socioeconomic disadvantage, and household socioeconomic disadvantage predict subsequent maternal postpartum weight retention.
Method
A total of 176 (N=176) racially diverse women were studied from the third trimester to 6 months after delivery. Neighborhood socioeconomic disadvantage was defined by information from the American Community Survey based on women's census tract and self-reports of neighborhood healthy food availability, safety, violence, and walking environment. Household socioeconomic disadvantage included food insecurity, income-to-needs ratio, and maternal education. Pregnancy health risk was operationalized using a summative index that included prepregnancy overweight/obesity, excessive gestational weight gain, and diagnosed hypertensive disorders during pregnancy. Postpartum weight retention was operationalized as a 6-month postpartum weight minus prepregnancy weight. Data were analyzed using structural equation modeling with bootstrapped CIs to estimate indirect effects.
Results
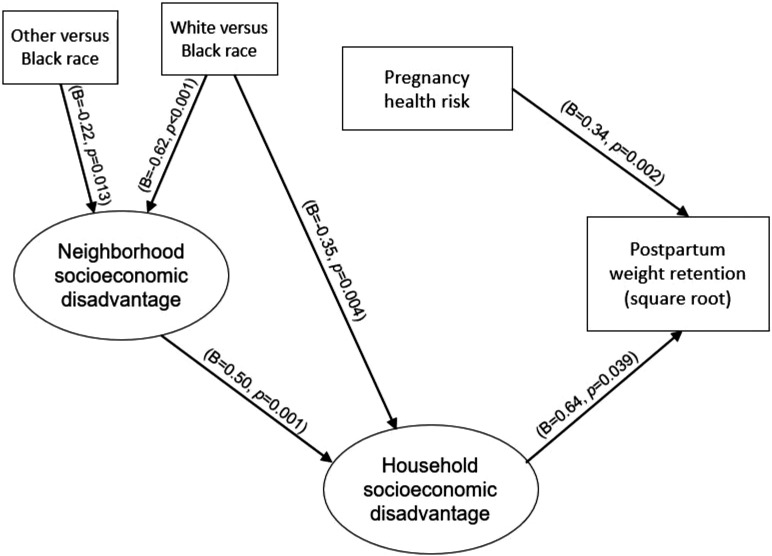
One third of participants retained more than 22 lbs. of pregnancy weight gain 6 months after delivery. Increased household socioeconomic disadvantage (β=0.64, p=0.039) and pregnancy health risk (β=0.34, p=0.002) were directly associated with higher postpartum weight retention. Maternal race/ethnicity had an indirect impact on postpartum weight retention through neighborhood socioeconomic disadvantage and household socioeconomic disadvantage. Non-Hispanic Black women had greater neighborhood socioeconomic disadvantage than non-Hispanic White women (White vs Black β= −0.62; p<0.001) and all other women (other vs Black β= −0.22; p=0.013). In addition, Black women had greater household socioeconomic disadvantage than White women (White vs Black β= −0.35; p=0.004), both of which in turn predicted higher postpartum weight retention.
Conclusions
To prevent postpartum weight retention, education on behavior change to lose weight is essential, but it must be offered in the context of basic resources, at both the neighborhood and household levels.
INTRODUCTION
Although weight gain is a natural occurrence in pregnancy, a higher postpartum weight may be related to gestational weight gain, which has health implications, including cardiometabolic disease.1 Postpartum weight retention (PWR) is linked to both prepregnancy weight and gestational weight gain, a modifiable risk factor for pregnancy complications and future cardiovascular disease.1,2 Evidence indicates that 48% of pregnant women gain more weight than recommended by the National Academy of Medicine guidelines,3 and most women retain above 5 kg by 1 year after delivery,4,5 altering the trajectory of lifetime weight gain and incidence of cardiovascular disease.6,7 Most previous research on pregnancy-related weight and intervention efforts to promote healthier patterns of weight gain and loss focus on individual factors such as diet and exercise,8,9 largely ignoring the role of systemic factors that contribute to weight problems and undermine weight loss efforts. Disparity in maternal preconception weight, gestational weight gain, and PWR persists, with Black women being at a higher risk.10, 11, 12
Previous evidence also indicates that there are racial differences in gestational weight gain and PWR13,14 such that Black women are more likely to be obese during the prepregnancy period and retain weight gained during pregnancy than other women.2 Such disparities are likely a function of structural racism, historical laws, zoning, and other restrictive measures aimed to maintain racial discrimination and segregation in the early twentieth century.15, 16, 17 Structural racism leads to neighborhood socioeconomic disadvantage (NSD), which influences crime rates, public services and resources, parks, recreational facilities, grocery stores, and fast food establishments.18,19 Our goal was to examine the extent to which racial disparities in NSD and household socioeconomic disadvantage (HSD) predicted PWR. Thus, we posit that race sets the stage for structural inequalities that confer risk for PWR through NSD and HSD.
The neighborhood, including the physical, social, economic, and built environment in which women live before, during, and after pregnancy, is essential for understanding weight gain and the pathways that predict PWR.20, 21, 22 In the U.S., neighborhoods’ SES determines the availability of many resources relevant to health and weight, including access to sidewalks, safety, and healthy food.15 These environmental features are also associated with weight-related pregnancy issues.22,23 Research on NSD usually focuses on gestational period complications such as weight gain and diabetes, with limited insight into postpartum health,22,24 showing the need for additional research on neighborhood impacts on PWR. Opportunities to engage in physical activity, such as taking one's baby for a walk, are likely undermined if one lives in a neighborhood with few sidewalks or with safety concerns related to violence or crime. Likewise, lower-income neighborhoods are characterized by deprivation of resources, including health-promoting services, because of the long-term impact of economic disinvestment.23,25,26 Thus, NSD may directly affect PWR by constraining opportunities to engage in behaviors that would support returning to prepregnancy weight.
Importantly, NSD is fairly stable over the lifespan and across generations.15,27 As such, characteristics of a woman's current neighborhood likely reflect, in part, the characteristics of her neighborhoods at earlier life stages. Living in neighborhoods characterized by high disadvantage constrains individual opportunities for subsequent economic prosperity.25,26 For example, high-school completion and college attendance rates are lower among residents of low-income neighborhoods, which has lasting implications for income during adulthood.15 Thus, we view current NSD as a proxy for lifetime NSD and posit that it will be positively associated with HSD. In turn, HSD may contribute to PWR because diet quality and physical space within the household are compromised in low-income households, and economic stress/strain can also undermine engagement in health-promoting behaviors.21,23,28 Consistent with this view, individual socioeconomic disadvantage is associated with compromised healthy pregnancy outcomes, including higher PWR.21,23,29,30 Thus, we posit that NSD will also have an indirect impact on PWR through HSD.
Given that weight retention predicts subsequent pregnancy-related cardiometabolic complications and postpartum cardiovascular disease risk,1,31,32 there is a need to delineate the role of NSD and HSD on the disparity in PWR to identify intervention target points. Therefore, this study investigates the pathways through which race, NSD, and HSD predict subsequent maternal PWR.
METHODS
Study Sample
Participants belonged to the cohort 1 (N=176) of the iGrow (Infant Growth and Development Study), a longitudinal study focused on prenatal and early life predictors of childhood obesity, described fully in the protocol paper.33 Pregnant women were recruited from childbirth education classes offered at the local women's hospital and public health department, from breastfeeding classes offered by the special supplemental nutrition assistance program for women, infants, and children and the local women's hospital, through flyers left in obstetrician/gynecologist clinics, and through social media. Eligibility criteria were being aged ≥18 years, expecting a singleton pregnancy, written English comprehension, and plans to remain in the region for 3 years. Women were recruited in Guilford County, North Carolina, a region that is diverse with respect to race/ethnicity and SES. For instance, according to census data, the racial/ethnic composition of the county is 55% White alone, 34% Black or African American alone, and 11% other/multiple races (including 8% Hispanic/Latino). The median income for families with children aged <18 years living in the household in 2018 was $56,794, and the poverty rate among these families was 20.3%. Among women aged 25‒34 years in the county, 8% had not completed high school/GED, and 47% had earned a bachelor's degree or higher.
Women completed written consent in their third trimester, completed questionnaires online, and visited our campus laboratory for assessments prenatally and 2 and 6 months after delivery. In addition, they provided details about their infants’ birth (i.e., birth weight, complications) through phone approximately 1 week after their due date. Mothers received $50‒$80 at each wave of data collection, were compensated for travel or transportation if needed, and were offered child care for siblings, and they and their infants received small gifts. The prenatal through 6-month postpartum data collections for cohort 1 occurred from February 2019 to October 2020. All procedures were approved by the university's IRB.
Measures
Neighborhood socioeconomic disadvantage
Participants provided their current address at the end of the recruitment call if they agreed to participate in the study. Addresses were used to extract neighborhood information at the census tract level for each participant using the 2018–2019 American Community Surveys. Census-based indicators of neighborhood disadvantage used in the current report include the percentage of individuals/households in the tract who were non-White, were receiving public assistance, and had incomes below the federal poverty threshold and included unemployed individuals who were aged ≥16 years, had less than a high-school degree, and lived in rental properties. In addition, participants rated the perceived quality of their neighborhood using the Neighborhood Scales34 through the online survey administered prenatally. A total of 4 subscales relevant to diet and exercise were used in the current report: availability of healthy food (4 items, α=0.55), safety (3 items, α=0.87), violence (4 items, α=0.84), and supportive walking environment (9 items, α=0.81).
Household socioeconomic disadvantage
Participants reported their highest level of education, household composition, and household income prenatally. An income-to-needs ratio was calculated by dividing household income by the federal household size poverty threshold. Participants also completed the 6-item short form of the U.S. Department of Agriculture Food Security Survey Module;35 high scores indicate higher household food insecurity.
Pregnancy health risk
Pregnancy health risk. Participants provided their prepregnancy weight through the prenatal online survey and final pregnancy weight through the 2-month online survey. Maternal height and weight were also measured during the prenatal visit in duplicate. Height was measured to the nearest 0.1 cm using a stadiometer (Model 213; Seca Corporation). If the 2 measurements differed by >0.5 cm, a third measurement was obtained. Weight was measured to the nearest 0.1 kg using a wireless professional weight scale (Model WB-8000RW; Tanita Corporation). If the 2 measurements differed by >0.1 kg, a third measurement was obtained. Self-reported prepregnancy weight and laboratory-assessed heights were used to calculate prepregnancy BMI, and women were categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2).36 Being normal weight was coded as 0, overweight as 1, and obese as 2. Rate of pregnancy weight gain per week was calculated as the difference between end-of-pregnancy weight and prepregnancy weight divided by the length of the pregnancy in weeks, and women were classified as having excessive weight gain (1) versus not (0) using the Institute of Medicine guidelines.3 Specifically, women whose prepregnancy weight status was underweight, normal weight, overweight, or obese were classified in the excessive weight gain group if they gained >0.59, 0.45., 0.32, or 0.27 kg per week, respectively. In the rare instances that the end-of-pregnancy weight was missing or a seemingly impossible value, it was estimated on the basis of weight assessed during the prenatal laboratory visit and the number of days between that visit and the infant's birthdate based on the assumption that weight gain continued. Participants noted the presence of pregnancy-related health risks and birth complications through the online surveys administered prenatally and at 2 months after delivery and through their answers to open-ended questions during the post-birth phone call. The presence of each of the following hypertensive disorders of pregnancy was recorded (0=not present, 1=present): preeclampsia, gestational hypertension, hemolysis, elevated liver enzymes, and low platelets syndrome, and gestational diabetes. Overall, pregnancy health risk was operationalized using a summative index that included prepregnancy overweight/obesity, excessive gestational weight gain, and diagnosed hypertensive disorders of pregnancy.
Postpartum weight retention
PWR was defined as 6-month postpartum weight minus the prepregnancy self-reported weight. Participants were weighed (as described earlier) during the 6-month visit.
Statistical Analysis
Descriptive statistics were estimated to describe the sample of women and their neighborhood and household characteristics as well as prenatal risk, including frequency (n) and percentage (%) for categorical data and mean and SD for continuous data. For NSD and HSD, a 1-way ANOVA for race group (non-Hispanic White, non-Hispanic Black, all other) comparisons was calculated for each dependent variable. This decision was made given overall Multiple Indicators and Multiple Causes modeling,37 with race for NSD- and HSD-revealed differences (p≤0.010). ANOVA posthoc pairwise multiple comparisons were done using Tukey's honestly significant difference (if equal variances could be assumed) or Tamhane's procedure (if unequal variances). Reliability through internal consistency was estimated using coefficient alpha (α). Multivariate analysis was performed using structural equation modeling, with bias-corrected bootstrapped CIs used to estimate the indirect effects. For interpreting greater socioeconomic disadvantage, healthy food, safety, supportive walking environment, and maternal education were reverse scored before structural equation modeling analysis. Income-to-needs ratio was also transformed by inverse and natural log. Significance for tests of direct effects was inferred using unstandardized coefficients (b), whereas effect sizes were estimated with standardized effects (β). For indirect effects, an effect was considered significant if zero was excluded from its corresponding 95% bias-corrected bootstrap CI after 10,000 iterations for its unstandardized estimate.38,39 Weighted least squares (mean- and variance-adjusted) estimation was used to specify any indicators or outcomes as ordinal that had <7 levels.40 Because of non-normality and outliers, PWR was square root transformed. All analyses were performed in Mplus, version 8.6,41 and SPSS, version 28 (IBM Corp, Armonk, NY). A 2-sided p<0.05 was considered statistically significant.
RESULTS
Of the 176 women participating in the study, the average age was 29.2 years (SD = 6.1 years), with 46% non-Hispanic White, 32% non-Hispanic Black, and 22% all other (Table 1). A total of 44% of the women had a 4-year college degree or higher education level, and approximately half (51%) were married or living with a partner. A little more than half of the women (52%) were overweight (21%) or obese (31%) before pregnancy. A total of 37% had excessive pregnancy weight gain, and more than one fourth had hypertensive disorders during pregnancy (26%). Almost two thirds of the women (65%) had 1 or more prenatal risk factors, with almost half having multiple (49%). For 6-month PWR, one third of participants retained more than 22 pounds of pregnancy weight gain.
Table 1.
Study Sample Demographics and Prenatal Risk (N = 176)
| Demographic characteristics | n (%) or mean ± SD |
|---|---|
| Age (years) | 29.2±6.1 |
| Race/ethnicity | |
| Non-Hispanic White | 81 (46) |
| Non-Hispanic Black | 57 (32) |
| All other | 38 (22) |
| Education level | |
| High-school degree or less | 37 (21) |
| Some college | 40 (23) |
| 2-year college degree | 17 (10) |
| 4-year college degree | 35 (20) |
| >4-year college degree | 43 (24) |
| Missing | 4 (2) |
| Married or living with partner | 90 (51) |
| Primiparous | 81 (46) |
| Prenatal risk | n (%) or mean ± SD |
|---|---|
| Pre-pregnancy weight status | |
| Overweight (25.0–29.9 kg/m2) | 37 (21) |
| Obese (≥30.0 kg/m2) | 55 (31) |
| Excessive gestational weight gaina | 65 (37) |
| Hypertensive disorders of pregnancy | 46 (26) |
| Total number of prenatal clinical risks (sum of above) | |
| 0 | 62 (35) |
| 1 | 28 (16) |
| 2 | 41 (23) |
| 3 | 29 (16) |
| 4 | 16 (9) |
Excessive gestational weight gain (weekly rate of weight gain >0.59, 0.45, 0.32, or 0.27 kg per week, for women classified as underweight, normal weight, overweight, or obese, respectively, before pregnancy)
Descriptive statistics for measures of socioeconomic disadvantage for both neighborhood and household are given by race/ethnicity in Table 2. Non-Hispanic Black women and women of other race/ethnicity lived in neighborhoods with substantially higher public assistance receipts than non-Hispanic White women. Their neighborhoods also had more poverty, more unemployment, more rental housing arrangements, and lower educational attainment. Additional NSD measures of healthy foods (Black, median=2.8 vs White, mean=3.2), safety (mean= 3.3 vs 4.0), violence (mean=1.5 vs 1.2), and walking environment (mean=3.3 vs 3.8) were more disadvantaged for non-Hispanic Black women than for White women. HSD measures showed a similar pattern for individual women's income-to-needs ratio (Black, mean=1.4 vs White, mean=4.4), education (mean=3.3 vs 5.1), and food insecurity (mean=1.6 vs 1.2). ANOVA and posthoc pairwise comparisons reveal that non-Hispanic Black women were significantly different from White women on all measures (p<0.050). Several other significant differences were found between other racial/ethnic groups, except for the percentage of those aged ≥16 years who were unemployed from Census/American Community Survey data, and included the neighborhood healthy food availability, neighborhood violence, and household food insecurity (only White versus Black differences with p<0.050).
Table 2.
Measures of Neighborhood and Household Socioeconomic Disadvantage
| Neighborhood and household characteristics | Non-Hispanic White (n = 81) | Non-Hispanic Black (n = 57) | All other (n = 38) | Group diffs | |||
|---|---|---|---|---|---|---|---|
| Census data/ACS | Mean | Min‒Max | Mean | Min‒Max | Mean | Min‒Max | |
| % Non-White | 32.6 | 2.4–85.3 | 69.2 | 30.3–100.0 | 52.8 | 12.0–93.1 | WB, WO, BO |
| % Public assistance | 9.8 | 0.0–34.4 | 25.4 | 3.0–51.6 | 19.0 | 2.2–51.1 | WB, WO |
| % In poverty | 13.5 | 0.8–44.0 | 25.2 | 4.2–60.3 | 19.0 | 3.7–50.0 | WB, BO |
| % Aged ≥16 years unemployed | 4.9 | 1.2–13.3 | 7.7 | 2.3–28.4 | 6.5 | 0.7–28.4 | WB |
| % < HS degree | 8.9 | 0.3–30.4 | 15.2 | 3.5–27.2 | 12.8 | 2.1–28.8 | WB, WO |
| % Rentals | 34.3 | 0.6–86.2 | 55.4 | 19.9–86.2 | 48.4 | 5.3–86.8 | WB, WO |
| Non-Census/ACS neighborhood socioeconomic disadvantage indicators | |||||||
| Healthy foods | 3.2 | 1.8–4.8 | 2.8 | 1.3–4.3 | 3.0 | 1.3–4.3 | WB |
| Safety | 4.0 | 2.0–5.0 | 3.3 | 1.0–5.0 | 3.5 | 2.0–5.0 | WB, WO |
| Violence | 1.2 | 1.0–3.0 | 1.5 | 1.0–3.5 | 1.3 | 1.0–2.3 | WB |
| Walking environment | 3.8 | 1.7–4.9 | 3.3 | 2.2–4.7 | 3.5 | 1.6–4.8 | WB, WO |
| Household socioeconomic disadvantage indicators | |||||||
| Income-to-needs | 4.4 | 0.1–16.6 | 1.4 | 0.0–9.3 | 2.7 | 0.0–11.0 | WB, WO, BO |
| Maternal education | 5.1 | 1.0–7.0 | 3.3 | 1.0–7.0 | 3.8 | 1.0–7.0 | WB, WO |
| Food insecurity | 1.2 | 1.0–3.0 | 1.6 | 1.0–3.0 | 1.4 | 1.0–3.0 | WB |
Note: Neighborhood Scales are from Mujahid, Diez Roux, Morenoff, & Raghunathan.35 Group diffs=significant pairwise comparisons (p<0.05) using Tukey's HSD or Tamhane's posthoc multiple comparisons procedure after significant ANOVA (or Welch ANOVA).
ACS, American Community Survey; BO, non-Hispanic Black versus all other; diff, difference; HS, high school; HSD, honestly significant difference; Max, maximum; Min, minimum; WB, non-Hispanic White versus Non-Hispanic Black; WO, non-Hispanic White versus all other.
Factor loadings for SD models ranged from adequate to very good: NSD range=0.464 and 0.856 and HSD range=0.423 and 0.808 (Table 3). Figures 1 and 2 provide the estimated path coefficients from structural modeling findings. NSD had a significant positive direct impact on HSD (β=0.50; p<0.001). There were significant positive direct impacts of pregnancy health risk (β=0.34; p=0.002) and HSD (β=0.63; p=0.039) on PWR. There were also race differences in NSD and HSD in this multivariate modeling. Specifically, non-Hispanic Black women had greater NSD than non-Hispanic White women (White vs Black β= −0.62; p<0.001) and all other women (other vs Black β= −0.22; p=0.013). In addition, Black women had greater HSD than White women (White vs Black β= −0.35; p=0.004).
Table 3.
Socioeconomic Disadvantage Measurement Model Findings
| Socioeconomic characteristics | Neighborhood SD (α=0.791) | Household SD (α=0.799) |
|---|---|---|
| % Non-White | 0.848 | |
| % Public assistance | 0.856 | |
| % In poverty | 0.776 | |
| % Aged ≥16 years unemployed | 0.635 | |
| % <HS degree | 0.684 | |
| % Rentals | 0.549 | |
| Healthy foodsRV | 0.541 | |
| SafetyRV | 0.688 | |
| Violence | 0.464 | |
| Walking environmentRV | 0.648 | |
| Income-to-needsIN | 0.808 | |
| Maternal educationRV | 0.738 | |
| Food insecurity | 0.423 |
Note: Numbers within the table represent (standardized) factor loadings.
HS, high school; IN, inverse and log-transformed; RV, reverse scored; SD, socioeconomic disadvantage.
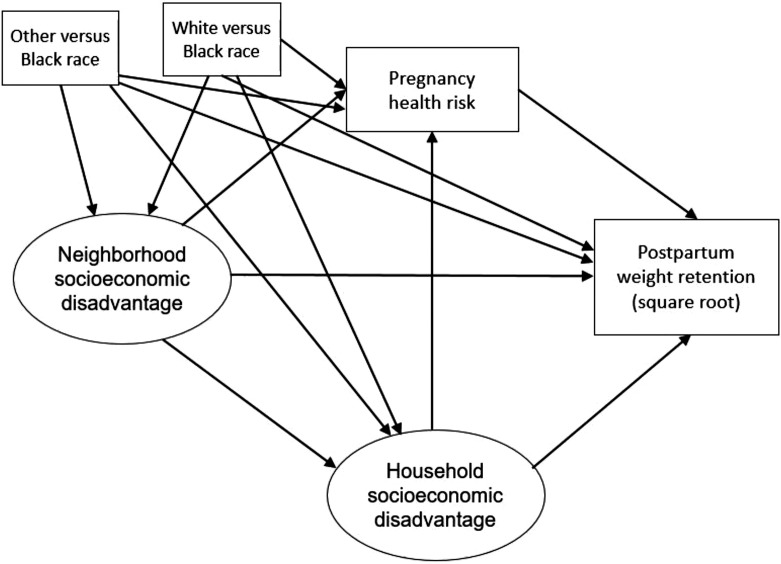
Figure 1.
Conceptual path diagram of structural modeling.
Figure 2.
Estimated path diagram of structural model significant findings.
Note: B denotes standardized coefficient; only significant direct effects are shown with p<0.05.
Several indirect effects were found as well. NSD was associated with higher PWR indirectly through HSD (β=0.32; b=0.73; 95% CI=0.29, 1.78). The total indirect effect for non-Hispanic Black women versus White women was significant (β= −0.45; b= −1.06; 95% CI= −2.07, −0.53). Specifically, Black women had higher PWR than White women indirectly through 2 cascading, intervening aspects of socioeconomic disadvantage: through HSD (β= −0.22; b= −0.52; 95% CI= −1.54, −0.18) and by NSD through HSD (β= −0.20; b= −0.46; 95% CI= −1.19, −0.18). The total indirect effect for Black women versus other was significant (β= −0.15; b= −0.42; 95% CI= −0.99, −0.009). In this study, Black women also had higher PWR than all other women indirectly by NSD through HSD (β= −0.07; b= −0.20; 95% CI= −0.63, −0.05).
DISCUSSION
This study investigated the pathways through which race, prenatal NSD, and HSD predict subsequent maternal PWR. Consistent with prediction and a structural racism perspective, non-Hispanic Black women were at risk for greater PWR as a function of racial differences in economic conditions, that is, non-Hispanic Black women were more likely to live in neighborhoods characterized by greater economic disadvantage than both White women and other minoritized women, which in turn predicted greater disadvantage at the household level, which in turn predicted greater weight retention 6 months after delivery. In addition, Black women had greater household disadvantage than White women, which in turn predicted greater PWR. These effects were over and above the positive association between pregnancy health risks and weight retention.
Our results are consistent with ample evidence that in the U.S., Black women and other ethnic minority women are socioeconomically disadvantaged, suggesting that race/ethnicity reflects neighborhood quality, resources, and opportunities, a result of system practices that lead to structural racism.16,42, 43, 44 Chambers et al.45 found that >50% of non-Hispanic Black and other minority pregnant women live in extremely deprived neighborhoods. These neighborhoods increase poor maternal health outcomes.46
To the best of our knowledge, there is no published study in the literature that directly examines the impacts of NSD and HSD on PWR. However, an intervention study by Headen and colleagues21 found that PWR was higher among women who live in wealthy neighborhoods. Our findings on NSD are inconsistent with their findings, given that we found that PWR is associated with poor neighborhoods. This inconsistency might be owing to the study methodologies and the different neighborhoods in which the participants were recruited. In addition, their study was an intervention, whereas our study was observational. They recruited the participants in California in the San Francisco Bay Area, whereas we recruited our sample from Greensboro, North Carolina, a geographic area with a unique history of structural racism that encompasses racial residential segregation, neighborhood integration, and resegregation.46, 47, 48, 49 Greensboro was established around planned separation principles. It serves as an example of several small-to-midsize American cities in the South. Thus, there is a need to address systemic disparities tailored to the geographic area and its history of structural racism.
The results of the intervention study by Headen et al.21 are reassuring given that our findings indicated that PWR is linked to disadvantaged neighborhoods because the findings suggest that with effective intervention, those who live in disadvantaged neighborhoods might have improved cardiometabolic outcomes after delivery. In fact, the mindfulness-based intervention to reduce stress and stress-based eating was more effective for women living in less advantaged neighborhoods. In addition, the intervention may have a bigger impact in disadvantaged neighborhoods if it particularly addressed issues more likely to be present in disadvantaged versus affluent neighborhoods.
As shown in this study, there are race/ethnicity differences in neighborhood disadvantage, which has repercussions for HSD. We found that NSD and HSD have a positive impact on PWR. The direct effect of race on HSD shows that factors other than neighborhood or aspects of the neighborhood not examined in this study must also be at play in putting Black women at risk for lower SES than White women. Lower SES may lead to a cascade of health events across the lifespan and generations. It is well documented in the literature that both cardiometabolic complications in pregnancy and future cardiovascular disease are prevalent in Black women.1,20,30 This study provides insight into the possible paths of the well-documented adverse health outcomes for Black women. It also helps to identify potential root causes that might explain why interventions focusing on individual factors have not resolved the disparities in maternal outcomes.50 Individual interventions will not be effective if the differences are the function of long-standing systemic inequality. Efforts must be made to address disparities in the neighborhood conditions that lead to severe maternal morbidity and mortality.
Our results also indicated a significant direct impact of higher pregnancy health risk and HSD on PWR. Our findings on pregnancy health risk, which consists of prepregnancy overweight/obesity, excessive gestational weight gain, and hypertensive disorders of pregnancy, are consistent with both individual-level findings on pregnancy complications and neighborhood associations with pregnancy complications, with Black women and other minorities suffering a disproportionate burden of pregnancy complications.23,30,51,52 Race is a predictor of an individual's physical, social, and built environment as well as the availability of resources to pregnant and postpartum women. Black women had greater NSD than non-Hispanic White women and all other women. Black women are at a greater health disadvantage than other women during prepregnancy, pregnancy, and after delivery.30 The findings from this study suggest that the risk factors and complications among Black women should be assessed at the macrolevel such as neighborhood because individual-level race/ethnicity explanation limits our understanding of the disease process and how to address it.
The results of this study indicated that PWR is greater among women living in disadvantaged neighborhoods or lower-income communities. In a population at greater risk for hypertensive disorders of pregnancy, which are the leading causes of maternal morbidity, mortality, and adverse infant outcomes, obesity is a precursor to some of these complications.53, 54, 55 Women who retain pregnancy weight are more likely to enter subsequent pregnancy overweight. Therefore, this study offers essential information for healthcare professionals, patient advocates, public health agencies, and policymakers to promote postpartum care for women by leveraging current recommendations, such as prenatal care. Postpartum transition care facilities in low-income areas that emphasize leading a healthy lifestyle and preventing postpartum health issues that could result in both short- and long-term cardiometabolic disease will be beneficial to communities at greater risk for maternal mortality. Likewise, efforts to reduce neighborhood-level barriers to adopting healthy behaviors must be implemented (e.g., promoting neighborhood changes that facilitate safe access to healthy food and exercise opportunities).
Limitations
This study adds to the limited literature on NSD in relation to postpartum health. It expands on the existing literature by considering both NSD with HSD to understand the physical and the built environment as a reflection of the individual household, pregnancy, and postpartum outcome. We measured NSD using multiple domains, including census-based indicators of neighborhood disadvantage and maternal-reported neighborhood conditions. We believe that using multiple sources of the NSD captured data that represent the neighborhoods reported in our study. Limitations include the relatively small sample size and cross-sectional design. In particular, NSD and HSD were assessed concurrently. The latter limits our ability to understand the complex cumulative impacts of the neighborhood and household on pregnancy and postpartum outcomes. Replication and extension using longitudinal designs and larger samples are warranted.
CONCLUSIONS
Neighborhood and household resources were constrained among non-Hispanic Black mothers in our sample, suggesting that structural racism plays a role in creating the conditions that confer risk for PWR. Pregnancy complications and postpartum health outcomes may provide a preview of the impact that daily encounters in disadvantaged neighborhoods have on women's overall health. Because their children will also live in the same neighborhood and household, there is the possibility of generational transmission of adverse health problems. As health professionals, we must advocate for the systematic dismantling of differences in neighborhood-level resources that stem from historic racist practices.
Declarations of interest
none.
Acknowledgments
ACKNOWLEDGMENTS
All procedures were approved by the university's IRB (protocol number 18–0198).
This work was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development (Grant Number R01HD093662, 2018).
CRediT AUTHOR STATEMENT
Avorgbedor Forgive: conceptualization, methodology, validation, writing original draft. Thomas McCoy: formal analysis, writing original draft, visualization. Laurie Wideman: writing-review & editing, funding acquisition. Lenka Shriver: writing-review & editing, funding acquisition. Cheryl Buehler: writing-review & editing, funding acquisition. Esther Leerkes: conceptualization, methodology, writing original draft, validation, funding acquisition, supervision.
REFERENCES
- 1.Kirkegaard H, Bliddal M, Støvring H, et al. Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: a cohort study. PLoS Med. 2021;18(4) doi: 10.1371/journal.pmed.1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev Med. 2013;56(6):372–378. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press (US); Washington, DC: 2009. Institute of Medicine (U.S.) and National Research Council (U.S.) Committee to Reexamine IOM Pregnancy Weight Guidelines, eds. [DOI] [PubMed] [Google Scholar]
- 4.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson EP. Childbearing and Obesity in Women: Weight Before, During, and After Pregnancy. Obstet Gynecol Clin North Am. 2009;36(2):317–332. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22(2):261–274. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 7.Mamun AA, Kinarivala M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr. 2010;91(5):1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 8.Bain E, Crane M, Tieu J, Han S, Crowther CA, Middleton P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2015;2015(4) doi: 10.1002/14651858.CD010443.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;2015(6) doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herring SJ, Albert JJ, Darden N, et al. Targeting pregnancy-related weight gain to reduce disparities in obesity: baseline results from the Healthy Babies trial. Contemp Clin Trials. 2019;87 doi: 10.1016/j.cct.2019.105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jevitt CM. Obesity and socioeconomic disparities: rethinking causes and perinatal care. J Perinat Neonatal Nurs. 2019;33(2):126–135. doi: 10.1097/JPN.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 12.Shrewsbury VA, Robb KA, Power C, Wardle J. Socioeconomic differences in weight retention, weight-related attitudes and practices in postpartum women. Matern Child Health J. 2009;13(2):231–240. doi: 10.1007/s10995-008-0342-4. [DOI] [PubMed] [Google Scholar]
- 13.Leonard SA, Abrams B, Main EK, Lyell DJ, Carmichael SL. Weight gain during pregnancy and the risk of severe maternal morbidity by prepregnancy BMI. Am J Clin Nutr. 2020;111(4):845–853. doi: 10.1093/ajcn/nqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widen EM, Whyatt RM, Hoepner LA, et al. Excessive gestational weight gain is associated with long-term body fat and weight retention at 7 y postpartum in African American and Dominican mothers with underweight, normal, and overweight prepregnancy BMI. Am J Clin Nutr. 2015;102(6):1460–1467. doi: 10.3945/ajcn.115.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 17.Thompson-Miller R, Feagin JR, Picca LH. Rowman & Littlefield; New York, NY: 2014. Jim Crow's Legacy: the Lasting Impact of Segregation. [DOI] [Google Scholar]
- 18.Anderson LM, Scrimshaw SC, Fullilove MT, Fielding JE. Task Force on Community Preventive Services. The Community Guide's model for linking the social environment to health. Am J Prev Med. 2003;24(3)(suppl):12–20. doi: 10.1016/s0749-3797(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 19.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culhane JF, Elo IT. Neighborhood context and reproductive health. Am J Obstet Gynecol. 2005;192(5)(suppl):S22–S29. doi: 10.1016/j.ajog.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 21.Headen I, Laraia B, Coleman-Phox K, Vieten C, Adler N, Epel E. Neighborhood typology and cardiometabolic pregnancy outcomes in the maternal adiposity metabolism and stress study. Obesity (Silver Spring) 2019;27(1):166–173. doi: 10.1002/oby.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez DD, Doebler DA, Kim KH, Amutah NN, Fabio A, Bodnar LM. Neighborhood socioeconomic disadvantage and gestational weight gain and loss. Matern Child Health J. 2014;18(5):1095–1103. doi: 10.1007/s10995-013-1339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Headen I, Mujahid M, Deardorff J, Rehkopf DH, Abrams B. Associations between cumulative neighborhood deprivation, long-term mobility trajectories, and gestational weight gain. Health Place. 2018;52:101–109. doi: 10.1016/j.healthplace.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janghorbani M, Stenhouse EA, Jones RB, Millward BA. Is neighbourhood deprivation a risk factor for gestational diabetes mellitus? Diabet Med. 2006;23(3):313–317. doi: 10.1111/j.1464-5491.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- 25.Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci U S A. 2016;113(47):13372–13377. doi: 10.1073/pnas.1607220113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osypuk TL, Galea S, McArdle N, Acevedo-Garcia D. Quantifying separate and unequal: racial-ethnic distributions of neighborhood poverty in metropolitan America. Urban Aff Rev Thousand Oaks Calif. 2009;45(1):25–65. doi: 10.1177/1078087408331119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharkey P, Elwert F. The legacy of disadvantage: multigenerational neighborhood effects on cognitive ability. Am J Sociol. 2011;116(6):1934–1981. doi: 10.1086/660009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman S, Gibbons J, Galvan C. Declining segregation through the lens of neighborhood quality: does middle-class and affluent status bring equality? Soc Sci Res. 2014;46:155–168. doi: 10.1016/j.ssresearch.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ananat EO. The wrong side(s) of the tracks: the causal effects of racial segregation on urban poverty and inequality. Am Econ J Appl Econ. 2011;3(2):34–66. doi: 10.1257/app.3.2.34. [DOI] [Google Scholar]
- 30.Howell EA, Egorova NN, Janevic T, et al. Race and ethnicity, medical insurance, and within-hospital severe maternal morbidity disparities. Obstet Gynecol. 2020;135(2):285–293. doi: 10.1097/AOG.0000000000003667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rong K, Yu K, Han X, et al. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr. 2015;18(12):2172–2182. doi: 10.1017/S1368980014002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahabi HA, Fayed AA, Tharkar S, Esmaeil SA, Bakhsh H. Postpartum weight retention and cardiometabolic risk among Saudi women: a follow-up study of RAHMA subcohort. BioMed Res Int. 2019;2019 doi: 10.1155/2019/2957429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leerkes EM, Buehler C, Calkins SD, Shriver LH, Wideman L. Protocol for iGrow (Infant Growth and Development Study): biopsychosocial predictors of childhood obesity risk at 2 years. BMC Public Health. 2020;20(1):1912. doi: 10.1186/s12889-020-10003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- 35.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89(8):1231–1234. doi: 10.2105/ajph.89.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.About adult BMI. Centers for Disease Control and Prevention. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. Updated June 3, 2022. Accessed July 20, 2022.
- 37.Kline RB. Principles and Practice of Structural Equation Modeling. 4th. Guilford Publications; New York, NY: 2016. [Google Scholar]
- 38.Hayes AF. Guilford Press; New York, NY: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [DOI] [Google Scholar]
- 39.Muthén BO, Muthén LK, Asparouhov T. Muthén & Muthén; Los Angeles, CA: 2017. Regression and Mediation Analysis Using Mplus. [Google Scholar]
- 40.Muthén B, du Toit SHC, Spisic D. Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. StatsModel. 1947. https://www.statmodel.com/download/Article_075.pdf. Published November 18, 2021. Accessed December 6, 2021.
- 41.Muthén LK., Muthén BO. Eighth. Muthén & Muthén; Los Angeles, CA: 2021. Mplus User’s Guide. [Google Scholar]
- 42.Bailey ZD, Feldman JM, Bassett MT. How structural racism works—racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384:768–773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell JF, Zimmerman FJ, Almgren GR, Mayer JD, Huebner CE. Birth outcomes among urban African-American women: a multilevel analysis of the role of racial residential segregation. Soc Sci Med. 2006;63(12):3030–3045. doi: 10.1016/j.socscimed.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Hogan VK, Culhane JF, Crews KJN, et al. The impact of social disadvantage on preconception health, illness, and well-being: an intersectional analysis. Am J Health Promot. 2013;27(3)(suppl):eS32–eS42. doi: 10.4278/ajhp.120117-QUAL-43. [DOI] [PubMed] [Google Scholar]
- 45.Chambers BD, Arabia SE, Arega HA, et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health. 2020;36(2):213–219. 10.1002/smi.2922. [DOI] [PMC free article] [PubMed]
- 46.Howland RE, Angley M, Won SH, et al. Determinants of severe maternal morbidity and its racial/ethnic disparities in New York City, 2008–2012. Matern Child Health J. 2019;23(3):346–355. doi: 10.1007/s10995-018-2682-z. [DOI] [PubMed] [Google Scholar]
- 47.Cutler DM, Glaeser EL, Vigdor JL. The rise and decline of the American ghetto. J Pol Econ. 1999;107(3):455–506. doi: 10.1086/250069. [DOI] [Google Scholar]
- 48.Lichter DT, Parisi D, Taquino MC. The geography of exclusion: race, segregation, and concentrated poverty. Soc Probl. 2012;59(3):364–388. doi: 10.1525/sp.2012.59.3.364. [DOI] [Google Scholar]
- 49.Massey DS, Tannen J. Suburbanization and segregation in the United States: 1970–2010. Ethn Racial Stud. 2018;41(9):1594–1611. doi: 10.1080/01419870.2017.1312010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geissler KH, Lubin B, Marzilli Ericson KM. Access is not enough: characteristics of physicians who treat Medicaid patients. Med Care. 2016;54(4):350–358. doi: 10.1097/MLR.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 51.Krukowski RA, Bursac Z, McGehee MA, West D. Exploring potential health disparities in excessive gestational weight gain. J Womens Health (Larchmt) 2013;22(6):494–500. doi: 10.1089/jwh.2012.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Gallagher AE, Carta CM, Torres ME, Moran R, Wilcox S. Racial differences in gestational weight gain and pregnancy-related hypertension. Ann Epidemiol. 2014;24(6):441–447. doi: 10.1016/j.annepidem.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuehn BM. Hypertensive disorders in pregnancy are on the rise. JAMA. 2022;327(24) doi: 10.1001/jama.2022.9510. 2387–2387. [DOI] [PubMed] [Google Scholar]
- 54.Avorgbedor F, Silva S, McCoy TP, et al. Hypertension and infant outcomes: North Carolina pregnancy risks assessment monitoring system data. Preg Hypertens. 2022;28:189–193. 10.1016/j.preghy. 2022.05.004. [DOI] [PubMed]
- 55.Bicocca MJ, Mendez-Figueroa H, Chauhan SP, Sibai BM. Maternal obesity and the risk of early-onset and late-onset hypertensive disorders of pregnancy. Obstet Gynecol. 2020;136(1):118–127. doi: 10.1097/AOG.0000000000003901. [DOI] [PubMed] [Google Scholar]