Abstract
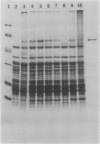
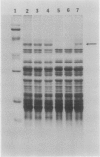
Translational alterations occur in maize (Zea mays L.) leaves stressed by pathogen infection or herbicide paraquat treatment. These translational changes include: (a) dissociation of large polysomes to small polysomes, monosomes, and subunits; (b) a decreased rate of total protein synthesis; and (c) a reduced synthesis of several proteins by polysomes in vitro. The polysome dissociation was neither due to an extraction artifact nor to degradation of RNA by RNase. The protein patterns of polysomes isolated from leaves inoculated with Bipolaris maydis at 6 to 48 hours showed an increase in the intensity of a 57 kilodalton protein. When inoculated with less virulent pathogens, such as B. zeicola, Exserohilum turcicum, or Colletotrichum graminicola, the protein was accumulated in polysomes of leaves at 24 to 48 hours after inoculation. The 57 kilodalton protein was also accumulated in polysomes of maize leaves responding to heat shock or herbicide paraquat treatments. The purified 57 kilodalton protein reassociated with polysomes isolated from healthy leaves and inhibited polysomal translation in vitro. Since the 57 kilodalton protein is rapidly accumulated in maize polysomes in response to various biological and environmental stresses and may affect protein synthesis, it may be involved in translational regulation of maize leaves during stress response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bag J. Free messenger ribonucleoprotein complexes of chicken primary muscle cells following modification of protein synthesis by heat-shock treatment. Eur J Biochem. 1983 Sep 15;135(2):187–196. doi: 10.1111/j.1432-1033.1983.tb07636.x. [DOI] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Hsiao T. C. Rapid Changes in Levels of Polyribosomes in Zea mays in Response to Water Stress. Plant Physiol. 1970 Aug;46(2):281–285. doi: 10.1104/pp.46.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy I. M., Burdon R. H., Leader D. P. Heat shock causes diverse changes in the phosphorylation of the ribosomal proteins of mammalian cells. FEBS Lett. 1984 Apr 24;169(2):267–273. doi: 10.1016/0014-5793(84)80331-2. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Marcus A., Efron D., Weeks D. P. The wheat embryo cell-free system. Methods Enzymol. 1974;30:749–754. doi: 10.1016/0076-6879(74)30073-0. [DOI] [PubMed] [Google Scholar]
- Matters G. L., Scandalios J. G. Changes in plant gene expression during stress. Dev Genet. 1986;7(4):167–175. doi: 10.1002/dvg.1020070402. [DOI] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. Effect of heat shock on ribosome structure: appearance of a new ribosome-associated protein. Mol Cell Biol. 1986 Jul;6(7):2527–2535. doi: 10.1128/mcb.6.7.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes P. R., Matsuda K. Water stress, rapid polyribosome reductions and growth. Plant Physiol. 1976 Nov;58(5):631–635. doi: 10.1104/pp.58.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON C. M. Chromatographic separation of ribonucleases in corn. Biochim Biophys Acta. 1963 Feb 26;68:177–184. doi: 10.1016/0006-3002(63)90133-1. [DOI] [PubMed] [Google Scholar]