Abstract
Background
ONC201 is a small molecule that can cause nonapoptotic cell death through loss of mitochondrial function. Results from the phase I/II trials of ONC201 in patients with refractory solid tumors demonstrated tumor responses and prolonged stable disease in some patients.
Methods
This single-arm, open-label, phase II clinical trial evaluated the efficacy of ONC201 at the recommended phase II dose (RP2D) in patients with recurrent or refractory metastatic breast or endometrial cancer. Fresh tissue biopsies and blood were collected at baseline and at cycle 2 day 2 for correlative studies.
Results
Twenty-two patients were enrolled; 10 patients with endometrial cancer, 7 patients with hormone receptor–positive breast cancer, and 5 patients with triple-negative breast cancer. The overall response rate was 0%, and the clinical benefit rate, defined by complete response (CR) + partial response (PR) + stable disease (SD), was 27% (n = 3/11). All patients experienced an adverse event (AE), which was primarily low grade. Grade 3 AEs occurred in 4 patients; no grade 4 AEs occurred. Tumor biopsies did not show that ONC201 consistently induced mitochondrial damage or alterations in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or the TRAIL death receptors. ONC201 treatment caused alterations in peripheral immune cell subsets.
Conclusion
ONC201 monotherapy did not induce objective responses in recurrent or refractory metastatic breast or endometrial cancer at the RP2D dose of 625 mg weekly but had an acceptable safety profile (ClinicalTrials.gov Identifier: NCT03394027).
Keywords: ONC201, breast cancer, endometrial cancer, phase II, clinical trial
This phase II clinical trial evaluated ONC201 monotherapy, using the previously established recommended phase II dose, in patients with recurrent or refractory metastatic breast or endometrial cancer.
Lessons Learned.
ONC201 was tolerable but did not have significant clinical activity in patients with advanced, heavily pretreated estrogen receptor positive (ER+), triple-negative breast cancer, or endometrial cancer when given at 625 mg PO weekly.
ONC201 did not consistently cause mitochondrial damage or other biochemical effects predicted by preclinical studies.
ONC201 induced changes in circulating immune cells consistent with immune suppressive effects.
These data suggest more frequent dosing or more potent ClpP agonists are needed to see on-target effects.
Discussion
ONC201 is an orally active small molecule initially identified by a phenotypic screen for small molecules which induced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) transcription.1 Subsequent work identified that ONC201 can cause nonapoptotic cell death through a TRAIL-independent pathway via loss of mitochondrial function.2 ONC201 progressed to phases I and II trials in patients with refractory solid tumors.3,4 The recommended phase II dose (RP2D) was determined to be 625 mg weekly, and no serious side effects were attributable to ONC201.3-5 This trial evaluated ONC201 monotherapy in breast or endometrial cancer.
Patients were eligible if they had histologically proven metastatic hormone-refractory hormone receptor (HR)-positive, HER2 nonamplified metastatic breast cancer previously treated with >2 lines of hormonal therapy (cohort 1), metastatic triple-negative breast cancer (TNBC; cohort 2), or advanced endometrial cancer (cohort 3). Measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 was required. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and adequate hematopoietic, hepatic, and renal function.
Twenty-two patients with endometrial cancer (n = 10), HR-positive breast cancer (n = 7), or TNBC (n = 5) were enrolled and treated with ONC201 625 mg every 7 days in a 28-day cycle between 2018 and 2020. All patients were female. Median age was 61 years, and all were heavily pretreated (median 3 prior therapies).
Patients were evaluated for response by imaging every 8 weeks. The median progression-free survival (PFS) was 8.7 weeks in cohort 1, 6.6 weeks in cohort 2, and 8.9 weeks in cohort 3. Seven patients were excluded from PFS analysis due to not completing a full cycle of ONC201, primarily due to rapid progression. These early progressions suggest a lack of clinical activity of ONC201 monotherapy and reflect the underlying advanced disease. Eleven patients had follow-up imaging to assess for radiological response per RECIST criteria. The overall response rate (ORR) in all cohorts was 0%. The clinical benefit rate (CBR) was 27% (n = 3/11) and the median duration of treatment overall was 8.7 weeks. Grade 3 adverse events (AEs) occurred in 4 patients; no grade 4 AEs occurred (Table 1). Tumor biopsies did not reveal mitochondrial damage or consistent alterations in TRAIL or the TRAIL death receptors. ONC201 treatment caused alterations in peripheral immune cell subsets compared to baseline.
Table 1.
Adverse events
| AE | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematological | ||||
| Lymphocyte count decreased | 0 | 1 | 1 | 0 |
| WBC decreased | 1 | 0 | 0 | 0 |
| Anemia | 0 | 1 | 0 | 0 |
| Gastrointestinal | ||||
| Diarrhea | 4 | 0 | 0 | 0 |
| Nausea | 4 | 0 | 0 | 0 |
| Abdominal pain | 2 | 1 | 0 | 0 |
| AST elevated | 2 | 0 | 1 | 0 |
| Anorexia | 2 | 1 | 0 | 0 |
| ALK phos elevated | 2 | 1 | 0 | 0 |
| ALT elevated | 1 | 1 | 0 | 0 |
| Bilirubin elevated | 0 | 0 | 1 | 0 |
| Vomiting | 1 | 0 | 0 | 0 |
| Chemistry | ||||
| Hypermagnesemia | 1 | 0 | 0 | 0 |
| Creatinine increased | 0 | 1 | 0 | 0 |
| Hypophosphatemia | 1 | 0 | 0 | 0 |
| Other | ||||
| Fatigue | 7 | 0 | 0 | 0 |
| Dizziness | 5 | 0 | 0 | 0 |
| Hyperhidrosis | 1 | 0 | 0 | 0 |
| Arthralgias | 1 | 0 | 0 | 0 |
| Flu-like symptoms | 1 | 0 | 0 | 0 |
| Paresthesia | 1 | 0 | 0 | 0 |
| Malaise | 1 | 0 | 0 | 0 |
| Confusion | 1 | 0 | 0 | 0 |
| Flushing | 1 | 0 | 0 | 0 |
| Hot flashes | 1 | 0 | 0 | 0 |
| Lethargy | 1 | 0 | 0 | 0 |
| Lymphedema | 1 | 0 | 0 | 0 |
| Stroke | 0 | 0 | 1 | 0 |
| Rash maculopapular | 1 | 0 | 0 | 0 |
| Headache | 1 | 0 | 0 | 0 |
Abbreviations: AE, adverse event; ALK phos, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transferase; WBC, white blood cell count.
| Trial Information | |
|---|---|
| Disease | Breast cancer and advanced endometrial carcinoma |
| Stage of disease/treatment | Recurrent/refractory metastatic |
| Prior therapy | Cohort 1: >2 prior lines of hormonal therapy Cohort 2/3: ≥1 line of chemotherapy for metastatic/recurrent disease |
| Type of study | Phase II, single arm in 3 cohorts. Cohort1: HR-positive breast cancer, Cohort 2: TNBC, Cohort3: endometrial cancer |
| Primary endpoint | Cohort 1: PFS Cohort 2/3: ORR |
| Secondary endpoints | Cohort 1: ORR, CBR, safety Cohort 2/3: PFS, CBR, safety |
| Investigator’s analysis | Level of activity did not meet planned endpoint. No evidence of effect on correlative endpoints. |
| Drug Information | |
|---|---|
| Generic/working name | Dordaviprone dihydrochloride/ONC201 |
| Company name | Chimerix, Inc. (Formerly Oncoceutics, Inc.) |
| Drug type | Small molecule |
| Drug class | ClpP agonist |
| Dose | 625 |
| Unit | Milligrams |
| Route | Oral |
| Schedule of administration | Weekly |
| Patient Characteristics | |
|---|---|
| Number of patients, male | 0 |
| Number of patients, female | 22 |
| Stage | IV |
| Age: median (range) | 61 (38-73) years |
| Number of prior systemic therapies: median (range) | 3 (1-11) |
| Performance status: ECOG | 0: 6 1: 16 2: 0 3: 0 4: 0 |
| Cancer types or histologic subtypes | HR-positive breast cancer, 7; TNBC, 5; endometrial cancer, 10 |
| Primary Assessment Method: HR-positive (Cohort 1) | |
|---|---|
| Number of patients screened | 10 |
| Number of patients enrolled | 7 |
| Number of patients evaluable for toxicity | 7 |
| Number of patients evaluated for efficacy | 3 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 0 (0%) |
| Response assessment, SD | 1 (33.3%) |
| Response assessment, PD | 2 (66.7%) |
| Median duration assessment, PFS | 8.7 weeks |
| Duration of treatment | 8.7 weeks |
| Outcome notes | Three patients did not complete 1 cycle due to clinical progression. One patient had clinical progression and did not have follow-up imaging. |
| Primary Assessment Method: TNBC (Cohort 2) | |
|---|---|
| Number of patients screened | 6 |
| Number of patients enrolled | 5 |
| Number of patients evaluable for toxicity | 5 |
| Number of patients evaluated for efficacy | 2 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 0 (0%) |
| Response assessment, SD | 1 (50%) |
| Response assessment, PD | 1 (50%) |
| Median duration assessment, PFS | 6.6 weeks |
| Duration of treatment | 6.6 weeks |
| Outcome notes | Two patients did not complete 1 cycle due to clinical progression. One patient had clinical progression and did not have follow-up imaging |
| Primary Assessment Method: Endometrial Cancer (Cohort 3) | |
|---|---|
| Number of patients screened | 14 |
| Number of patients enrolled | 10 |
| Number of patients evaluable for toxicity | 10 |
| Number of patients evaluated for efficacy | 6 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 0 (0%) |
| Response assessment, SD | 2 (33.3%) |
| Response assessment, PD | 4 (66.7%) |
| Median duration assessments | 8.9 weeks |
| Duration of treatment | 8.9 weeks |
| Outcome notes | Two patients did not complete 1 cycle; of these, 1patient did not complete 1 cycle due to clinical progression and 1 patient did not complete a cycle due to intercurrent illness (a cerebrovascular accident) after 2 of 4 weekly doses in cycle 1 thought not to be related to ONC201. Two patients had clinical progression and did not have follow-up imaging. |
| Pharmacodynamic Analysis: Results |
|---|
| Mitochondrial DNA (mtDNA) copy number analysis, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), immunoblotting and electron microscopy were performed on the paired tumor biopsy samples in 9 patients (Fig. 1D). There were not consistent changes in mitochondrial structure, mitochondrial DNA copy number, protein levels of ClpP target subrates Tfam and Tufm. Gene expression of TRAIL, DR4, DR5, ATF4, and DDIT3/CHOP were also mixed. |
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator’s assessment | Inactive because results did not meet primary endpoint |
This open-label, single-arm, phase II clinical trial evaluated ONC201 monotherapy in 3 cohorts: (1) hormone-refractory HR-positive, HER2 nonamplified metastatic breast cancer, (2) metastatic TNBC, and (3) advanced endometrial cancer. The primary endpoint for cohort 1 was PFS. The primary endpoint for both cohort 2 and cohort 3 was ORR. Secondary endpoints included safety, CBR (defined as complete response [CR] + partial response [PR] + stable disease [SD]), ORR (cohort 1), and PFS (cohorts 2 and 3). All statistical tests were 2-sided with 95% confidence intervals (CIs) and an alpha level of .05. PFS was estimated using the Kaplan–Meier method (Fig. 1). All statistical analyses were performed using GraphPad Prism software v6.0 (GraphPad Software Inc., La Jolla, CA).
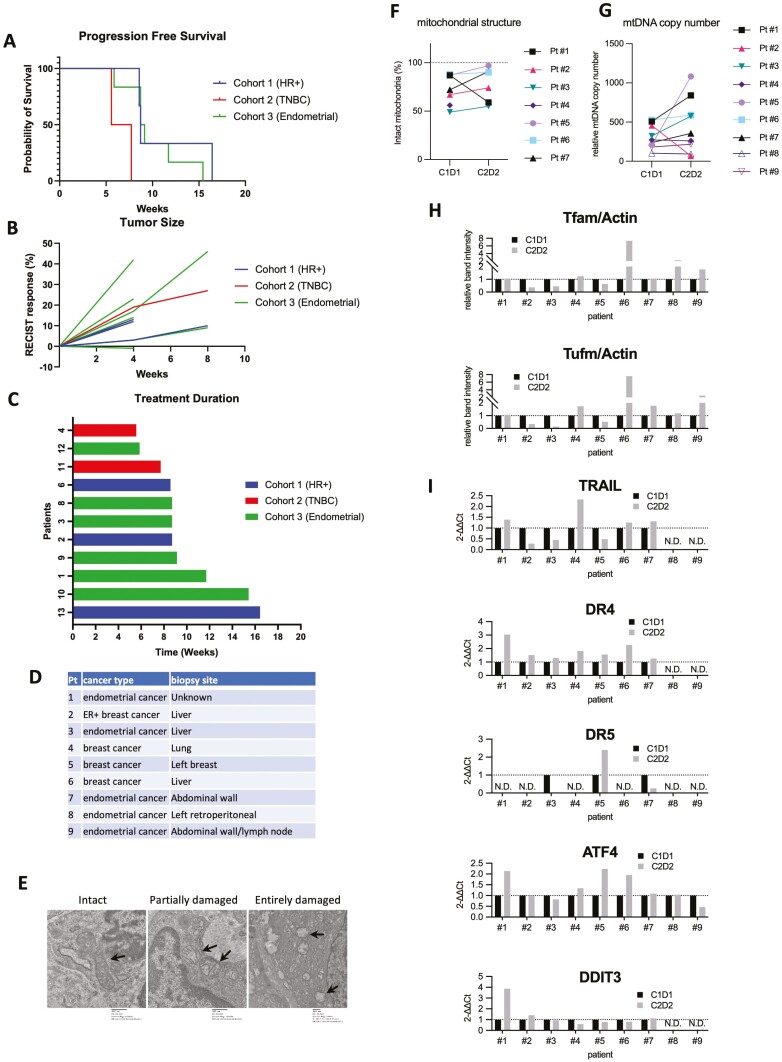
Figure 1.
(A) Progression-free survival. (B) Response by RECIST v1.1. (C) Duration of ONC201 treatment. (D) Patients whose biopsy samples compared between cycle 1 day1 and Cycle 2 Day 28. (E) Representative images showing intact, partially damaged, and severely damaged mitochondria. Intact mitochondria was from Pt #1 (cycle 1 day 1, 10000×), partially damaged mitochondria Pt #1(cycle 2 day 28, 10000×), severely damaged mitochondria Pt #3 (Cycle 1 Day 1, 5000×). (F) Mitochondrial structural analysis by electron microscopy images. (G) mtDNA copy number qPCR. (H) Immunobloting analysis of Tfam and Tufm normalized with actin as an internal control. (I) qPCR analysis of TRAIL,DR4, DR5 and ATF4, and DDIT3, normalized with GAPDH. ATF4, activating transcription factor 4; DR, death receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mtDNA, mitochondrial DNA; N.D., not determined due to no detectable amplification;qPCR, quantitative polymerase chain reaction; RECIST, Response Evaluation Criteria in Solid Tumors; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Out of 22 patients enrolled, 15 patients completed at least 1 cycle of ONC201. Six patients had clinical progression prior to cycle 2. Four patients were excluded from response rate evaluations due to not having follow-up imaging because of clinical progression. Four patients had stable disease at first restaging, but all had progression by next restaging.
ONC201 was well tolerated though all patients experienced an AE. The most common low-grade AEs were fatigue (31.8%), dizziness (22.7%), nausea (18.2%), and diarrhea (18.2%). There were 4 grade 3 AEs (decreased lymphocyte count, elevated AST, elevated bilirubin, and stroke) and no grade 4 AEs.
ONC201 was hypothesized to induce TRAIL expression resulting in autocrine apoptotic death.6 Subsequent work identified that ONC201 causes nonapoptotic cell death through a TRAIL-independent pathway by inducing mitochondrial structural damage and functional impairment.2 ONC201 was found to bind and activate mitochondrial caseinolytic protease P (ClpP), a serine protease that participates in the degradation of damaged mitochondrial proteins.7,8 ClpP is overexpressed in many hematologic and solid malignancies including breast cancers, and thus is of interest as an anticancer target.9-11 Patients with endometrial cancer had tumor regressions in a phase I study of ONC201 prompting their inclusion in this study.4
To compare the effect of ONC201 on tumors before and after the treatment, paired tumor biopsy samples were performed prior to the first dose (cycle 1, day 1) and after the fifth dose (Cycle 2, Day 2) in 9 patients. Cancer types and biopsy sites are summarized in Fig. 1D. Note that patient #1 experienced the overall best clinical response of SD, though had rising CA125. Mitochondrial DNA (mtDNA) copy number analysis, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), immunoblotting, and electron microscopy were performed on the samples as previously described.2 In mitochondrial structural analysis of 7 patients using electron microscopy, only patient #1 showed a decrease in the fraction of intact mitochondria from 87% to 59% (Fig. 1E, 1F), while other paired samples did not show a reduction. mtDNA copy number qPCR analysis did not show a consistent trend in 9 patients between pre- and post-ONC201 (Fig. 1G). The protein levels of Tfam and Tufm, 2 established ClpP target substrates, were compared with immunoblotting analysis. Several patients showed a reduction of both Tfam and Tufm (#2, 3, 5) after ONC201 treatment. Meanwhile, other patients (#4, 6, 7, 8, 9) showed an increase of these proteins, or no change (patient #1, Fig. 1H). In summary, there was no consistent ONC201-induced changes in Tfam and Tufm levels.
The original study that identified ONC201 described it as a TRAIL-inducing small molecule compound.6 Subsequent studies reported that ONC201 induces TRAIL death receptor 5 (DR5) and upregulates ATF4 and DDIT3/CHOP as an integrated stress response.12-14 We previously reported that ONC201 does not significantly induce TRAIL, DR4/5 in breast cancer cell lines and that cytotoxicity of ONC201 is independent of TRAIL-DR pathway, while ATF4 and DDIT3 are significantly upregulated.2 We analyzed the expression level of these genes in the 9 paired-biopsy samples by RT-qPCR. The TRAIL and DR4/5 qPCR presented mixed results upon ONC201 treatment. TRAIL mRNA was decreased in 3 patients (#2, 3, 5), increased in 4 patients (#1, 4, 5, 7), and not detectable in 2 patients (#8, 9). The level of DR4 was increased upon treatment in Patients #1–7, but not detected in Patients #8 or #9. DR5 was not detectable in many patients, but Patients #3 and #7 showed a reduction, while patient #5 showed an increase after ONC201 treatment. Both ATF4 and DDIT3/CHOP were increased in patient #1, potentially associated with noticeable mitochondrial damage (Fig. 1I) and the best overall clinical response. Induction of ATF4 was observed in 3 patients (#4, 5, 6), but not in other patients. DDIT3 induction was only observed in patient #1. Together, the molecular and electron microscopy analysis from paired-biopsy samples demonstrated that ONC201 625 mg per week for 5 doses did not show consistent changes in these potential pharmacodynamic targets of ONC201 in these tissue sites.
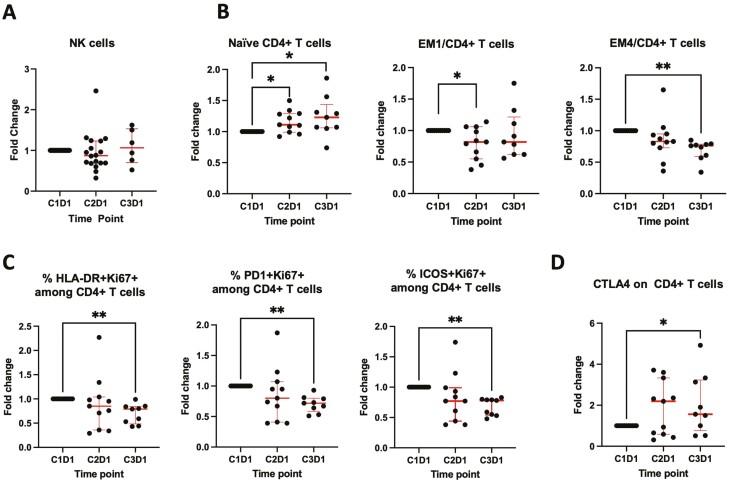
ONC201 has been shown to upregulate natural killer (NK) cells and CD3+ cells within tumors and peripheral blood in preclinical mouse models.15 Peripheral NK cells have further been shown to be increased following treatment with ONC201 in a cohort of patients with prostate cancer.15 There were no consistent changes in the peripheral NK cells during treatment with ONC201 in our study (Fig. 2A). Among total peripheral CD4+ T cells, naïve cells were increased and subsets of effector memory cells (EM1 and EM4) were decreased (Fig. 2B). Activated (HLA-DR+ or PD-1+ or ICOS+) and proliferating (Ki67+) CD4+ T cells (Fig. 2C) and CD8+ T cells (data not shown) were decreased after 2 cycles of treatment. CTLA4 expression on CD4+ T cells was increased after treatment (Fig. 2D). These changes in the peripheral immune cells are consistent with an immunosuppressive effect. This suggests combining ClpP agonists with anti-CTLA4 therapy may increase efficacy.
Figure 2.
(A) Natural killer cells did not change significantly at C2D1 and C3D1. (B) Naïve cells were increased and effector memory (EM)1 and EM4 cells were decreased among total peripheral CD4+ T cells (*P < .05; **P < .005). (C) Activated (HLA-DR+ or PD-1+ or ICOS+) and proliferating (Ki67+) cells were decreased among total peripheral CD4+ T cells. (D) CTLA4 expression on peripheral CD4+ T cells was increased.
ONC201 monotherapy at 625 mg weekly did not demonstrate clinical benefit with no durable responses in this phase II study of patients with breast or endometrial cancer. Correlative studies from tumor biopsies suggest that ONC201 did not consistently reach its target within the tumor given that no consistent changes in mitochondrial structure, loss of mtDNA, or ClpP substrates (Fig. 1F–H), or upregulation of TRAIL, DR4, or DR5 (Fig. 1I).
Contributor Information
Sarah L P Atkins, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Yoshimi Endo Greer, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Sarah Jenkins, University of Tennessee Medical Center, Knoxville, TN, USA.
Margaret E Gatti-Mays, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA; Division of Hematology/Medical Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Nicole Houston, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Sunmin Lee, Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Min-Jung Lee, Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Shraddha Rastogi, Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Nahoko Sato, Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Christina Burks, Electron Microscopy Laboratory, NCI, NIH, Frederick, MD, USA.
Christina M Annunziata, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Jung-Min Lee, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Kunio Nagashima, Electron Microscopy Laboratory, NCI, NIH, Frederick, MD, USA.
Jane B Trepel, Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Stanley Lipkowitz, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Alexandra S Zimmer, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute , National Institutes of Health, Bethesda, MD, USA.
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (ZIA SC 007263). This study was sponsored by the National Cancer Institute and Chimerix Inc. (formerly Oncoceutics, Inc.). ONC201 was provided by Chimerix Inc. (formerly Oncoceutics, Inc.).
Conflict of Interest
The authors indicated no financial relationships.
Data Availability
The data underlying this article are available in the article.
References
- 1. Allen JE, Kline CLB, Prabhu VV, et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget 2016;7(45):74380-74392. 10.18632/oncotarget.11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greer YE, Porat-Shliom N, Nagashima K, et al. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget 2018;9(26):18454-18479. 10.18632/oncotarget.24862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein, M., et al. , First-in-human trial of ONC201 in patients with refractory solid tumors. ASCO Annual Meeting. 2016.
- 4. Stein MN, Bertino JR, Kaufman HL, et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017;23(15):4163-4169. 10.1158/1078-0432.ccr-16-2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stein MN, et al. , Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J ImmunoTher Cancer. 2019;7(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171):171ra17. 10.1126/scitranslmed.3004828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graves PR, Aponte-Collazo LJ, Fennell EMJ, et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem Biol. 2019;14(5):1020-1029. 10.1021/acschembio.9b00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35(5):721-737. 10.1016/j.ccell.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nouri K, Feng Y, Schimmer AD.. Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis. 2020;11(10):841. 10.1038/s41419-020-03062-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole A, Wang Z, Coyaud E, et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2015;27(6):864-876. 10.1016/j.ccell.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seo JH, Rivadeneira DB, Caino MC, et al. The mitochondrial unfoldase-peptidase complex ClpXP controls bioenergetics stress and metastasis. PLoS Biol. 2016;14(7):e1002507. 10.1371/journal.pbio.1002507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kline CLB, Van den Heuvel APJ, Allen JE, et al. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci Signal. 2016;9(415):ra18. 10.1126/scisignal.aac4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen JE, Crowder R, El-Deiry WS.. First-in-class small molecule ONC201 induces DR5 and cell death in tumor but not normal cells to provide a wide therapeutic index as an anti-cancer agent. PLoS One. 2015;10(11):e0143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishizawa J, Kojima K, Chachad D, et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9(415):ra17. 10.1126/scisignal.aac4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner J, Kline CL, Zhou L, et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J Clin Investig. 2018;128(6):2325-2338. 10.1172/jci96711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.