Abstract
Concurrent outbreaks of circulating vaccine-derived poliovirus serotypes 1 and 2 (cVDPV1, cVDPV2) were confirmed in the Republic of the Philippines in September 2019 and were subsequently confirmed in Malaysia by early 2020. There is continuous population subgroup movement in specific geographies between the two countries. Outbreak response efforts focused on sequential supplemental immunization activities with monovalent Sabin strain oral poliovirus vaccine type 2 (mOPV2) and bivalent oral poliovirus vaccines (bOPV, containing Sabin strain types 1 and 3) as well as activities to enhance poliovirus surveillance sensitivity to detect virus circulation. A total of six cVDPV1 cases, 13 cVDPV2 cases, and one immunodeficiency-associated vaccine-derived poliovirus type 2 case were detected, and there were 35 cVDPV1 and 31 cVDPV2 isolates from environmental surveillance sewage collection sites. No further cVDPV1 or cVDPV2 have been detected in either country since March 2020. Response efforts in both countries encountered challenges, particularly those caused by the global COVID-19 pandemic. Important lessons were identified and could be useful for other countries that experience outbreaks of concurrent cVDPV serotypes.
Keywords: Poliovirus outbreak, Circulating vaccine-derived poliovirus, Immunodeficiency-associated vaccine-derived poliovirus, Philippines, Malaysia
1. Introduction
The international spread of polioviruses was declared a Public Health Emergency of International Concern (PHEIC) by the Director-General of the World Health Organization (WHO) in May 2014, after multiple instances of importations of wild poliovirus type 1 (WPV1) from endemic countries during 2013–2014 and subsequent spread, including long-distance from Nigeria to the Horn of Africa and from south Asia to the middle East [1–4]. The emergence and spread of circulating vaccine-derived polioviruses (cVDPVs) have also become a major concern for the International Health Regulations (2005) (IHR) Emergency Committee under the polio PHEIC, particularly since 2017. We report concurrent outbreaks of cVDPV serotypes 1 and 2 (cVDPV1 and cVDPV2) that moved across two neighboring, multi-island countries, the Republic of the Philippines (“Philippines”) and Malaysia in 2019–2020 with activities continuing in 2021. Chronic security issues in the southern Philippines limited access to essential immunizations and forced families to flee to more stable areas in the northern Philippines. Domestic transmission of both cVDPVs was observed along this established path of population movement. High-risk mobile populations move predominantly by sea across the Sulu archipelago between the southern Philippines and Sabah state on Borneo Island in Malaysia. Low reach of essential (routine) immunization has created sizable cohorts of children susceptible to polio, especially among non-citizens in Sabah state.
2. Background
All oral poliovirus vaccine (OPV)-using countries stopped use of type 2-containing OPV in essential and supplementary immunization activities in a globally coordinated “switch” from trivalent OPV (tOPV, containing Sabin strains types 1, 2 and 3) to bivalent OPV (bOPV, containing Sabin strains types 1 and 3) in 2016. Prior to the switch, at least one dose of trivalent inactivated poliovirus vaccine (IPV) was to be introduced to provide protection against paralytic polio caused by type 2 [5]. IPV does not induce intestinal mucosal immunity in OPV-naïve individuals which is necessary in areas where poliovirus transmission is predominantly fecal-oral [6]. In the Philippines, the essential immunization schedule consists of bOPV at 6, 10, and 14 weeks of age and IPV at 14 weeks of age (i.e., with the third dose of bOPV). Phased IPV introduction began in 2015 and was not nationwide until 2017 due to a global supply shortage [7]. Malaysia switched to IPV-only for essential immunizations in 2008, given as a pentavalent with diphtheria, tetanus toxoids, acellular pertussis (DTaP), and Hemophilus influenzae type b vaccines at 2, 3, and 5 months (primary series), and 18 months of age (booster); hexavalent as of the end of 2020 with the addition of hepatitis B. An OPV booster dose was also administered at age 7 but was discontinued in 2016.
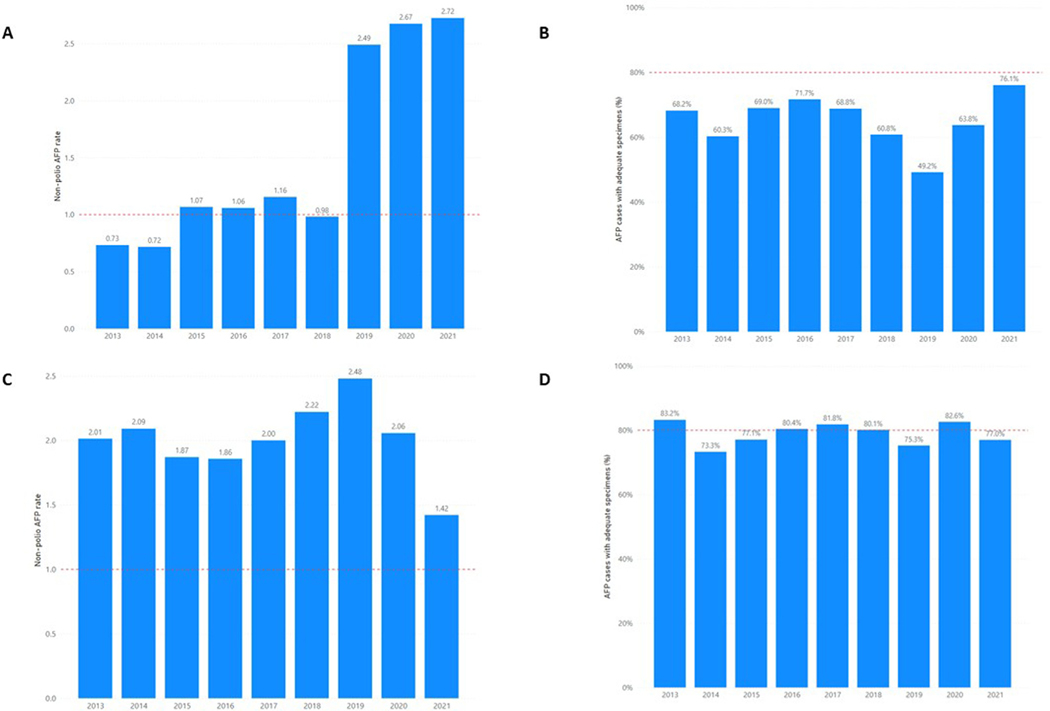
Concerns about the risk for cVDPV outbreaks in the Philippines had been raised for almost a decade during annual meetings of WHO’s Western Pacific (WP) Polio Regional Certification Commission (RCC) because of declining polio immunization coverage. National vaccination coverage estimates to three doses of OPV (POL3) declined from 88% in 2012 to 66% in 2019; [8] by 2017, all 17 regions in the country reported < 80% POL3 coverage. Challenges in vaccine procurement led to frequent vaccine stockouts; in addition, there was increased vaccine hesitancy in the population [9]. National and subnational performance indicators of acute flaccid paralysis (AFP) surveillance quality also indicated likelihood of delayed detection of poliovirus transmission. During 2013–2018, the national non-polio AFP (NPAFP) rate infrequently achieved WHO’s target of ≥1 per 100,000 children < 15 years of age and stool adequacy percentage1 never reached the targeted ≥ 80% since 2013, steadily declining from a high of 72% in 2016 (Fig. 1). Subnational variability was noted over these years; for example, only one region in 2018 met both indicator targets. Environmental surveillance (ES) through systematic sewage sampling was established in 2017 in three sites in two regions to supplement AFP surveillance and by 2019 included a network of 11 sites in six regions.
Fig. 1.
Annual non-polio acute flaccid paralysis (NPAFP) rate* and stool adequacy percentage in the Philippines (A and B) and Malaysia (C and D), 2013–2021**. *Non-polio acute flaccid paralysis rate expressed as per 100,000 children under 15 years old per year. **Data as of 21 February 2022. Red hashed line indicates surveillance indicator target: NPAFP rate target of 1 per 100,000 children under 15 years old and 80% for stool adequacy.
Malaysia never detected cVDPV and had been consistently assessed as low risk for a polio outbreak by the WP Polio RCC based on overall high vaccination coverage for the primary course of polio vaccines and relatively strong surveillance indicators. National coverage for three doses of IPV has been ≥98% since 2015 [8]. However, reported immunization coverage may not be accurate; a joint review in 2019 of essential immunization by the Malaysia Ministry of Health (MoH) and WHO found that reported immunization coverage data had limitations to reliably measure programme performance and immunity gaps in communities. Coverage may be under-estimated due to missing or incomplete data from private healthcare facilities and sometimes inaccurate documentation of late doses, including vaccine doses given to non-citizens. Coverage may also be over-estimated because the number of children targeted for vaccination are based on the number of estimated live births, which incompletely captures live births among non-citizens.
Gaps exist in coverage between citizens and non-citizens especially in Sabah which attracts many undocumented arrivals; an estimated 29% of the population are non-citizens (source: Malaysia MoH). A coverage survey conducted in a community during outbreak investigations found none of the 101 non-citizen children < 15 years of age had been vaccinated (source: Malaysia MoH). Non-citizen populations can access fee-for-service immunizations at government health facilities whereas access is free for citizen children; access to private health facilities is available to all. There is also reluctance by non-citizens to visit government health facilities for essential immunizations, reportedly on fear of deportation. This combined with the switch to IPV-only in 2008 indicate that among children < 13 years of age, protection from paralytic polio (i.e., humoral immunity) is high among citizen children but low in non-citizen children. Furthermore, all children < 13 years of age have little or no intestinal mucosal immunity to polioviruses.
3. Outbreak details
3.1. Outbreak detection
cVDPV1.
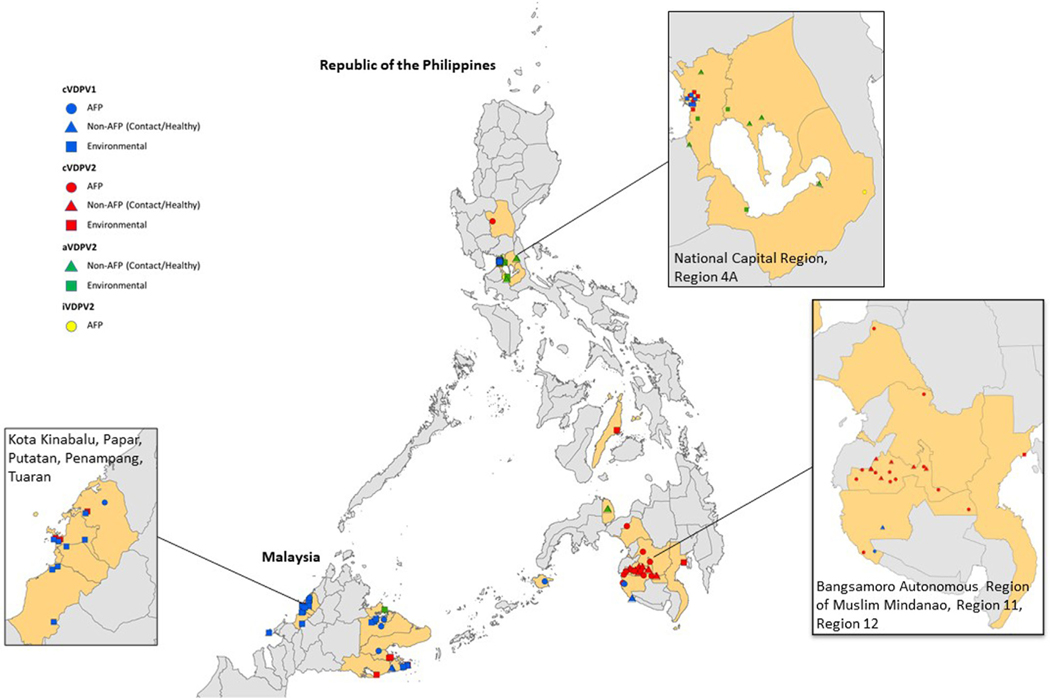
In the Philippines, an ES sample collected on 1 July 2019 from the Tondo district site, Manila, (National Capital Region, NCR) was confirmed on 19 July as VDPV1 positive with 32 nucleotide (nt) substitutions from the Sabin 1 virus in the VP1 coding section of the genome (3.5% divergence) (Fig. 2, Table 1). Genetically-linked VDPV12 continued to be isolated from the Tondo ES site for more than two months leading to the confirmation of a cVDPV1 outbreak on 27 September, which was preceded by the declaration of a cVDPV2 outbreak on 19 September [10]. cVDPV1 isolates genetically linked to the virus in Manila were confirmed in one AFP case from Basilan island (onset 19 October 2019) and Sultan Kudarat, Mindanao (onset 14 September 2019) in the southern Philippines by January 2020 (Fig. 3A).
Fig. 2.
Vaccine-derived poliovirus type 1 (VDPV1) and vaccine-derived poliovirus type 2 (VDPV2) isolated from acute flaccid paralysis (AFP) case-patients, other human samples (non-AFP)*, and environmental samples in the Philippines and Malaysia, 2019–2020. *Other human samples (non-AFP): samples collected from contacts of AFP case-patients or healthy children. Data as of 8 April 2021. AFP case-patients and other humans are placed randomly within province (Philippines) & district (Malaysia) boundaries. Environmental specimen locations are based on exact coordinates of collection sites.
Table 1.
Summary of vaccine-derived poliovirus type 1 (VDPV1) and vaccine-derived poliovirus type 2 (VDPV2) detections from acute flaccid paralysis case-patients, environmental samples, and other human samples in the Philippines and Malaysia, 2019–2020.
| cVDPV1 | AFP cases | Environmental samples | Other human samples |
|---|---|---|---|
|
| |||
| Philippines | 2* | 14 | 1 |
| Malaysia | 4 | 21 | 1 |
| cVDPV2 | |||
| Philippines | 13 | 23 | 7 |
| Malaysia | 0 | 8 | 0 |
| iVDPV2 | |||
| Philippines | 1 | 0 | 0 |
| Malaysia | 0 | 0 | 0 |
| aVDPV2 | |||
| Philippines | 1* | 3 | 6 |
| Malaysia | 0 | 1 | 0 |
cVDPV: circulating vaccine-derived poliovirus; iVDPV: immunodeficiency-associated vaccine-derived poliovirus; aVDPV: ambiguous vaccine-derived poliovirus.
One acute flaccid paralysis case-patient epidemiologically linked to a laboratory confirmed polio infection in a contact.
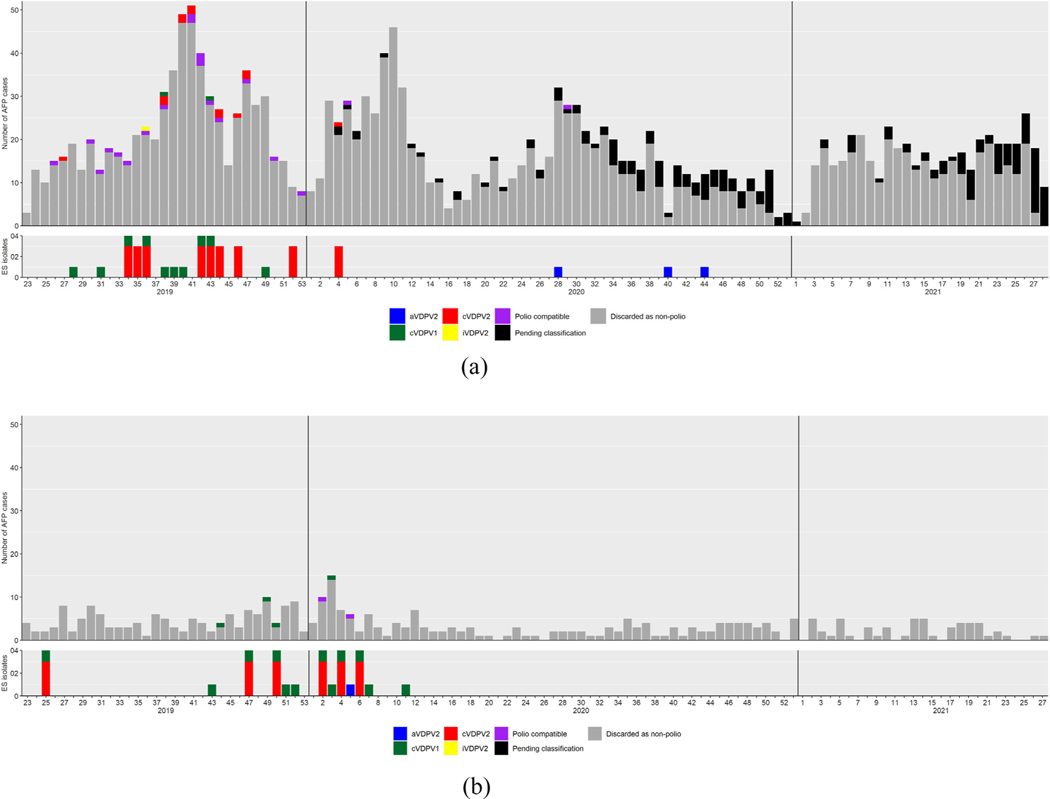
Fig. 3.
Acute flaccid paralysis (AFP) case-patients by week of paralysis onset and final classification status, and environmental surveillance (ES) isolates by week of collection in the Philippines (A) and Malaysia (B), June 2019–February 2021.
In Sabah, Malaysia, a three-month-old male citizen had paralysis onset on 30 October 2019 and confirmed positive for VDPV1 on 6 December 2019. The virus had 34 nt substitutions from Sabin 1 virus (3.8% divergence) and was classified as cVDPV1 with genetic linkage to the cVDPV1 in the Philippines. He had received his first dose of combination DTaP-IPV-Hib < 1 month before onset. Three additional cases were confirmed by March 2020, all in non-citizens who resided in Sabah communities and were unvaccinated for polio (Fig. 3B); two were older children, 8 and 11 years of age.
In Malaysia, cVDPV1 and cVDPV2 isolates were also identified on 28 December 2019 from a sewage sample collected on 19 November 2019 from the Central Treatment Plant in Semporna, Sabah State. The cVDPV1 isolate had 33 nt changes (3.6% divergence from Sabin 1 virus) and was genetically linked to the cVDPV1 in the Philippines. Details of the cVDPV2 isolates are included in the next section. Thereafter on 9 January 2020, MoH confirmed cVDPV1 and cVDPV2 detected in a sewage sample taken on 18 June 2019 from Sempelang 2 Sewage Pump Station in Kota Kinabalu; sequencing was delayed as the initial testing laboratory was not a WHO-accredited laboratory within the Global Polio Laboratory Network. Both viruses were genetically linked to cVDPV1 and cVDPV2 elsewhere in Sabah and the Philippines.
cVDPV2.
In the Philippines, ES samples collected on 13 August 2019 from the Tondo ES site and 22 August from the Davao City ES site were confirmed as positive for VDPV2 on 3 September and 6 September 2019, respectively. The genetically linked viruses had 61–65 nt changes from Sabin 2 virus (6.8–7.2% divergence) in the VP1 coding sequence (Fig. 2, Table 1) and was immediately classified as cVDPV2 and the outbreak formally declared on 19 September [11]. In addition, a VDPV2 isolate with 69 nt substitutions (7.1% divergence) was identified from an AFP case in a child in Laguna with onset of paralysis on 25 August 2019; this isolate was remotely genetically linked (11 nt in common) to the cVDPV2. Upon confirmation that the case-patient had humoral immunodeficiency, this virus was classified as immunodeficiency-associated vaccine-derived poliovirus type 2 (iVDPV2).
In Malaysia, the cVDPV2 isolate from a sewage sample taken on 19 November 2019 from the Central Market Sewage Treatment Plant in Semporna, Sabah state, had 62 nt changes from Sabin (6.9% divergence) and was genetically linked to the cVDPV2 circulating in the Philippines.
3.2. Virologic analyses
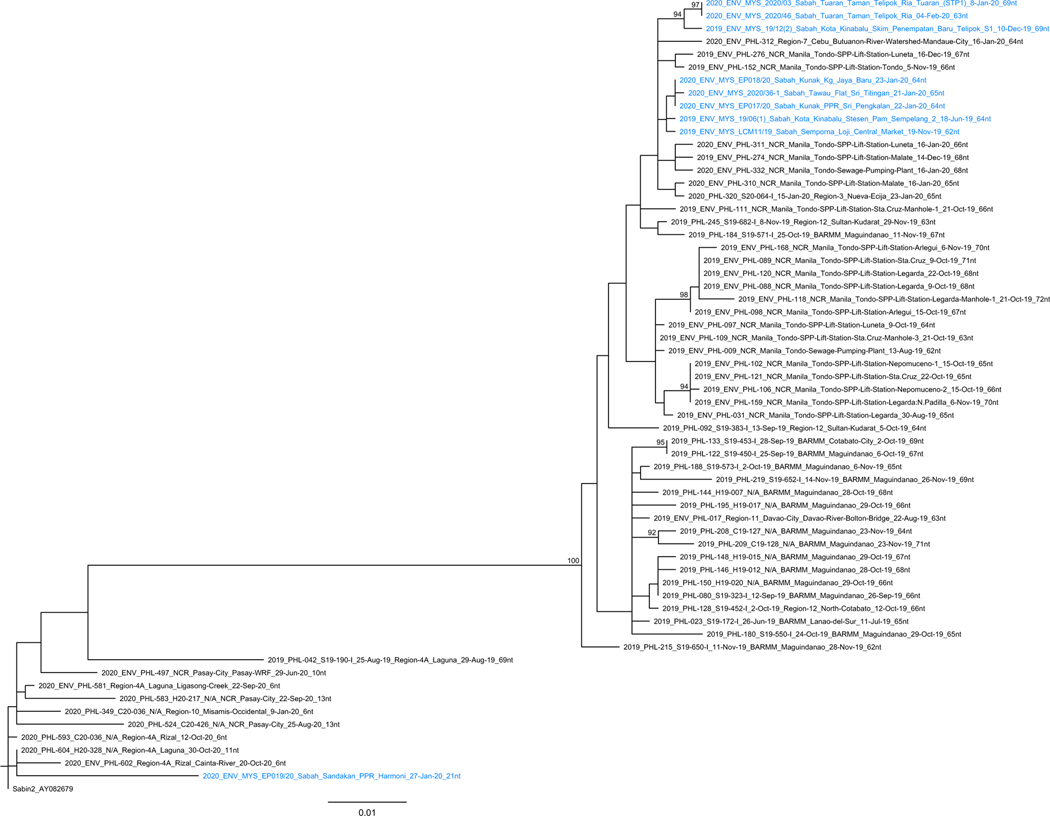
The number of VP1 nt substitutions from Sabin 1 in the isolates of cVDPV1 PHL-NCR-2 emergence group in the Philippines and Malaysia ranged 30–40 (3.3–4.4% divergence) indicating at least three years of replication without detection (Fig. 4). Based on Bayesian phylogenetic analysis and assuming a constant rate of evolution of 1.1% per year, the mean estimated date of the seeding dose was early 2016 [12].
Fig. 4.
Maximum-likelihood based tree from poliovirus VP1 coding region genomic sequencing analysis depicting the phylogenetic relationships between vaccine-derived poliovirus type 1 (VDPV1) isolates collected from the Philippines and Malaysia in 2019 and 2020. The tree was constructed using the PhyML program within Geneious using the K80 + G model of evolution with optimised parameters based on 906nt VP1 sequences. The Sabin1 prototype strain AY082688 was used as an out-group to root the tree. Bootstrap analysis was performed on 1000 replicates of the dataset to provide a statistical measure of the reliability of the clades depicted. Bootstrap values indicate the number of times, out of 1000 replicates, that all members of the clade descended from the individual node were grouped together, with values >90% indicated. Isolates from the Philippines are indicated in black and those from Malaysia are indicated in blue. Prepared by Victorian Infectious Disease Reference Laboratory, Australia and National Institute of Infectious Diseases, Japan.
The number of VP1 nt substitutions from Sabin 2 virus in the cVDPV2 PHL-NCR-1 emergence group detected in both countries ranged 60–72 (6.8–8.0% divergence) (Fig. 5). Based on Bayesian phylogenetic analysis, this suggests more than six years of replication without detection [12].
Fig. 5.
Maximum-likelihood based tree from poliovirus VP1 coding region genomic sequencing analysis depicting the phylogenetic relationships between vaccine-derived poliovirus type 2 (VDPV2) isolates collected from the Philippines and Malaysia in 2019 and 2020. The tree was constructed using the PhyML program within Geneious using the K80 + G model of evolution with optimised parameters based on 903nt VP1 sequences. The Sabin2 prototype strain AY082679 was used as an out-group to root the tree. Bootstrap analysis was performed on 1000 replicates of the dataset to provide a statistical measure of the reliability of the clades depicted. Bootstrap values indicate the number of times, out of 1000 replicates, that all members of the clade descended from the individual node were grouped together, with values >90% indicated. Isolates from the Philippines are indicated in black and those from Malaysia are indicated in blue. Prepared by Victorian Infectious Disease Reference Laboratory, Australia and National Institute of Infectious Diseases, Japan.
Closely related viruses are represented on the phylogenetic trees of cVDPV1 (Fig. 4) and cVDPV2 (Fig. 5) as short horizontal branch connections between sequences, while long branches represent gaps in detection among polio cases and ES samples. Two long branches in Fig. 5 clearly indicate undetected, prolonged circulation of cVDPV2. The upper long branch represents a large cluster of cVDPV2 from AFP cases and ES in Malaysia and the Philippines while the lower long branch represents iVDPV2 from a prolonged excreter in the Philippines that is distantly related to the circulating cluster.
3.3. Public health response to cVDPV outbreaks
The Global Polio Eradication Initiative (GPEI) provides technical assistance and some financial support in countries’ response efforts and outlines expectations of activities and timelines in Standard Operating Procedures (SOPs) for responding to a poliovirus event or outbreak [10]. Two key components of response activities are to 1) conduct at least two high-quality supplemental immunization activities (SIAs) targeting children < 5 years of age (or older based on the epidemiology), and 2) enhance the sensitivity of surveillance to detect poliovirus. For cross-border outbreaks, countries are encouraged to hold synchronized SIAs and implement complementary surveillance strengthening activities. There is no guidance on sequential or co-administration of monovalent OPV type 2 (mOPV2) and bOPV in the event of co-circulating cVDPV types 1/2 or types 2/3 outbreaks.
Declaration of the coronavirus disease (COVID-19) pandemic in March 2020 led to unprecedented actions within and across countries to curtail its spread. As risk mitigation efforts to protect polio staff and volunteers, GPEI recommended in mid-March that all planned SIAs be postponed until July 2020 and only essential polio surveillance activities be undertaken which curtailed case detection and field investigations [13,14]. Government mandates such as movement control orders limited activities further such that ES sample collection was stopped or reduced at certain sites and transport of collected ES samples and stool specimens were delayed.
3.4. Philippines public health response
Outbreaks of cVDPV1 and cVDPV2 were officially declared in September 2019. The Philippines Department of Health (DOH) established an incident command structure with a national emergency operating center (EOC) in Manila, a subnational EOC Hub in Davao City, and an EOC in each of the seven outbreak-affected regions [15]. International GPEI consultants (including alumni of the Stop Transmission of Polio program) were recruited to work with DOH staff at the national and regional-levels to support implementation of polio SIAs and strengthen poliovirus surveillance.
Polio SIAs.
In response to the initial detections of VDPV1 from ES in Manila, DOH rapidly implemented vaccinator trainings and preparations for a focused SIA in Manila that began on 19 August 2019, before an outbreak was officially declared. While only 54% of the targeted children < 5 years old were vaccinated, the experience provided key details for DOH to improve their overall SIA strategies (predominantly house-to-house but included fixed-posts). This led to revisions to improve the details and accuracy of the microplans, the need for targeted social mobilization and risk communications efforts, and engagement of key stakeholders to facilitate access to children.
Once co-circulation of cVDPV1 and cVDPV2 was confirmed, sequential mOPV2 and bOPV SIAs were conducted (Table 2). SIAs targeting children < 5 years old (expanded in specific geographies to children < 10 years old) were successfully implemented until March 2020 when the risk mitigation efforts of the COVID-19 pandemic were introduced and all SIAs were halted. The bOPV SIA in Mindanao scheduled for March was delayed by four months. Two mOPV2 SIAs that were scheduled to begin in March for Region 3 and Region 4A were also delayed until July 2020. Overall, reported vaccination coverage for each of the rounds in NCR and southern Philippines surpassed the targeted ≥ 95% after the initial SIA in Manila but ranged 71–85% in Regions 3 and 4A where a geographically-phased approach was used upon SIA resumption in July. Areas selected for intra-campaign monitoring indicated that overall, 1%−4% of children checked by external monitors were unvaccinated with the percent of unvaccinated children slightly higher among children < 1 year of age than ≥ 1 year.
Table 2.
Summary of bivalent oral poliovirus vaccine (bOPV) and monovalent oral poliovirus vaccine type 2 (mOPV2) Supplemental Immunization Activities in the Philippines and Malaysia, 2019–2020.
| First detection | Last detection | Rounds (MM/YY) | Vaccine | Locations | Target Age | Target population | Vaccination coverage |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Metro Manila (also known as National Capital Region) | |||||||
| cVDPVl | |||||||
| ES sample collected 1 July 2019 | ES sample collected on 28 Nov 2019 | Round 0 (Aug 2019) | bOPV | Manila City only | < 5 years | 197,138 | 54% |
| Round 1 (Oct 2019) | bOPV | Metro Manila (17 cities) | < 5 years | 1.2 million | 96% | ||
| Round 2 (Nov 2019) | bOPV | Metro Manila (17 cities) | < 5 years | 1.2 million | 110% | ||
| cVDPV2 | |||||||
| ES sample collected 13 Aug 2019 | ES sample collected 16 Jan 2020 | Round 1 (Jan 2020) | mOPV2 | Metro Manila (17 cities) | <5 years | 1,403,861 | 99% |
| Round 2 (Feb 2020) | mOPV2 | Metro Manila (17 cities) | <5 years | 1,403,861 | 102% | ||
| Southern Philippines | |||||||
| cVDPVl | |||||||
| AFP case-patient with paralysis onset 19 Oct 2019 | AFP contact with specimen collection on 31 Oct 2019 | Round 0 (Jan 2020) | bOPV | Mindanao Island - selected cities | <10 years | 739,640 | 95% |
| Round 1 (Feb 2020) | bOPV | Mindanao Island - selected cities | <10 years | 739,640 | 99% | ||
| Mindanao Island - remaining areas | <5 years | 3 million | |||||
| Round 2 (July 2020) | bOPV | Mindanao Island - selected cities | <10 years | 476,544 | 98% | ||
| Mindanao Island - remaining areas | <5 years | 3 million | |||||
| cVDPV2 | |||||||
| ES sample collected on 22 Aug 2019 | AFP case-patient with paralysis onset 8 Nov 2019 | Round 0 (Oct 2019) | mOPV2 | Mindanao Island - selected cities | <5 years | 459,381 | 92% |
| Round 0 (cont, Nov 2019) | mOPV2 | Mindanao Island - additional selected cities | <5 years | 17,541 | 95% | ||
| Round 1 (Nov 2019) | mOPV2 | Mindanao Island | <5 years | 3,102,973 | 95% | ||
| Round 2 (Jan 2020) | mOPV2 | Mindanao Island | <5 years | 3,102,973 | 99% | ||
| Philippines - other regions | |||||||
| cVDPV2 | |||||||
| AFP case-patient with paralysis onset 15 Jan 2020 | Round 1 (Jul 2020) | mOPV2 | Central Luzon - selected cities and provinces | <5 years | 2,532,010 | 81% | |
| Round 1 (cont, Aug 2020) | Central Luzon - remaining provinces | (phased SIA | 81% | ||||
| Calabarzon - selected province | due to | 82% | |||||
| Round 1 (cont, Aug 2020) | Calabarzon - remaining provinces | COVID-19) | 71% | ||||
| Round 2 (Sept 2020) | mOPV2 | Central Luzon - all provinces | <5 years | 2,532,010 | 85% | ||
| Calabarzon - all provinces | 80% | ||||||
| East Malaysia | |||||||
| cVDPVl | |||||||
| AFP case-patient with paralysis onset of 20 Oct 2019 | ES sample collected 13 Mar 2020 | Round 1 (Jan 2020) | bOPV | Sabah State | <13 years | 853,265 | 97% |
| Round 2 (Feb 2020) | bOPV | Sabah State | <13 years | 853,265 | 95% | ||
| Round 1 (Jun 2020) | bOPV | Labuan Federal Territory | <13 years | 32,131 | 78% | ||
| Round 2 (Aug 2020) | bOPV | Labuan Federal Territory | <13 years | 32,131 | 75% | ||
| cVDPV2 | |||||||
| ES sample collected on 19 Nov 2019 | ES sample collected 4 Feb 2020 | Round 1 (Jun 2020) | mOPV2 | Sabah State | <13 years | 853,265 | 94% |
| Round 2 (Aug 2020) | mOPV2 | Sabah State | <13 years | 853,265 | 92% | ||
Poliovirus Surveillance Strengthening.
Improvements in AFP surveillance were noted in the 12 months after the declaration of the outbreaks (October 2019-September 2020) compared to the 12 months prior (October 2018–September 2019) throughout the country although targets for performance indicators were not met. At the national level, the NPAFP rate increased from 1.83 per 100,000 children < 15 years of age prior to the outbreak to 3.21 after the outbreak and stool adequacy improved from 41% to 63%. Among the seven initial outbreak-affected regions, all reported increased NPAFP rates with four regions achieving the outbreak response zone target of ≥3. Six of the seven regions improved stool adequacy percentage by 12–48% but only one met the ≥80% target. In the remaining 10 regions, nine regions improved NPAFP rates and met the target of ≥2 per 100,000 children < 15 years of age. Stool adequacy percentage increased in seven of the ten regions by 7–67% and two regions achieved ≥80% target.
These improvements reflect prioritization and allocation of resources by the government with support from external partners. Surveillance strengthening activities undertaken were: 1) AFP surveillance guidelines updated and distributed; 2) large health facility staff and members of the Philippines Pediatric and Neurological Societies sensitized; 3) six-month retrospective medical record reviews conducted in high priority health facilities to identify unreported cases; 4) searches conducted in communities where children with cVDPV cases resided to identify unreported AFP cases; 5) workforce capacity increased by hiring 17 dedicated AFP surveillance officers; 6) surveillance refresher trainings held for all regions including vaccine-preventable disease (VPD) surveillance staff in the Epidemiology Bureau; 7) the National Expert Review Committee reconstituted with new members; and 8) Regional Expert Review Committees established to expedite AFP case classification.
The established ES system was expanded to supplement AFP surveillance during the outbreaks in detecting virus transmission across the country. After the initial detection of cVDPV1 in Manila, ES collection sites upstream were established to better pinpoint the area(s) of the city where infected individuals may have been located. ES expansion was fast-tracked with 34 new sites in 2019 and 23 additional sites in 2020. The accelerated expansion of ES in non-outbreak regions led to the detection of cVDPV2 in Cebu City, outside the original outbreak response areas. The National Polio Laboratory at the Research Institute for Tropical Medicine urgently implemented a surge capacity response plan, including staff recruitment, and equipment and supplies procurement, to cope with the almost 4,900 stool specimens and sewage samples collected during 2019–2020.
Essential Immunization.
Efforts to improve essential immunization were made in parallel with SIAs to improve bOPV and IPV coverage. A general advisory was made to accelerate essential immunizations, particularly IPV. DOH issued guidelines that recommended IPV administration to infants ≥ 14 weeks of age at any essential immunization visit and not only with the third bOPV dose. In addition, a two-phase, national measles-rubella (MR) and bOPV SIA began in October 2020 to improve population immunity against both VPDs.
Overall response.
The outbreak affected seven of 17 regions by the end of 2019 and 10 of 17 regions by the end of 2020. Substantial gains in increasing population immunity and enhancing poliovirus surveillance were slowed or halted due to the COVID-19 pandemic in March 2020. DOH overcame a number of these obstacles by shifting to virtual activities (e.g., AFP surveillance training, regular bi-monthly meetings of regional surveillance officers, and SIA planning), continued to operate essential AFP surveillance activities, and continued to collect ES samples in NCR. As of 26 February 2022, no breakthrough3 cVDPV1 or cVDPV2 have been detected among AFP cases or ES samples after completion of polio SIAs in September 2020. The last isolations detected were in ES samples collected 28 November 2019 and 16 January 2020, respectively. Nine distinct VDPV2 with 6–13 nt changes in the VP1 gene from Sabin virus (0.7%−1.4% divergence), classified as ambiguous VDPV2 (aVDPV2)4, were isolated after mOPV2 SIAs and no VDPV1 has been isolated since the bOPV SIAs (Table 1, Fig. 2). One aVDPV2 was isolated in Mindanao preceding the second Mindanao-wide mOPV2 campaign. Three aVDPV2 were detected in Pasay City from September-October 2020, several months after mOPV2 campaigns were completed in NCR. In Region 4A, five aVDPV2 were isolated (two ES sample, one AFP contact, two healthy children) between July-December 2020. No evidence of community transmission was identified. A response plan was developed in the event community transmission is confirmed.
3.5. Malaysia public health response
Upon outbreak confirmation in the Philippines, Malaysia conducted a nation-wide risk assessment and identified only Sabah state as high risk for circulation with 14 of 25 districts specifically classified as high-risk. Malaysia activated EOCs at district, state and national levels. Detailed epidemiological investigations of cases were conducted, including active case searches, AFP contact sampling, healthy children stool sampling, and rapid expansion of ES in all states particularly in Sabah.
Polio SIAs.
Two rounds of bOPV and mOPV2 were planned for Sabah state and Labuan Federal Territory (FT) (Table 2). MoH prioritized efforts to clearly communicate the complex SIA schedule to all communities to improve vaccine reach, including use of local languages and engaging with community networks, especially non-citizen groups. The first bOPV round started on 27 December 2019 in affected districts as a polio case response round, but later extended to become statewide on 27 January 2020. Initially the age group targeted was <5 years old but following a more detailed risk assessment, expanded to <13 years old due to the expected absence of intestinal mucosal immunity. The SIA work force consisted primarily of state-employed health care workers, with limited use of volunteers, mainly vaccinating at fixed posts, supplemented by some house-to-house and outreach. The bOPV response was stopped on 18 March due to COVID-19 related restrictions and resumed on 2 June 2020. The modalities of the SIAs were modified in the wake of COVID-19; SIA start dates were staggered by district, and appointment-based and drive-through vaccinations commenced.
High overall vaccination coverage (>95%) reported for the two bOPV rounds conducted pre-pandemic suggests that coverage was high enough to stop transmission in both citizens (95–96%) and non-citizens (93–98%) (Table 2). Likewise, the two bOPV rounds in Labuan FT reported high overall vaccination coverage (>90%) following review of target population numbers. The two mOPV2 campaigns conducted after the initial pause from March to June 2020 reached 94% and 92% coverage among all populations, respectively. There have been no breakthrough cases or detections as of 26 February 2022. The last detections were ES samples collected 13 March 2020 for cVDPV1 and 4 February 2020 for cVDPV2.
Poliovirus Surveillance Strengthening.
In an effort to increase the sensitivity of surveillance, the target NPAFP rate was raised to 3.0 per 100,000 children < 15 years old per year in Sabah and Labuan FT. The national NPAFP rate decreased from 2.48 per 100,000 children < 15 years of age in 2019 to 2.06 in 2020 (Fig. 1). The national stool adequacy percentage increased from 75% in 2019 to 83% in 2020. At the state level, the NPAFP rate and stool adequacy were above targets in 2020 in Sabah, 3.54 and 82%, and Labuan FT, 6.56 and 100%. The number of active ES sites increased from six to 62 across the country, including 39 sites in Sabah and six in Labuan FT. The increased number of AFP stool specimens and rapid increase in the number of ES sites necessitated careful coordination by the National Public Health Laboratory as three laboratories were involved in processing and testing stool and environmental samples.
Essential Immunization.
Essential immunization coverage for IPV in Malaysia is high, with national coverage for the primary series at 98% and over 95% reported for Sabah State [8]. However, a few districts in Sabah reported lower coverage, indicating pockets of under-immunized children; Semporna district reported <70% in 2016–2017. In 2019, nine out of the 22 districts in Sabah had coverage for the primary IPV series under 95%, with two of these reporting under 80%. The national schedule discontinued the OPV booster as of 2016; high IPV coverage will not protect against transmission of polioviruses where fecal-oral transmission predominates.
Overall response.
Ongoing advocacy with federal and state health authorities combined with release of 2.5 million doses of mOPV2 for reaching all children including the non-citizen communities were critical for the quality of the response. The COVID-19 pandemic hampered response activities, delaying the second bOPV SIA and both mOPV2 SIAs until June due to movement restrictions imposed to limit the spread of SARS-CoV-2. SIAs were continued until October 2020 with extended catch-up activities through January 2021.
3.6. Joint country response efforts
In light of known population movement between the Philippines to Malaysia and Indonesia, neighboring countries were alerted upon confirmation of the cVDPV outbreaks in the Philippines. Cross-border coordination calls were held in 2019–2020 to discuss overall response efforts including progress and scope of the SIAs and coordination of vaccination among populations moving between the countries, as well as enhancing sensitivity of polio surveillance along bordering areas. Laboratory testing and results from the two National Polio Laboratories, the Regional Reference Laboratory in Australia, and the Global Specialized Laboratories in Japan and US were coordinated and reported in a timely manner to WHO and National Programs in Malaysia and the Philippines. Synchronized SIAs were not conducted due to the difference in timing of virus detection between the countries and the need to quickly resume SIAs once COVID-19 population movement restrictions were lifted.
4. Discussion
These concurrent cVDPV outbreaks with well-known risk factors had several unique features and provide several generalizable lessons. This is the first identified outbreak of cVDPV in Malaysia and is the second confirmed cVDPV1 outbreak with international spread after the first identification and documentation in 2000–2001 in the Dominican Republic and Haiti [16]. Furthermore, the outbreaks of cVDPV1 and cVDPV2 in Malaysia are the only documented cVDPV outbreaks known to have occurred in a country with an IPV-only vaccination schedule. Most IPV-only countries are in the WHO European Region and Region of the Americas. In the WHO Regions of South East Asia and Western Pacific, only five countries have an IPV-only schedule (Australia, Japan, Malaysia, New Zealand, and South Korea) and Malaysia ranks lowest of the five on sanitation indices [17]. The limited role of IPV in inducing intestinal mucosal immunity to stop fecal-oral transmission of poliovirus combined with Sabah State’s proximity and regular population movements to/from surrounding OPV-using countries and areas with lower levels of sanitation may have made it particularly vulnerable to cVDPV emergence and transmission.
The IHR provide the overarching framework to reduce the risk of international spread of infectious diseases through international travel. While large vessels are well regulated, it is much harder to adequately regulate sea crossings of small commercial and family seacraft, such as the population movement across the Sulu Archipelago. However, these concurrent outbreaks suggest that oversight of such settings need to be considered by maritime nations in their planning to reduce the risk of poliovirus importation and spread of other outbreak-prone diseases. Population movement across Borneo Island also continued to be a concern jeopardizing poliovirus interruption. It is important for countries to engage in cross-border strategies, both land and sea, to ensure a thorough and comprehensive outbreak response that includes immunization of all population subgroups – citizens and non-citizens – to minimize the gaps in population immunity that allow ongoing transmission. Polio-affected countries should implement the IHR Emergency Committee’s temporary recommendations including polio vaccination (particularly for type 1 and 3 polioviruses) of international travelers before departure to the extent possible [18].
Co-circulation of cVDPV1 and cVDPV2 is indicative of long-standing low OPV coverage and therefore overall low population intestinal mucosal immunity to all poliovirus serotypes. The chronically under-vaccinated populations in the Philippines and subpopulations in Malaysia created vulnerability to the emergences and transmission of cVDPV. The outbreaks in the Philippines demonstrate that there is a critical need in all countries to ensure essential immunization programs are fully functioning and vaccines are well-stocked. Programs need to be sufficiently flexible to reach all communities including population subgroups that are mobile and/or geographically hard-to-reach. Likewise, attention needs to be focused on urban and peri-urban areas where migrant communities can be underserved; the reasons for being underserved include limited health facility utilization, language or other cultural barriers, and insufficient human and other resources in health centers and outreach services to serve population numbers larger than those registered. A subgroup of non-citizens, the stateless populations, pose an additional set of challenges that would benefit from a regional approach to ensure plans and resources for implementing and sustaining essential immunizations are secured. Vaccine hesitancy has and will continue to be a challenge in the Philippines but the >95% administrative vaccination coverage of most SIAs indicates that social mobilization and communication interventions are effective approaches to overcome this challenge. Efforts should be made to build upon these gains to improve vaccination coverage of other VPDs.
The cVDPV2 isolated in Malaysia was more closely related to the virus circulating in Manila than in the lineage circulating in Mindanao. While the country of emergence and direction of subsequent spread of each cVDPV remains unknown, these outbreaks highlight severe suboptimal poliovirus surveillance performance. Based on the poliovirus molecular clock (rate of 1.1% nt substitutions per year), genetic analyses estimated that the cVDPV1 seeding dose was in 2016 and >6 years of replication without detection for cVDPV2. It is likely that the cVDPV2 emerged from a subpopulation with poor tOPV coverage during 2012–2014. However, replication in a primary immunodeficient person at some point cannot be excluded as early isolates from the Philippines identified 13 amino acid substitutions, high compared to the number of nt substitutions [19]. No outbreaks caused by community circulation of iVDPV2 have been confirmed to date, yet the possibility exists. Surveillance of individuals diagnosed with primary immunodeficiency disease (PID) is recommended as a supplement to AFP surveillance in countries with a high prevalence of PID to ensure early detection of iVDPV and potential antiviral therapy [20].
Although cVDPV2 was predominantly detected in the outbreak-affected areas of Manila, southern Philippines, and Sabah State, Malaysia, it was also detected in two other regions in the Philippines. This highlights the need to strengthen and maintain surveillance nationwide in outbreak-affected countries, after the initial focused effort in outbreak-affected areas. Furthermore, most detections were through ES and not facility-based AFP surveillance, highlighting the need for quickly strengthening AFP surveillance. The value of ES as a supplement to AFP surveillance for poliovirus detection was amply demonstrated in both countries. Without ES, cVDPV2 would not have been detected in Malaysia and in Manila given the absence of clinical polio cases. ES may be crucial to implement in countries where maintaining sensitive AFP surveillance systems can be challenging, especially in WHO regions that have been polio-free for more than a decade, and as a supplement to AFP surveillance even where performance indicators are met.
The Global Polio Laboratory Network (GPLN) and its sequencing and molecular epidemiologic analyses capabilities were instrumental to understanding the epidemiology of the outbreak and informing outbreak response activities. Sequencing results and phylogenetic trees were necessary to link isolates from the countries and elucidate discreet population movement that guided response activities. Specificity of the outbreak was increased by clearly delineating ongoing virus transmission (cVDPV1/cVDPV2), new emergences (VDPV1/VDPV2), and unrelated detections (i.e., iVDPV2). GPLN will continue to play a critical role in the efforts to end poliovirus transmission.
There have been increasing numbers of cVDPV2 outbreaks since 2017; additionally, there have also been co-circulations of two serotypes identified since the switch: WPV1 and cVDPV2 in Pakistan and Afghanistan (2019 – ongoing) [21] and cVDPV2 and cVDPV3 in Somalia (2017 – ongoing) [22]. As illustrated in this outbreak, the operational challenges of responding to mixed serotype outbreaks with bOPV and mOPV2 are considerable. Anticipating the possibility of further such outbreaks, tOPV has now been re-introduced for mixed serotype co-circulation control, most recently used in Afghanistan and Pakistan, which considerably simplifies the immunization response by decreasing the overall duration, number, and operational costs of SIAs in comparison with separate SIAs with different OPV content.
Polio outbreak response activities were severely hampered by the COVID-19 pandemic, but it did not stop response activities due to the ingenuity and dedication of staff in both countries. Activities were re-imagined and timelines were altered to protect polio staff and volunteers as well as communities.
The concurrent outbreaks of cVDPV1 and cVDPV2 in the Philippines and Malaysia should serve as a caution to the global community on its quest to achieve a world free of polio due to WPV and VDPV. The risk of cVDPV outbreaks will persist as long as OPV is used; cessation of all OPV use is an essential step towards achieving a polio-free world. As an IPV-only country and with a highly vulnerable population, Malaysia highlights how easily the virus can circulate in populations with no intestinal mucosal immunity to any of the three poliovirus serotypes. Essential polio eradication activities will be vulnerable to competing public health priorities, especially in WPV-free regions and countries. Early virus detection will be key and the introduction of a comprehensive VPD surveillance system as outlined in the Immunization Agenda 2030 (IA2030) will help sustain efforts [23]. Timely response with an OPV that is more genetically stable than mOPV, such as the novel OPV (nOPV), will also be critical to reduce the risk of seeding new VDPV emergences. Presently, nOPV type 2 is used in SIAs under emergency use listing and nOPV types 1 and 3 are under development [24,25]. cVDPV outbreaks in the long standing WPV-free WHO regions of Europe, South- East Asia and the Western Pacific are a reminder that every country must stay vigilant if we are to successfully eradicate polio.
5. Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention and respective agencies.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Mayan Lumandas of the Department of Virology and all the staff of the National Polio Laboratory - Philippines; Dr Kouichi Kitamura and Dr Hiroyuki Shimizu of the Global Specialized Laboratory for enteroviruses at the National Institute of Infectious Diseases, Japan; staff of the WHO Polio Regional Reference Laboratory, Australia; Dr Ousmane Diop, Department of Polio Eradication, Detection and Interruption Unit, WHO; and Kelley Bullard and the Molecular Epidemiology Surveillance Laboratory team, Polio and Picornavirus Laboratory Branch, CDC for their tireless efforts to ensure accurate testing, communication, and interpretation of poliovirus laboratory results. The authors would also like to acknowledge the contributions of Dr Myrna C. Cabotaje and Dr Abdulla Jr Dumama, Under Secretaries for Health of the Department of Health of the Republic of the Philippines, and Dr Noor Hisham Abdullah, Director General of Health Malaysia, for ensuring implementation of SIAs and surveillance strengthening activities; and Mr Benjamin Bayutas, WHO’s Western Pacific Regional office for providing the tables and figures for the Philippines and Malaysia. The authors would like to extend our appreciation to all those who tirelessly led and supported the polio outbreak response efforts especially during the unprecedented COVID-19 pandemic. These include the public health staff and consultants, healthcare providers and healthcare workers, local leaders, volunteers, and non-government organizations in the Philippines and Malaysia.
Footnotes
CRediT authorship contribution statement
Cynthia J. Snider: Investigation, Writing – original draft, Writing – review & editing, Visualization. Liliane Boualam: Investigation, Writing – original draft, Writing – review & editing, Visualization. Graham Tallis: Investigation, Writing – original draft, Writing – review & editing, Visualization. Yoshihiro Takashima: Investigation, Writing – review & editing, Supervision. Rabindra Abeyasinghe: Investigation, Writing – review & editing, Supervision. Ying-Ru Lo: Investigation, Writing – review & editing, Supervision. Varja Grabovac: Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Tigran Avagyan: Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Syeda Kanwal Aslam: Investigation, Data curation, Writing – review & editing, Visualization. Abu Obeida Eltayeb: Investigation, Writing – review & editing. Khin Devi Aung: Investigation, Writing – review & editing. Xiaojun Wang: Investigation, Data curation, Writing – review & editing. Achyut Shrestha: Investigation, Data curation, Writing – review & editing. Carla Ante-Orozco: Investigation, Writing – review & editing. Maria Wilda T. Silva: Investigation, Data curation, Writing – review & editing, Supervision. Nemia Lapastora-Sucaldito: Investigation, Data curation, Writing – review & editing, Supervision. Lea Necitas G. Apostol: Investigation, Data curation, Writing – review & editing, Supervision. Muhammad Bin Hj. Jikal: Investigation, Data curation, Writing – review & editing, Supervision. Waheed Miraj: Investigation, Data curation, Writing – review & editing. Faisal Lodhi: Investigation, Data curation, Writing – review & editing. Hyung Joon Kim: Investigation, Writing – review & editing. Norhayati Rusli: Investigation, Data curation, Writing – review & editing, Supervision. Bruce R. Thorley: Investigation, Data curation, Writing – review & editing, Visualization. Matthew B. Kaye: Investigation, Data curation, Writing – review & editing, Visualization. Yorihiro Nishimura: Investigation, Data curation, Writing – review & editing, Visualization. Minetaro Arita: Investigation, Data curation, Writing – review & editing, Visualization. Jamiatul Aida Md. Sani: Investigation, Data curation, Writing – review & editing, Supervision. Christina Rundi: Investigation, Data curation, Writing – review & editing, Supervision. Keith Feldon: Investigation, Data curation, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.022.
Calculated among AFP cases <15 years old and defined as two stool specimens collected within 14 days of paralysis onset, ≥24 h apart, and arrived at a WHO-accredited laboratory in good condition (i.e., reverse cold chain maintained and no evidence of leakage or desiccation).
VDPVs that share a high degree of sequence identity in the VP1 region of the capsid (typically >99.5%) to another previously sequenced VDPV.
Interim definition of breakthrough transmission: Any WPV or cVDPV detected in an AFP case, healthy child or environmental sample with the date of onset (for AFP cases) or the date of sample collection (for the healthy child or environmental sample) >28 days after the first day of the last supplemental immunization activity (SIA) round in an area where at least two outbreak SIA rounds have been conducted.
A VDPV isolate from individuals or from environmental samples, without evidence of circulation and from individuals with no known immunodeficiency.
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization or UNICEF or Bill and Melinda Gates Foundation. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
References
- [1].Anis E, Kopel E, Singer SR, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill 2013;18(38). [DOI] [PubMed] [Google Scholar]
- [2].Arie S Polio virus spreads from Syria to Iraq. BMJ 2014;348(apr02 3):g2481. [DOI] [PubMed] [Google Scholar]
- [3].Mbaeyi C, Ryan MJ, Smith P, Mahamud A, Farag N, Haithami S, et al. Response to a Large Polio Outbreak in a Setting of Conflict - Middle East, 2013–2015. MMWR Morb Mortal Wkly Rep 2017;66(8):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hagan JE, Wassilak SG, Craig AS, et al. Progress toward polio eradication - worldwide, 2014–2015. MMWR Morb Mortal Wkly Rep 2015;64(19):527–31. [PMC free article] [PubMed] [Google Scholar]
- [5].Meeting of the Strategic Advisory Group of Experts on Immunization. November 2012 – conclusions and recommendations. Wkly Epidemiol Rec 2013;88(1):1–16. [PubMed] [Google Scholar]
- [6].Sutter R, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine-live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 7th ed. Philadelphia, PA: Elsevier, Inc.; 2018. p. 866–917. [Google Scholar]
- [7].Gurung S, Harris JB, Eltayeb AO, Hampton LM, Diorditsa S, Avagyan T, et al. Experience With Inactivated Polio Vaccine Introduction and the “Switch” From Trivalent to Bivalent Oral Polio Vaccine in the World Health Organization’s Western Pacific Region. J Infect Dis 2017;216(suppl_1):S101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].WHO. WHO/UNICEF estimates of national immunization coverage (WUENIC); 2020. https://www.who.int/teams/immunization-vaccines-and-biologicals/data-statistics-and-graphics2020. [Google Scholar]
- [9].Republic of the Philippines Department of Health. DOH identifies vaccine hesitancy as one of the reasons for measles outbreak; 2019. https://doh.gov.ph/node/16721 [accessed 1 June 2021]. [Google Scholar]
- [10].GPEI . Standard operating procedures: respond to a poliovirus event or outbreak; 2020. http://polioeradication.org/wp-content/uploads/2020/04/POL-SOP-V3.1-20200424.pdf3.1. [Google Scholar]
- [11].Republic of the Philippines Department of Health. Polio case confirmed in the Philippines: DOH to mount mass immunization campaign; 2019. https://doh.gov.ph/node/18012 [accessed June 1 2021]. [Google Scholar]
- [12].Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 2008;82 (9):4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].GPEI. Interim Guidance for the poliomyelitis (polio) surveillance network in the context of coronavirus disease (COVID-19); 2020. http://polioeradication.org/wp-content/uploads/2020/06/Interim-Guidance-Polio-Surveillance-in-the-context-of-COVID19-20200514.pdf [accessed 14 May 2020]. [Google Scholar]
- [14].GPEI. Call to action to support COVID-19 response; 2020. http://polioeradication.org/wp-content/uploads/2020/04/POB-COVID-19-Statement-20200402.pdf [accessed 03 April 2020]. [Google Scholar]
- [15].Bauri M, Wilkinson AL, Ropa B, Feldon K, Snider CJ, Anand A, et al. Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 1 and Outbreak Response - Papua New Guinea, 2018. MMWR Morb Mortal Wkly Rep 2019;68(5):119–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Zacarıás Garib, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 2002;296(5566):356–9. [DOI] [PubMed] [Google Scholar]
- [17].2020 Environmental Performance Index – Sanitation & Drinking Water; 2021. https://epi.yale.edu/epi-results/2020/component/h2o2021. [Google Scholar]
- [18].WHO. Statement of the Twenty-Third IHR Emergency Committee Regarding the International Spread of Poliovirus; 2020. https://www.who.int/news/item/07-01-2020-statement-o-the-twenty-third-ihr-emergency-committee-regarding-the-international-spread-of-poliovirus [accessed March 25 2021]. [Google Scholar]
- [19].Zhao K, Jorba J, Shaw J, Iber J, Chen Qi, Bullard K, et al. Are Circulating Type 2 Vaccine-derived Polioviruses (VDPVs) Genetically Distinguishable from Immunodeficiency-associated VDPVs? Comput Struct Biotechnol J 2017;15:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].GPEI. Guidelines for Implementing Poliovirus Surveillance among Patients with Primary Immunodeficiency Disorders (PIDs); 2020. https://polioeradication.org/wp-content/uploads/2020/12/Guidelines-for-Implementing-PID-Suveillance-3.3-20201215.pdf [accessed 3 June 2021]. [Google Scholar]
- [21].Hsu CH, Kader M, Mahamud A, Bullard K, Jorba J, Agbor J, et al. Progress Toward Poliomyelitis Eradication – Pakistan, January 2018-September 2019. MMWR Morb Mortal Wkly Rep 2019;68(45):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mbaeyi C, Alleman MM, Ehrhardt D, Wiesen E, Burns CC, Liu H, et al. Update on vaccine-derived poliovirus outbreaks – democratic republic of the Congo and Horn of Africa, 2017–2018. MMWR Morb Mortal Wkly Rep 2019;68 (9):225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Agenda Immunization 2030 – a global strategy to leave no one behind; 2020. http://www.immunizationagenda2030.org/. [DOI] [PubMed] [Google Scholar]
- [24].GPEI. nOPV2; 2021. https://polioeradication.org/nopv2/ [accessed 12 December 2021]. [Google Scholar]
- [25].Van Damme P, De Coster I, Bandyopadhyay AS, Revets H, Withanage K, De Smedt P, et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 2019;394(10193):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.