Abstract
According to manufacturers, inactivated poliovirus vaccines (IPVs) are freeze sensitive and require storage between 2°C and 8°C, whereas oral poliovirus vaccine requires storage at −20 °C. Introducing IPV into ongoing immunization services might result in accidental exposure to freezing temperatures and potential loss of vaccine potency. To better understand the effect of freezing IPVs, samples of single-dose vaccine vials from Statens Serum Institut (VeroPol) and multi-dose vaccine vials from Sanofi Pasteur (IPOL) were exposed to freezing temperatures mimicking what a vaccine vial might encounter in the field. D-antigen content was measured to determine the in vitro potency by ELISA. Immunogenicity testing was conducted for a subset of exposed IPVs using the rat model. Freezing VeroPol had no detectable effect on in vitro potency (D-antigen content) in all exposures tested. Freezing of the IPOL vaccine for 7 days at −20 °C showed statistically significant decreases in D-antigen content by ELISA in poliovirus type 1 (p < 0.0001) and type 3 (p = 0.048). Reduction of poliovirus type 2 potency also approached significance (p = 0.062). The observed loss in D-antigen content did not affect immunogenicity in the rat model. Further work is required to determine the significance of the loss observed and the implications for vaccine handling policies and practices.
Keywords: Oral poliovirus vaccine, Freeze damage, Antigenicity, Vaccine cold chain, Polio eradication, D-antigen
1. Introduction
As part of the polio endgame strategy, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization recommended phased cessation of oral poliovirus vaccine (OPV) use, starting with a switch from trivalent OPV (tOPV) to bivalent OPV (bOPV) types 1 and 3 only. The switch from tOPV to bOPV occurred in April 2016 and all 155 OPV-using countries ceased use of tOPV by May 2016 [1,2]. Additionally, at least one dose of trivalent inactivated poliovirus vaccine (IPV) was recommended to prevent a rapid rise in type 2 susceptibility in birth cohorts born after the switch. As of August 31, 2016, 89% of WHO Member States included at least one dose of IPV in their immunization schedules [1]. The annual global demand for IPV could increase to 190–245 million doses for a 2 full dose-routine immunization schedule in a post-eradication era; however, existing IPV production capacity cannot cover the global need and some OPV-using countries have had to delay IPV introduction to late 2017 [3]. In the post-eradication era, each country will determine its own immunization strategy against the eradicated poliomyelitis disease, which will affect the global demand for IPV.
Increase of vaccine availability could be achieved by reducing wastage during storage and transportation, for instance by improving cold chain management. Vaccines are often inadvertently exposed to freezing temperatures during storage or transport to peripheral vaccination sites, resulting in vaccine spoilage or delivery of sub-potent vaccine [3–5]. This is concerning because there are several circumstances that could lead to accidental freezing of IPV: (1) Expanded Programme on Immunization staff familiar with OPV storage requirements might mistakenly place IPV in freezers; (2) for IPV transport in vaccine carriers, cold packs might be frozen instead of properly conditioned, leading to freezing temperatures within the carrier; and (3) the revised WHO-recommended policy for retaining opened multi-dose IPV vials (up to 28 days) could lead to inadvertent freeze-thaw cycles as the vials that are repeatedly taken to vaccination sessions until all doses in a vial have been administered might increase the risk of circumstance 1 or 2 occurring [6].
Freezing appears to alter the potency of IPV in combined diphtheria-tetanus-pertussis (DTP) vaccine/IPV products, mainly due to the adjuvanted formulation of DTP/IPV, but data on potency and immunogenicity of standalone IPVs exposed to freezing conditions are limited [7]. Data on DTP vaccine, which is also freeze sensitive, indicate varying loss in potency among various components after freezing and freeze-thaw cycles [8,9]. There is also evidence of diminished immunogenicity to pertussis components in DTP after storage at −3 °C [10]. Manufacturers recommend discarding any vial of IPV that has been or is suspected to have been frozen, but detecting vials exposed to freezing conditions is difficult in low-resource settings where electronic temperature data loggers or functioning thermometers are frequently unavailable. The “shake test” cannot be used with IPV [1,11]. In contrast, reference IPV preparations used for determining the D-antigen content, which present a higher D-antigen titer than commercialized vaccine, are labeled for storage at −70 °C [12,13], indicating IPV preparations at higher concentrations are stable when stored at −70 °C. With the global introduction and use of IPV, inadvertent freezing and freeze-thaw cycles are likely to occur. This might lead to high wastage of effective vaccines or a reduction in IPV effectiveness, depending on the effect of freezing. Therefore, more data on the effects of freezing on IPV potency and immunogenicity are needed.
No systematic study has been previously performed to investigate IPV inactivation by freezing, prompting us to initiate this work. This report describes a laboratory-based proof-of-concept study in which field scenarios were replicated to assess the effects of freezing temperatures on IPV potency and immunogenicity. In an in vitro study, IPV vials were exposed to simulated field conditions, then alterations in IPV potency were assessed by D-antigen ELISA [14]. In an in vivo study, a subset of exposed IPVs were used to immunize rats, and antibodies to poliovirus types 1, 2, and 3 were measured by the gold-standard polio microneutralization assay [15,16].
2. Materials and methods
Preliminary freezing experiments were conducted with IPOL IPV (lot #K1329–1; expiration June 18, 2016). Vaccine vials were untreated (storage at 2 °C–8 °C) or frozen at −20 °C overnight or for two weeks. In addition, the effect of freeze-thaw was tested after freezing at −20 °C and thawing one, three, or five times. D-antigen content was measured by an ELISA specific for each serotype performed by the US Food and Drug Administration (FDA) [14]. Samples were tested in duplicate with repeats on different days (total of 4–16 measurements per point).
2.1. Vaccine procurement
Two IPVs were selected to represent vaccines that supply the global market. Single-dose vaccine vials, VeroPol (lot #199; no listed expiration date because the vaccine was not intended for release to the market but met potency as required for markets), were provided by Statens Serum Institut (100 vaccine vials). Additionally, multi-dose vaccine vials manufactured by Sanofi Pasteur, IPOL (lot #M14531 M; expiration September 8, 2018), were purchased (48 vaccine vials). Attempts were made to secure additional IPV supplies, but this was not possible because of the intense global demand for IPV associated with the switch from tOPV to bOPV.
2.2. Freezing point determination
Before conducting in vitro and in vivo studies, the freezing point was measured for each vaccine by using differential scanning calorimetry (DSC). Briefly, 10 μl of vaccine was added to a cleaned DSC sample cell, and the cell was placed in the μDSC7 evo (SETARAM Instrumentation, Caluire, France) for analysis, with an empty DSC sample cell as a control. The sample cell was held at 10 °C for 10 min, ramped at 1 °C/min to −30 °C, held for 10 min, and then ramped at 1 °C/min to 10 °C. The freezing point onset was recorded from the ramp to −30 °C, and the onset melting temperature was recorded from the ramp to 10 °C. Three replicates were tested from three vials of each vaccine.
2.3. In vitro study
Four different treatments were designed to mimic freezing conditions that an IPV vial may encounter in the field. The treatments represented two different distribution steps in which freezing may occur: vaccine storage (scenarios 1 and 2) and vaccine transport to a field site (scenarios 3 and 4). A total of 15 vials of each vaccine were used for each treatment evaluated. An additional 15 vaccine vials from each manufacturer were held at 2 °C–8 °C as untreated controls.
2.3.1. Freezing during storage
In scenario 1, IPV vials were placed in a freezer at −20 °C for 24 h. Vaccine vials were briefly removed from the freezer at 8 h, visually inspected for freezing, tapped on the bench to induce freezing if not frozen due to supercooling, and then returned to the freezer. Supercooling describes the process in which a liquid can remain in a fluid state at less than the freezing temperature without becoming a solid. Introducing energy by tapping the vaccine vial will induce freezing and solid crystal formation. The vaccine was inspected again after the 24-h period and held in a refrigerator at 2 °C–8 °C prior to testing by D-antigen ELISA (approximately 2 weeks). In scenario 2, IPV vials were placed in the freezer at −20 °C for 7 days. Vaccine was briefly removed from the freezer at 3 days, visually inspected for freezing, tapped on the bench to induce freezing if not frozen, then returned to the freezer. The vaccine was inspected again after the 7-day period and held in a refrigerator at 2 °C to 8°Cprior to D-antigen ELISA testing (approximately 2 weeks) and in vivo immunogenicity testing.
2.3.2. Freezing during transport
To properly condition frozen ice packs prior to use, health workers must remove them from the freezer and allow them to thaw until a mixture of water and ice is present. In scenario 3, we studied improperly conditioned ice packs that were frozen at −20 °C for 24 h and then placed in a vaccine carrier (WHO-approved AVC-46, AOV International LLP, Noida, Uttar Pradesh, India) without any thawing or conditioning. Vials were placed in direct contact with the frozen ice pack, the carrier was closed, and held in an environmental chamber (EPX-3H, Espec North America, Hudsonville, Michigan) at 10 °C for 8 h. Holding vaccine carriers at 10 °C was selected to test a worst case scenario and to model the use of vaccine in cold climates. WHO Performance, Quality and Safety performance specifications for vaccine carriers were referenced as well [17]. After 8 h, the vaccines were removed, visually inspected for evidence of freezing, and stored in a refrigerator at 2 °C–8 °C prior to D-antigen ELISA testing (approximately 2 weeks). In scenario 4, vials were placed in a vaccine carrier with improperly conditioned ice packs multiple times (multiple freeze-thaw cycles), simulating real-world scenarios in which vials are transported by carrier and returned to refrigerators multiple times [18]. Ice packs were frozen at −20 °C for 24 h and then placed in a vaccine carrier. Vials were placed in direct contact with a frozen ice pack, the carrier was closed, and the carrier was kept in an environmental chamber at 10 °C for 8 h. After 8 h, the frozen ice packs were removed, the vials were visually inspected for evidence of freezing, and refrigerated ice packs were added to the carrier and then stored at 2 °C–8 °C for 16 h. This cycle was repeated three times on three consecutive days. Vaccines were held at 2 °C–8 °C prior to D-antigen ELISA testing (approximately 2 weeks) and in vivo immunogenicity testing.
Due to limitations in vaccine availability, IPOL was subjected to only the two treatments selected for the in vivo portion of the study (scenario 2 and scenario 4). Vaccine freezing was monitored using both temperature loggers (TempTale, Sensitech United Technologies, Beverly, Massachusetts) and thermocouples attached to each vaccine vial. Type T thermocouples (OMEGA Engineering, Stamford, Connecticut) were attached using cryolabels (Diversified Biotech, Dedham, Massachusetts) on the outside of the vials to accurately measure the temperature. Thermocouples were connected to a NI-DAQ controller (National Instruments, Austin, Texas), and data were collected with National Instruments' LabVIEW software. The impact of freezing on IPV potency was measured by D-antigen ELISA, and immunogenicity was evaluated in an animal model.
The D-antigen ELISA was developed, with assistance from Dr. Konstantin Chumakov (FDA Center for Biologics Evaluation and Research, Silver Spring, MD), to detect the D-antigen content of IPV types 1, 2, and 3. Polyclonal rabbit serotype-specific immunoglobulin (IgG) (produced by Spring Valley Laboratories for PATH) was evaluated for use in a D-antigen ELISA using three approaches: block ELISA, IPV neutralization, and monovalent IPV binding ELISA by PATH, USFDA, and USCDC. In addition to the D-antigen specificity testing of polyclonal rabbit IgG with trivalent vaccine shown in Fig. 1, additional testing was conducted by heat-treating monovalent IPV1, IPV2 and IPV3 for 1 h at 56 °C. Once identified serotype-specific polyclonal rabbit IgG was used to capture D-antigen to the ELISA plate by coating overnight at 4 °C in 0.05 M carbonate-bicarbonate buffer (Sigma-Aldrich, St. Louis, MO; cat #C3041–110CAP). Plates were washed three times with Dulbecco's phosphate-buffered saline (DPBS) with 0.05% Tween 20 (Fisher Scientific, Waltham, MA; cat #SH30028.03 and cat #BP337–500) and blocked with DPBS and 1% bovine serum albumin fraction V (Sigma-Aldrich; cat #3117332001) for 1 h at room temperature (RT). Test samples and the European Directorate for the Quality of Medicines & HealthCare (EDQM) polio vaccine standard1 were diluted in DPBS with 1% bovine serum albumin and 0.05% Tween 20 (assay buffer) to the approximate starting concentration of 15–17 DU/ml (values listed are for the type 1 component; vaccines were mixed at a ratio of 40/8/32 D-antigen units for types 1, 2, and 3, respectively). Test samples and standards were diluted from this initial dilution six times for a total of seven dilutions tested per sample. Plates were incubated at RT for 2 h. They were washed five times, and bound antigen was detected using poliovirus specific biotin-conjugated rabbit IgG diluted in assay buffer incubated for 2 h at RT. ExtrAvidin-Peroxidase (Sigma-Aldrich; cat #E2886) was used to detect biotinylated antibodies by diluting 1:10,000 in assay buffer and incubating for 1 h at RT. Tetramethylbenzidine substrate (KPL, Milford, MA; cat #50-76-00) was added, and plates were incubated in the dark for 30 min before being read at 450 nm using a SpectraMax M2 plate reader (Molecular Devices, Sunnyvale, CA). The final results and test validity were determined by statistical analysis of dose response curves using a four-parameter logistic fit in Molecular Devices' SoftMax Pro and parallel line analysis.
Fig. 1.
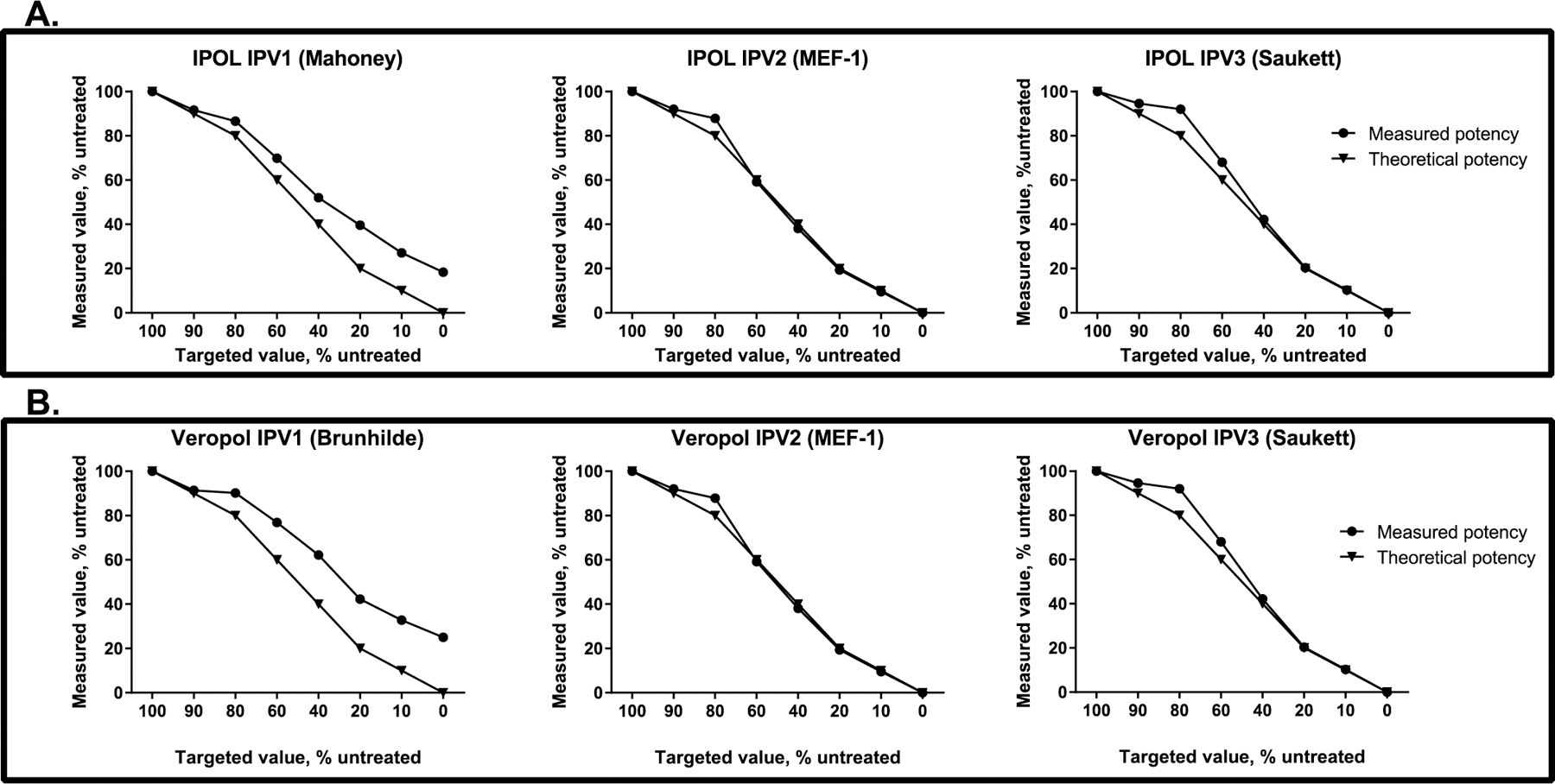
D-antigen ELISA selectivity. IPOL (panel A) and VeroPol (panel B) were treated by heating at 56 °C for 1 h to convert D-antigen to H-antigen, mixed with untreated vaccine at different ratios, and tested by ELISA. Plots depict the theoretical and the measured D-antigen content of IPV 1, IPV 2, and IPV 3 demonstrate D-antigen selectivity for each vaccine studied.
The assay reagents and procedures were specific to each IPV serotype (minimum sensitivity of 8 D-antigen units/ml for type 1, 1.6 D-antigen units/ml for type 2, and 6.4 D-antigen units/ml for type 3) and were not cross-reactive with other serotypes (data not shown). High reactivity was observed with the D-antigen relative to the H-antigen. The assay was reproducible between users and in agreement with an existing manufacturer's IPV potency assays.
The D-antigen ELISA was adapted to measure the change in potency for each vaccine by completing testing for parallelism to the EDQM standard, titration range determination, assay variability, and sensitivity to D-antigen damage. Both vaccines produced parallel titration curves with the EDQM standard over the range of 0.25–16 DU/ml (values listed are for poliovirus type 1). Four vials of VeroPol and three vials of IPOL were tested on three consecutive days by the same operator to determine the variability of the D-antigen ELISA. The assay coefficient of variation was less than 10% for each vaccine. To ensure the D-antigen ELISA was sensitive enough to detect damage to IPV from each manufacturer, an aliquot of each vaccine was treated to convert D-antigen to H-antigen by heating at 56 °C for 1 h. Mixing studies were done with untreated (full D-antigen) and treated (full H-antigen) vaccines. Untreated vaccine (D) and heat-treated vaccine (H) were mixed at different ratios—i.e., 100:0, 90:10, 80:20, 60:40, 40:60, 20:80, 10:90, 0:100 (D:H)—and then tested by ELISA.
2.4. In vivo studies
Vaccine from two of the freezing treatments (scenarios 2 and 4) as well as untreated controls for both vaccines were used in the in vivo immunogenicity study. Pooled vaccine samples from 15 vials (0.25 ml from each vial) from the same manufacturer were aliquoted and used for the prime and booster immunization of animals. Briefly, each group consisted of 15 naïve 8- to 10-week-old female Wistar rats that each received two intramuscular doses of 0.1 ml of vaccine (one-fifth of a human dose), on days 1 and 29. Serum samples were collected 14 days after vaccination, on days 15 and 43.
Serum samples from day 43 were tested in the microneutralization antibody assay using a previously published method [19]. Because IPV contains different strains of poliovirus type 1 (Mahoney for IPOL and Brünhilde for VeroPol), assays for poliovirus type 1 were conducted with both the Mahoney and Brünhilde strains. Data shown are from the homologous poliovirus type 1 strain for each vaccine. To ensure the endpoint titer was achieved, neutralization assays were performed over an extended dilution range of 1:8 to 1:262,144.
2.5. Statistical analysis
For the D-antigen ELISA and the microneutralization results, an overall analysis of variance (ANOVA) of the treatment groups was performed to determine whether any of the groups differed from the others. The significant ANOVAs were followed with pairwise comparisons of all the groups. For these comparisons, adjusted p-values using Tukey's procedure were used. These are adjusted for doing multiple comparisons, which increases the likelihood of getting a spurious significant difference. Analyses were performed using R statistical software version 3.2.4. The pairwise comparisons were done using the R package multcomp.
3. Results
Before initiating the large-scale study, we conducted a pilot experiment in which vials of IPOL (Sanofi Pasteur) were subjected to five cycles of freeze-thawing, followed by determining vaccine potency using D-antigen ELISA. The results shown in Table 1 demonstrate that there was a small decline in potency after the first freeze-thaw cycle, specifically for poliovirus type 1, and potency remained relatively stable after two freeze thaw cycles. This encouraging result led to a more detailed study that started by determining some physical characteristics of IPV from two sources, Sanofi Pasteur and Statens Serum Institut. Table 2 shows the average freezing point and melting temperature for each vaccine. Freezing point is a more variable measurement due to the effects of supercooling but might be closer to what is encountered in the field. The melting point determines the temperature at which a vaccine is susceptible to freezing.
Table 1.
Preliminary experiments on the effect of freezing on IPOL D-antigen content.
| Treatment | Average D-antigen content Percentage of untreated (95% confidence interval) |
||
|---|---|---|---|
| IPV 1 | IPV 2 | IPV 3 | |
| Untreated (2–8 °C) | 100% | 100% | 100% |
| 1 freeze-thaw cycle (−20 °C) | 86% (82–90%) | 94% (90–98%) | 97% (95–99%) |
| 3 freeze-thaw cycles (−20 °C) | 70% (56–84%) | 89% (85–93%) | 89% (81–97%) |
| 5 freeze-thaw cycles (−20 °C) | 72% (58–86%) | 89% (85–93%) | 93% (81–105%) |
| 2 weeks storage (−20 °C) | 71% (61–81%) | 87% (73–101%) | 91% (83–99%) |
Table 2.
Freezing and melting point measurements by differential scanning calorimetry.
| Freezing point |
Melting point |
|||
|---|---|---|---|---|
| VeroPol | IPOL | VeroPol | IPOL | |
| Average | −16.2 | −17.7 | −2.4 | −3.0 |
| % CVa | 10.1 | 14.8 | 4.0 | 3.1 |
CV, coefficient of variation.
Prior to using the ELISA in this study, the assay variability and sensitivity to damage were determined for each of the vaccines tested. The coefficient of variation was less than 10% for each of the vaccines, for all three types tested (Table 2). In addition, the ability of the ELISA to detect damage to the D-antigen in both vaccines was measured (Fig. 1). In general, the ELISAs were able to detect damage to the D-antigen and quantify the D-antigens at all the ratios tested, with similar results for both vaccines. However, the selectivity for type 1 was lower than that for types 2 and 3, suggesting either cross-reactivity with the H-antigen or that type 1 D-antigen was not completely converted to H-antigen during treatment. Thus, loss detected for type 1 may be underestimated by this ELISA for each of the vaccines studied.
3.1. Freeze treatment scenarios
In scenario 1 (1 day at −20 °C), 14 of 15 vials of VeroPol were frozen after 8 h, but one required tapping to complete the freezing process because of supercooling (data not shown). In scenario 2 (7 days at −20 °C), all 15 vials of IPOL and 7 of 15 vials of VeroPol were frozen after 24 h (Fig. 2). After 72 h, 5 of the remaining 8 vials of VeroPol required tapping to induce freezing. The lowest temperature reached by the vials in scenario 2 was −21 °C. In scenarios 3 and 4, none of the vaccine vials froze. The lowest temperature reached by vaccine vials in scenario 4 was −11 °C (Fig. 2). There was no observable difference in the assays between samples that froze and those that underwent supercooling.
Fig. 2.
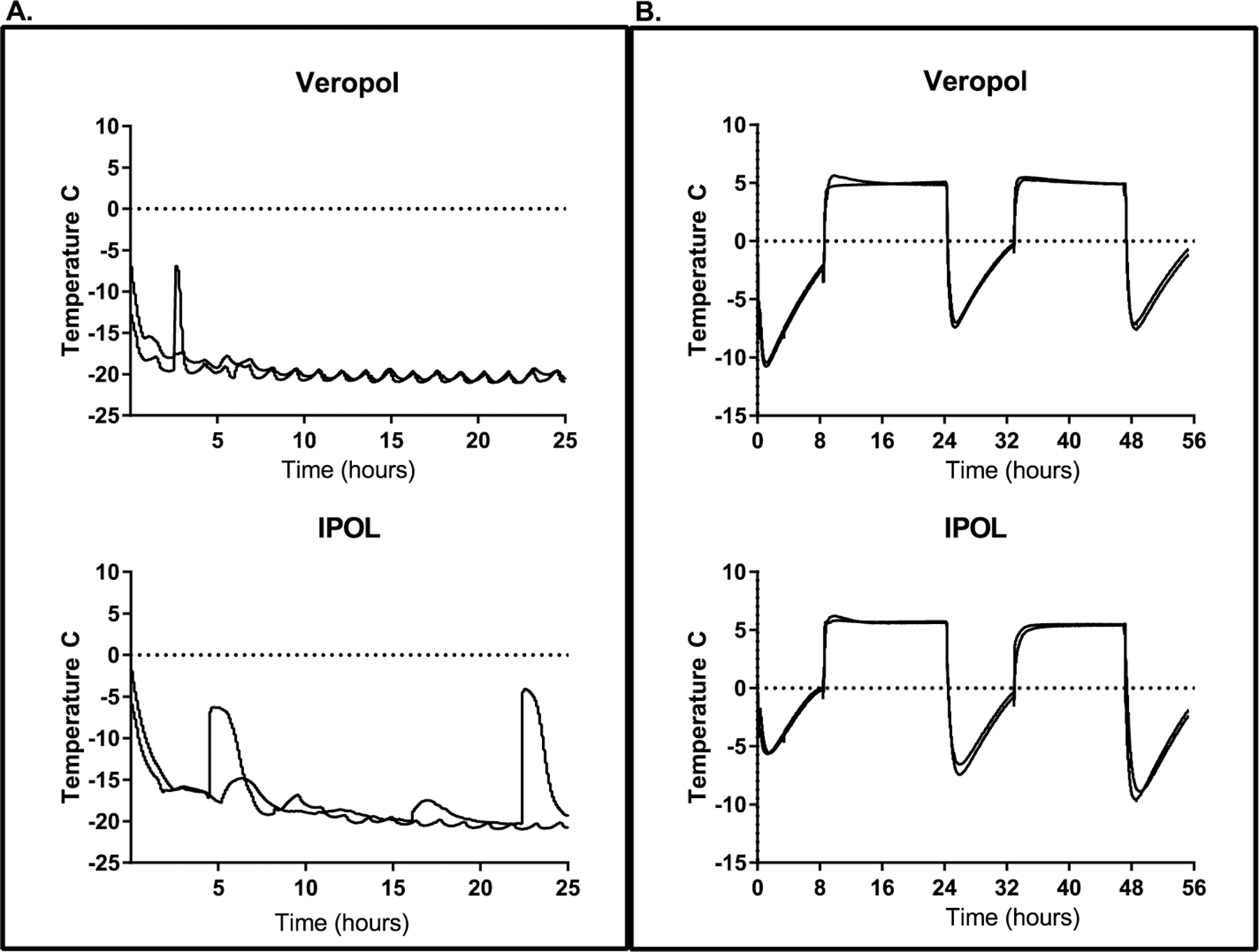
Representative freeze profiles from two vaccine vials from each manufacturer. Panel A: Representative graphs for vaccine vial temperatures during the first 24 h of storage freezing conditions (scenario 2). Panel B: Representative graphs for vaccine vial temperatures during transport conditions (scenario 4).
3.2. In vitro vaccine potency
The effects of the freezing treatments on vaccine in vitro potency were evaluated using the D-antigen ELISA. Although no statistically significant differences were found in vaccine D-antigen content after scenario 1 (−20 °C for 24 h), scenario 2 (7 days at −20 °C) led to significant decreases in D-antigen for types 1 (p < 0.0001) and 3 (p = 0.048) of the IPOL vaccine, with type 2 approaching significance (p = 0.062). No statistically significant differences were observed for VeroPol D-antigen potency after 7 days at −20 °C (Fig. 3) and no statistically significant differences were found with treatment scenarios 3 or 4 for either vaccine (Fig. 4).
Fig. 3.
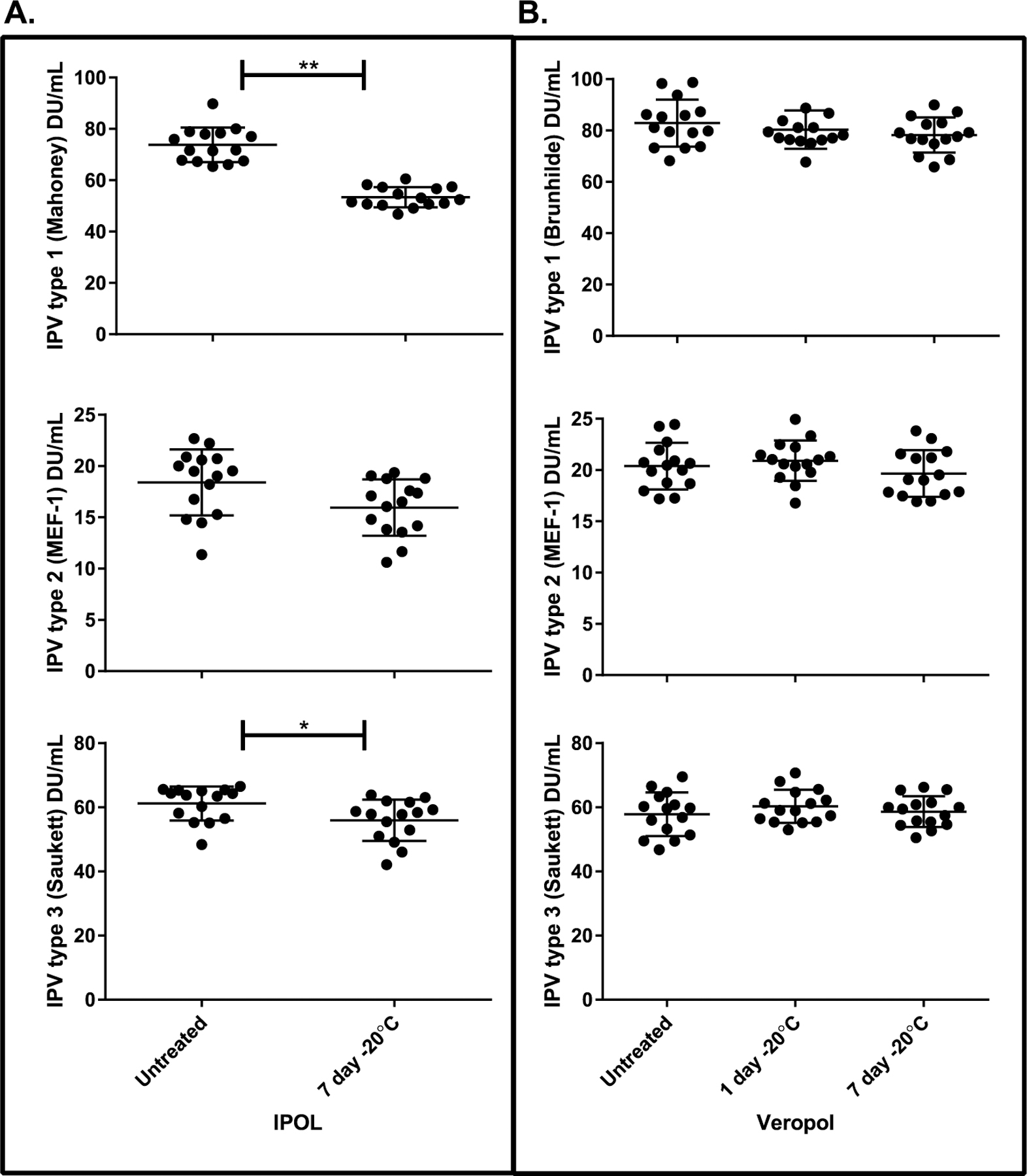
D-antigen ELISA results of storage freezing treatments. IPOL (panel A) and VeroPol (panel B) were subjected to freezing treatments mimicking vaccine storage (1 day at −20 °C [scenario 1] and 7 days at −20 °C [scenario 2]) and tested by ELISA, with untreated vaccine included as a control. Error bars indicate the standard deviation. Statistically significant differences found by ANOVA and calculated p-values based on multiple comparisons analysis are indicated for IPOL in panel A: **p < 0.0001 and * p = 0.048.
Fig. 4.

D-antigen ELISA results of transport freezing treatments. IPOL (panel A) and VeroPol (panel B) were subjected to freezing treatments mimicking vaccine transport (1 freeze-thaw cycle [scenario 3] or 3 freeze-thaw cycles [scenario 4] and tested by ELISA with untreated vaccine included as a control. Error bars indicate the standard deviation.
3.3. In vivo vaccine potency
The effects of scenarios 2 and 4 on immunogenicity were studied in the Wistar rat model and assessed by microneutralization assays. For all three serotypes, no statistically significant differences in immunogenicity were found for either scenario 2 (storage) (Fig. 5) or scenario 4 (transport) (Fig. 6) freeze treatments in comparison with the untreated vaccine vials.
Fig. 5.

Neutralizing antibody titers against IPV. Microneutralization results for storage freeze treatments, scenario 2, from day 43 rat sera are shown for IPOL (A) and VeroPol (B).
Fig. 6.
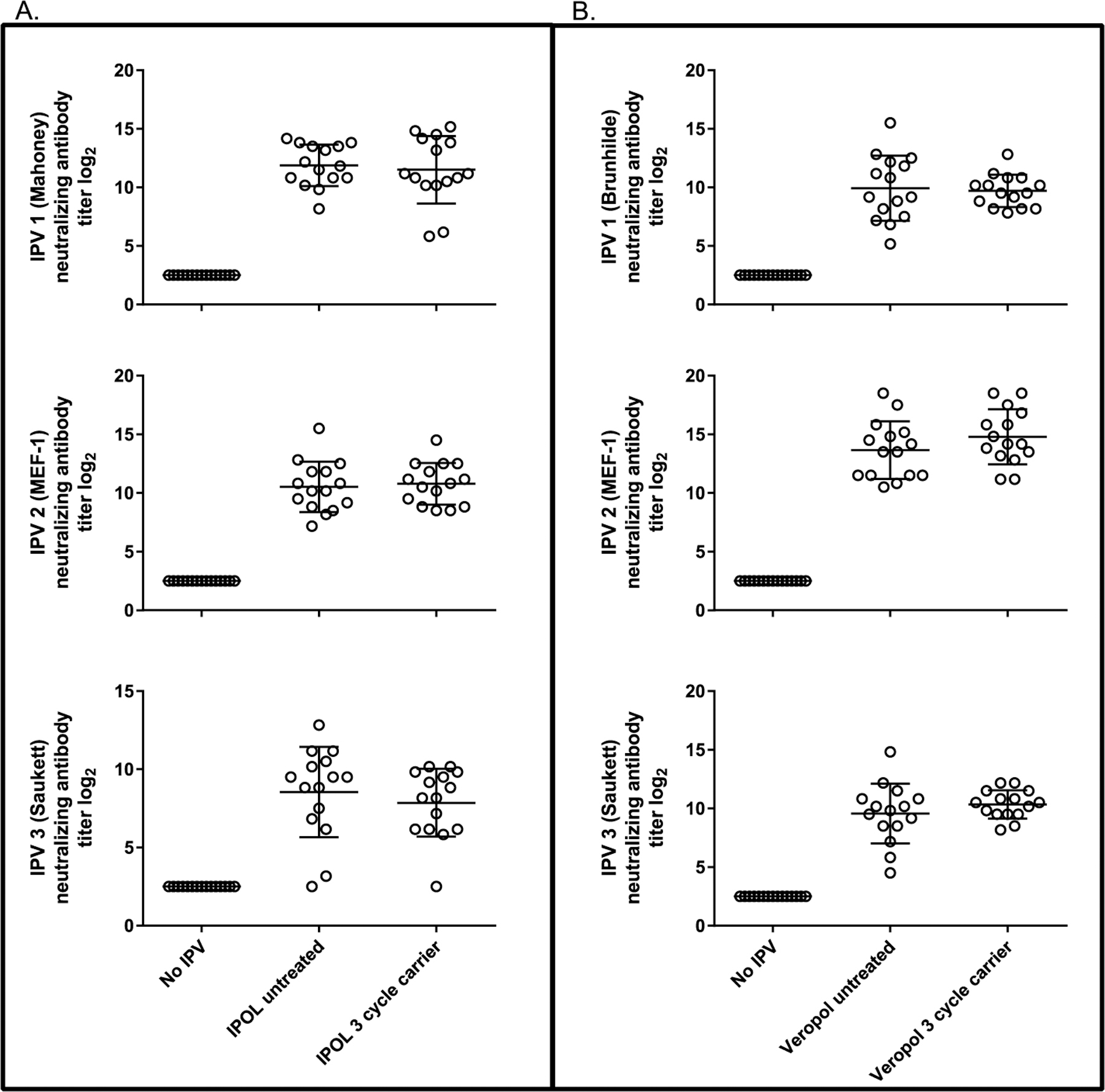
Neutralizing antibody titers against IPV. Microneutralization results for transport freeze treatments, scenario 4, from day 43 rat sera are shown for IPOL (A) and VeroPol (B).
4. Discussion
In this study, two IPVs prequalified by WHO were tested for their freeze sensitivity during field-simulated storage and transport conditions. Freezing VeroPol had no detectable effect on in vitro potency as measured by D-antigen ELISA in all the exposures tested, for all three types. However, freezing IPOL vaccine for 7 days at −20 °C led to statistically significant decreases in D-antigen content in IPV types 1 and 3 compared with untreated vaccine, and reduction of IPV type 2 D-antigen potency approached significance. Immunogenicity testing showed no significant difference in neutralization titer against freeze-treated vaccines for either vaccine. However as discussed in more detail below, the limited vaccine available prevented us from testing a dose response curve in animals which significantly limits the in vivo potency study sensitivity.
Differences were observed in the freeze sensitivity of the two vaccines after 7 days at −20 °C. The potential difference in freeze sensitivity could be due to several factors; e.g., the vaccine presentation size (single-dose versus multi-dose), the production process, the presence of 2-phenoxyethanol as a preservative in IPOL, or the difference in the serotype 1 strain included in the vaccine formulation (Mahoney for IPOL versus Brünhilde for VeroPol). An additional factor that potentially contributed to this difference is the time required for a vaccine to freeze. Even though IPOL constituted a larger volume, all 15 vials were frozen after 24 h at −20 °C, whereas a portion of the VeroPol vials took up to 72 h to freeze. In addition, the freezing curves for the IPOL vaccines showed a broader temperature peak, indicating this vaccine maintained a partially frozen state for a longer period of time before freezing. This difference in freezing time could be due to excipients included in the vaccine formulation. Examples of the differences in freezing curves for both vaccines are shown in Fig. 2A. These differences highlight the fact that these findings are specific to the two types of vaccine tested and cannot be extrapolated to other IPV manufacturers or presentations.
Discrepant results between the in vitro D-antigen ELISA potency measurements and the in vivo rat immunogenicity study make it difficult to determine the implications of the measured loss of D-antigen content. This study was intended as an initial proof of concept to provide vaccine manufacturers with motivation to include freeze sensitivity testing in their product development work, which could eventually lead to changes in labeled product storage conditions. As proof-of-concept work, the animal study was smaller than the WHO-outlined method for IPV validation and did not include immunizations with seral dilutions of vaccine or reference material. Animal studies only included testing at one vaccine dose, which is not the recommended method for in vivo poliovirus potency and may limit our ability to see differences in immunogenicity. In addition, in vitro testing with IPOL was limited to two of the four testing scenarios. These limitations might have affected our ability to see differences in D-antigen content reflected in immunogenicity. In addition, to better characterize in vivo studies which are inherently more variable, including test samples with deliberately denatured D-antigen would demonstrate how a loss in neutralization titer (in vivo) correlated to a loss in D-antigen content (in vitro). Further work is required with individual standalone IPV to determine whether a change in the labeled vaccine storage condition is warranted. Studies are needed to understand if the IPOL D-antigen loss observed by ELISA occurs as a result of freezing and is apparent after 1 day at −20 °C or is a factor of the amount of time the vaccine is frozen. In addition, as this work was completed with vaccine at the beginning of its shelf life different results may be obtained with IPV tested at the end of shelf its life. In conclusion, the results of this work suggests that if individual vaccine manufacturers conducted complete IPV in vivo potency validation studies, this might lead to validation that some IPV products are freeze stable resulting in revisions to the product labeling allowing storage and transportation under a broader range of conditions.
Several of the approaches being pursued to stretch the very limited IPV supply are changing the labeling of IPV, improving cold chain management, and increasing manufacturing. In addition, the use of fractional doses of IPV given via the intradermal route is being evaluated. Fractional doses are being employed in India and Sri Lanka for routine use [20]. In October 2016, the WHO Strategic Advisory Group of Experts on Immunization strongly recommended that outbreak response requiring IPV be conducted with fractional doses only [21]. These studies may need to be repeated for vaccines intended for intradermal administration compared to intramuscular administration because the mechanism for immune response via each of these routes differs. Combining the use of fractional doses of IPV, the open vial policy, and this potential increased product stability have the ability to greatly increase the IPV supply.
Acknowledgements
We acknowledge Statens Serum Institut for kindly providing IPV for use in this study. The authors wish to thank Olga Mirochnitchenko, Deborah Moore, Yiting Zhang, Sharla McDonald, Will Hendly, Patricia Mitchell, Mario Nicolas, Yuhua Ji, Taj Munson, and Jeff Sedita for technical assistance. In addition, the authors wish to thank John Ballenot and Leslie Barrett for assistance in the development of this article. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the US Centers for Disease Control and Prevention and other contributing agencies.
Funding
This work was supported by the US Centers for Disease Control and Prevention, Atlanta, GA (contract number 200-2015-87932).
Abbreviations:
- IPV
inactivated poliovirus vaccine
- OPV
oral poliovirus vaccine
- tOPV
trivalent oral poliovirus vaccine
- bOPV
bivalent oral poliovirus vaccine
- DTP
diphtheria-tetanus-pertussis
- DSC
differential scanning calorimetry
- RT
room temperature
- EDQM
European Directorate for the Quality of Medicines & HealthCare
Footnotes
Conflicts of interest
None.
Ethics statement
All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of SNBL USA, Ltd.
Poliomyelitis Vaccine (Inactivated), Types 1, 2, 3 [Biological Reference Preparation] batch 2. Catalogue code: P2160000 (batch 2: validity until 28 February 2017). European Directorate for the Quality of Medicines & HealthCare European Pharmacopoeia (Ph. Eur.) 7, Allée Kastner CS 30026, F-67081 Strasbourg (France).
References
- [1].Global Polio Eradication Initiative, World Health Organization (WHO). Preparing for the withdrawal of all oral polio vaccines (OPVs): replacing trivalent OPV (tOPV) with bivalent OPV (bOPV) – briefing note February 2015 Geneva: WHO; 2015. Available from: http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/OPVswitch-briefing-note-Feb2015.pdf?ua=1 [accessed 15 August, 2017]. [Google Scholar]
- [2].Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, et al. Cessation of use of trivalent oral polio vaccine and introduction of inactivated poliovirus vaccine worldwide. Wkly Epidemiol Rec 2016;91(36/37):421–32. Available from: http://apps.who.int/iris/handle/10665/250045 [accessed 15 August, 2017].27623614 [Google Scholar]
- [3].Pragya S, Akanksha R, Taneja DK, Suneela G. Implications of IPV introduction in national immunization schedule and strategies to combat shortages. Int J Vaccine Res 2016;1(2):3. [Google Scholar]
- [4].US Centers for Disease Control and Prevention. Combined use of inactivated and oral poliovirus vaccines in refugee camps and surrounding communities – Kenya, December 2013. MMWR (Morb Mortal Wkly Rep) 2014;63(11):237–41. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6311a4.htm [accessed 15 August, 2017]. [PMC free article] [PubMed] [Google Scholar]
- [5].Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine 2007;25(7):1328–33. 10.1016/j.vaccine.2006.09.092. [DOI] [PubMed] [Google Scholar]
- [6].World Health Organization (WHO). WHO policy statement: multi-dose vial policy (MDVP): handling of multi-dose vaccine vials after opening Revision 2014 Geneva: WHO; 2014. Available from: http://apps.who.int/iris/handle/10665/135972 [accessed 15 August, 2017]. [Google Scholar]
- [7].Milstien JB, Galazka AM, Kartoglu U, Zaffran M. World Health Organization (WHO). Temperature sensitivity of vaccines Geneva: WHO; 2006. Available from: http://apps.who.int/iris/handle/10665/69387 [accessed 15 August, 2017]. [Google Scholar]
- [8].World Health Organization (WHO) Expanded Programme on Immunization. The effects of freezing on the appearance, potency, and toxicity of adsorbed and unadsorbed DPT vaccines. Wkly Epidemiol Rec 1980;55(50):385–92. Available from: http://apps.who.int/iris/handle/10665/223308 [accessed 15 August, 2017]. [Google Scholar]
- [9].Kartoglu U Temperature sensitivity of the diphtheria containing vaccines. In: Priti R, editor. Insight and control of infectious disease in global scenario Rijeka, Croatia: In Tech; 2012. p. 271–88. Available from: http://www.intechopen.com/books/insight-and-control-of-infectious-disease-in-global-scenario/temperature-sensitivity-of-the-diptheria-containing-vaccines [accessed 15 August, 2017]. [Google Scholar]
- [10].Boros CA, Hanlon M, Gold MS, Roberton DM. Storage at −3 degrees C for 24 h alters the immunogenicity of pertussis vaccines. Vaccine 2001;19(25–26):3537–42. [DOI] [PubMed] [Google Scholar]
- [11].Dimayuga R, Scheifele D, Bell A. Effects of freezing on DPT and DPT-IPV vaccines, adsorbed. Can Comm Dis Rep 1995;21(11):101–3. [PubMed] [Google Scholar]
- [12].European Directorate for the Quality of Medicines & HealthCare (EDQM). BRP information leaflet, European Pharmacopoeia reference standard: poliomyelitis vaccine (inactivated) BRP batch 3 Strasbourg: EDQM; 2016. Available from: https://crs.edqm.eu/db/4DCGI/View=P2160000 [accessed 15 August, 2017]. [Google Scholar]
- [13].National Institute for Biological Standards and Control (NIBSC). International standard for IPV 12/104 (3rd International standard). Potters Bar, UK: NIBSC; 2014. Available from: www.nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=12/104 [accessed 15 August, 2017]. [Google Scholar]
- [14].Rezapkin G, Dragunsky E, Chumakov K. Improved ELISA test for determination of potency of inactivated poliovirus vaccine (IPV). Biologicals 2005;33(1):17–27. 10.1016/j.biologicals.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [15].van Steenis G, Van Wezel AL, Sekhuis VM. Potency testing of killed polio vaccine in rats. Dev Biol Stand 1981;47:119–28. [PubMed] [Google Scholar]
- [16].World Health Organization (WHO) Expert Committee on Biological Standardization. Recommendations to assure the quality, safety and efficacy of poliomyelitis vaccines (inactivated) Sixty-fifth report WHO technical report series 993, Annex 3 Geneva: WHO; 2015. p. 89–174. Available from: http://apps.who.int/iris/handle/10665/173739 [accessed 15 August, 2017]. [Google Scholar]
- [17].World Health Organization (WHO). Vaccine Carrier with freeze-prevention technology Geneva: WHO; 2014. Available from: http://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/catdocumentation.aspx?id_cat=18 [accessed 15 August, 2017]. [Google Scholar]
- [18].World Health Organization (WHO). Vaccine Carrier Geneva: WHO; 2010. Available from: http://www.who.int/immunization_standards/vaccine_quality/pqs_e004_vc01_vp2.pdf?ua=1 [accessed 15 August, 2017]. [Google Scholar]
- [19].Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Meth Mol Biol 2016;1387:145–76. 10.1007/978-1-4939-3292-4_8. [DOI] [PubMed] [Google Scholar]
- [20].World Health Organization (WHO). Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Wkly Epidemiol Rec 2010;85(23):213–28. Available from: http://apps.who.int/iris/handle/10665/241581 [accessed 15 August, 2017]. [PubMed] [Google Scholar]
- [21].World Health Organization Department of Immunization, Vaccine and Biologicals. Meeting of the strategic advisory group of Experts on immunization, october 2016–conclusions and recommendations. Wkly Epidemiol Rec 2016;91(48):561–84. Available from: http://apps.who.int/iris/bitstream/10665/251810/1/WER9148.pdf?ua=1 [accessed 15 August, 2017].27922031 [Google Scholar]


